Transition-Metal-Free Activation of Amide Bond by Arynes
Abstract
:1. Introduction
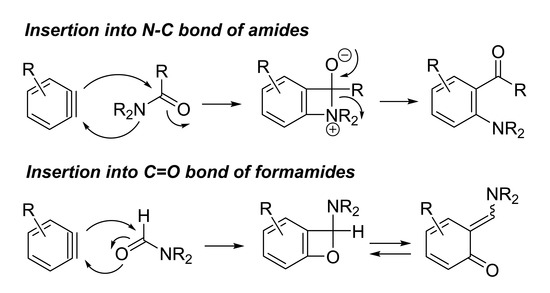
2. N–C Bond Activation
3. C=O Bond Activation
4. Activation of Relative Bonds
5. Concluding Remarks
Funding
Conflicts of Interest
References
- Saito, S.; Yamamoto, Y. Recent advances in the transition-metal-catalyzed regioselective approaches to polysubstituted benzene derivatives. Chem. Rev. 2000, 100, 2901–2915. [Google Scholar] [CrossRef] [PubMed]
- Wenk, H.H.; Winkler, M.; Sander, W. One century of aryne chemistry. Angew. Chem. Int. Ed. 2003, 42, 502–528. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, H.; Santelli, M. The use of arynes in organic synthesis. Tetrahedron 2003, 59, 701–730. [Google Scholar] [CrossRef]
- Peña, D.; Pérez, D.; Guitián, E. Aryne-mediated synthesis of heterocycles. Heterocycles 2007, 74, 89–100. [Google Scholar] [CrossRef]
- Yoshida, H.; Ohshita, J.; Kunai, A. Aryne, ortho-quinone methide, and ortho-quinodimethane: Synthesis of multisubstituted arenes using the aromatic reactive intermediates. Bull. Chem. Soc. Jpn. 2010, 83, 199–219. [Google Scholar] [CrossRef]
- Bhunia, A.; Yetra, S.R.; Biju, A.T. Recent advances in transition-metal-free carbon-carbon and carbon-heteroatom bond-forming reactions using arynes. Chem. Soc. Rev. 2012, 41, 3140–3152. [Google Scholar] [CrossRef] [PubMed]
- Tadross, P.M.; Stoltz, B.M. A comprehensive history of arynes in natural product total synthesis. Chem. Rev. 2012, 112, 3550–3577. [Google Scholar] [CrossRef] [PubMed]
- Gampe, C.M.; Carreira, E.M. Arynes and cyclohexyne in natural product synthesis. Angew. Chem. Int. Ed. 2012, 51, 3766–3778. [Google Scholar] [CrossRef] [PubMed]
- Miyabe, H. Synthesis of Oxygen Heterocycles via Aromatic C–O Bond Formation Using Arynes. Molecules 2015, 20, 12558–12575. [Google Scholar] [CrossRef] [PubMed]
- Goetz, A.E.; Shah, T.K.; Garg, N.K. Pyridynes and indolynes as building blocks for functionalized heterocycles and natural products. Chem. Commun. 2015, 51, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Bhojgude, S.S.; Bhunia, A.; Biju, A.T. Employing arynes in Diels-Alder reactions and transition-metal-free multicomponent coupling and arylation reactions. Acc. Chem. Res. 2016, 49, 1658–1670. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, R.; Lee, D. Reactions of arynes promoted by silver ions. Chem. Soc. Rev. 2016, 45, 4459–4470. [Google Scholar] [CrossRef] [PubMed]
- García-López, J.-A.; Greaney, M.F. Synthesis of biaryls using aryne intermediates. Chem. Soc. Rev. 2016, 45, 6766–6798. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, Y.; Li, Y. Aryne multifunctionalization with benzdiyne and benztriyne equivalents. Chem. Soc. Rev. 2017, 46, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Diamond, O.J.; Marder, T.B. Methodology and applications of the hexadehydro-Diels-Alder (HDDA) reaction. Org. Chem. Front. 2017, 4, 891–910. [Google Scholar] [CrossRef]
- Guitián, E.; Pérez, D.; Peña, D. Palladium-catalyzed cycloaddition reactions of arynes. Top. Organomet. Chem. 2005, 14, 109–146. [Google Scholar] [CrossRef]
- Worlikar, S.A.; Larock, R.C. Pd-catalyzed reactions involving arynes. Curr. Org. Chem. 2011, 15, 3214–3232. [Google Scholar] [CrossRef]
- Himeshima, Y.; Sonoda, T.; Kobayashi, H. Fluoride-induced 1,2-elimination of o-trimethylsilylphenyl triflate to benzyne under mild conditions. Chem. Lett. 1983, 12, 1211–1214. [Google Scholar] [CrossRef]
- Bai, W.-J.; David, J.G.; Feng, Z.-G.; Weaver, M.G.; Wu, K.-L.; Pettus, T.R.R. The domestication of ortho-quinone methides. Acc. Chem. Res. 2014, 47, 3655–3664. [Google Scholar] [CrossRef] [PubMed]
- Van De Water, R.W.; Pettus, T.R.R. o-Quinone methides: Intermediates underdeveloped and underutilized in organic synthesis. Tetrahedron 2002, 58, 5367–5405. [Google Scholar] [CrossRef]
- Yoshida, H.; Shirakawa, E.; Honda, Y.; Hiyama, T. Addition of ureas to arynes: Straightforward synthesis of benzodiazepine and benzodiazocine derivatives. Angew. Chem. Int. Ed. 2002, 41, 3247–3249. [Google Scholar] [CrossRef]
- Saito, N.; Nakamura, K.; Shibano, S.; Ide, S.; Minami, M.; Sato, Y. Addition of cyclic ureas and 1-methyl-2-oxazolidone to pyridynes: A new approach to pyridodiazepines, pyridodiazocines, and pyridooxazepines. Org. Lett. 2014, 15, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.K.; Medina, J.M.; Garg, N.K. Expanding the strained alkyne toolbox: Generation and utility of oxygen-containing strained alkynes. J. Am. Chem. Soc. 2016, 138, 4948–4954. [Google Scholar] [CrossRef] [PubMed]
- Mesgar, M.; Daugulis, O. Silylaryl halides can replace triflates as aryne precursors. Org. Lett. 2016, 18, 3910–3913. [Google Scholar] [CrossRef]
- Liu, Z.; Larock, R.C. Intermolecular C–N addition of amides and S–N addition of sulfinamides to arynes. J. Am. Chem. Soc. 2005, 127, 13112–13113. [Google Scholar] [CrossRef] [PubMed]
- Pintori, D.G.; Greaney, M.F. Insertion of benzene rings into the amide bond: One-step synthesis of acridines and acridones from aryl amides. Org. Lett. 2010, 12, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Rogness, D.C.; Larock, R.C.; Shi, F. Formation of acridones by ethylene extrusion in the reaction of arynes with β-lactams and dihydroquinolinones. J. Org. Chem. 2012, 77, 6262–6270. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.C.; Haley, C.K.; Lapointe, G.; Stoltz, B.M. Synthesis of aryl ketoamides via aryne insertion into imides. Org. Lett. 2016, 18, 2793–2795. [Google Scholar] [CrossRef] [PubMed]
- Yaroslavsky, S. Reaction of aryldiazonium salts with dimethylformamide. Tetrahedron Lett. 1965, 6, 1503–1507. [Google Scholar] [CrossRef]
- Yoshioka, E.; Kohtani, S.; Miyabe, H. Sequential reaction of arynes via insertion into the π-bond of amides and trapping reaction with dialkylzincs. Org. Lett. 2010, 12, 1956–1959. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.; Miyabe, H. Insertion of arynes into the carbon-oxygen double bond of amides and its application into the sequential reactions. Tetrahedron 2012, 68, 179–189. [Google Scholar] [CrossRef]
- Liu, F.; Yang, H.; Hu, X.; Jiang, G. Metal-free synthesis of ortho-CHO diaryl ethers by a three-component sequential coupling. Org. Lett. 2014, 16, 6408–6411. [Google Scholar] [CrossRef] [PubMed]
- Okuma, K.; Nojima, A.; Nakamura, Y.; Matsunaga, N.; Nagahora, N.; Shioji, K. Reaction of benzyne with formamides and acetylimidazole. Bull. Chem. Soc. Jpn. 2011, 84, 328–332. [Google Scholar] [CrossRef]
- Yoshioka, E.; Kohtani, S.; Miyabe, H. A multicomponent coupling reaction induced by insertion of arynes into C=O bond of formamide. Angew. Chem. Int. Ed. 2011, 50, 6638–6642. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Ito, Y.; Ohshita, J. Three-component coupling using arynes and DMF: straightforward access to coumarinsvia ortho-quinone methides. Chem. Commun. 2011, 47, 8512–8514. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.-R.; Man, N.-N.; Yuan, W.-K.; Li, M. Direct construction of 2-aryliminochromenes from arynes, N,S-keteneacetals, and DMF. J. Org. Chem. 2016, 81, 5942–5948. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.; Tamenaga, H.; Miyabe, H. [4 + 2] cycloaddition of intermediates generated from arynes and DMF. Tetrahedron Lett. 2014, 55, 1402–1405. [Google Scholar] [CrossRef]
- Yoshioka, E.; Tanaka, H.; Kohtani, S.; Miyabe, H. Straightforward synthesis of dihydrobenzofurans and benzofurans from arynes. Org. Lett. 2013, 15, 3938–3941. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.; Miyabe, H. Three-component coupling reactions of arynes for the synthesis of benzofurans and coumarins. Molecules 2014, 19, 863–880. [Google Scholar] [CrossRef] [PubMed]
- Neog, K.; Das, B.; Gogoi, P. 2,3-Diaroyl benzofurans from arynes: sequential synthesis of 2-aroyl benzofurans followed by benzoylation. Org. Biomol. Chem. 2018, 16, 3138–3150. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.; Kohtani, S.; Miyabe, H. 2,3,4,9-Tetrahydro-9-(3-hydroxy-1,4-dioxo-1H-dihydronaphthalen-2-yl)-8-methoxy-3,3-dimethyl-1H-xanthen-1-one. Molbank 2015, 2015, M841. [Google Scholar] [CrossRef]
- Yoshioka, E.; Nishimura, M.; Nakazawa, T.; Kohtani, S.; Miyabe, H. Multicomponent coupling reaction using arynes: Synthesis of xanthene derivatives. J. Org. Chem. 2015, 80, 8464–8469. [Google Scholar] [CrossRef] [PubMed]
- Gorobets, E.; Parvez, M.; Derksen, D.J.; Keay, B.A. Generation of benzyne species from diphenylphosphoryl derivatives: Simultaneous exchange of three functional groups. Chem. Eur. J. 2016, 22, 8479–8482. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Yang, G.; Zhang, W. Insertion of arynes into arylphosphoryl amide bonds: One-step simultaneous construction of C–N and C–P bonds. Org. Lett. 2013, 15, 5722–5725. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.; Zeng, X. Aminocyanation by the addition of N–CN bonds to arynes: Chemoselective synthesis of 1,2-bifunctional aminobenzonitriles. Org. Lett. 2014, 16, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Holden, C.M.; Sohel, S.M.A.; Greaney, M.F. Metal free bi(hetero)aryl synthesis: A benzyne Truce–Smiles rearrangement. Angew. Chem. Int. Ed. 2016, 55, 2450–2453. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; Greaney, M.F. Insertion of arynes into thioureas: A new amidine synthesis. Org. Lett. 2011, 13, 4946–4949. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, X.; Wei, Z.; Wu, C.; Shi, F. Reaction of arynes with vinylogous amides: Nucleophilic addition to the ortho-quinodimethide intermediate. Org. Lett. 2013, 15, 4366–4369. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Tang, H.; Fu, H.; Ren, H.; Wang, X.; Wu, C.; Wu, C.; Shi, F. Arynes double bond insertion/nucleophilic addition with vinylogous amides and carbodiimides. J. Org. Chem. 2014, 79, 1344–1355. [Google Scholar] [CrossRef] [PubMed]

























© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyabe, H. Transition-Metal-Free Activation of Amide Bond by Arynes. Molecules 2018, 23, 2145. https://doi.org/10.3390/molecules23092145
Miyabe H. Transition-Metal-Free Activation of Amide Bond by Arynes. Molecules. 2018; 23(9):2145. https://doi.org/10.3390/molecules23092145
Chicago/Turabian StyleMiyabe, Hideto. 2018. "Transition-Metal-Free Activation of Amide Bond by Arynes" Molecules 23, no. 9: 2145. https://doi.org/10.3390/molecules23092145
APA StyleMiyabe, H. (2018). Transition-Metal-Free Activation of Amide Bond by Arynes. Molecules, 23(9), 2145. https://doi.org/10.3390/molecules23092145