Ionic Liquids: Efficient Media for the Lipase-Catalyzed Michael Addition
Abstract
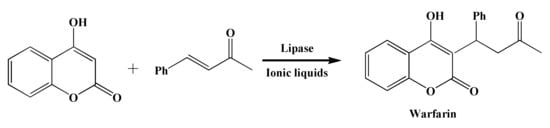
:1. Introduction
2. Results and Discussion
2.1. Selection of ILs
2.2. Optimization of the Warfarin Yield
2.3. Reusability of Lipase and [C1C3OHim]NTf2
2.4. Separation of Warfarin from the Reaction Mixture
3. Materials and Methods
3.1. Reagents
3.2. Determination of Warfarin
3.3. Determination of the Hydrophobic Parameters of the ILs
3.4. Synthesis of Warfarin
3.5. Measurements of Fourier Transform Infrared (FT-IR) Spectra
3.6. Measurements of the Specific Rotation of the Synthesized Warfarin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ghaffari-Moghaddam, M.; Eslahi, H.; Aydin, Y.A.; Saloglu, D. Enzymatic processes in alternative reaction media: A mini review. J. Biol. Meth. 2015, 2, e25. [Google Scholar] [CrossRef]
- Solano, D.M.; Hoyos, P.; Hernáiz, M.J.; Alcántara, A.R.; Sánchez-Montero, J.M. Industrial biotransformations in the synthesis of building blocks leading to enantiopure drugs. Bioresour. Technol. 2012, 115, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yang, L.; Liu, S.; Yang, J.; Zhang, H.; Yan, J.; Hu, X. Enzyme-catalyzed Michael addition for the synthesis of warfarin and its determination via fluorescence quenching of L-tryptophan. Spectrochim. Acta A 2017, 176, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Steunenberg, P.; Sijm, M.; Zuilhof, H.; Sanders, J.P.M.; Scott, E.L.; Franssen, M.C.R. Lipase-catalyzed aza-Michael reaction on acrylate derivatives. J. Org. Chem. 2013, 78, 3802–3813. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Saito, S.; Hirose, Y.; Kohari, Y.; Nakano, H.; Seki, C.; Tokiwa, M.; Takeshita, M.; Uwai, K. Development of a novel method for warfarin synthesis via lipase-catalyzed stereoselective Michael reaction. Heterocycles 2013, 87, 1269–1278. [Google Scholar] [CrossRef]
- Stergiou, P.Y.; Foukis, A.; Filippou, M.; Koukouritaki, M.; Parapouli, M.; Theodorou, L.G.; Hatziloukas, E.; Afendra, A.; Pandey, A.; Papamichael, E.M. Advances in lipase-catalyzed esterification reactions. Biotechnol. Adv. 2013, 31, 1846–1859. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, M.; Osuga, J.I.; Yahagi, N.; Okazaki, H.; Tamura, Y.; Igarashi, M.; Takase, S.; Harada, K.; Okazaki, S.; Iizuka, Y.; et al. Hormone-sensitive lipase is involved in hepatic cholesteryl ester hydrolysis. J. Lipid Res. 2008, 49, 1829–1838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canet, A.; Bonet-Ragel, K.; Benaiges, M.D.; Valero, F. Lipase-catalysed transesterification: Viewpoint of the mechanism and influence of free fatty acids. Biomass Bioenergy 2016, 85, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Danziger, J. Vitamin K-dependent proteins, warfarin, and vascular calcification. Clin. J. Am. Soc. Nephrol. 2008, 3, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Anderes, E.; Nand, S. Commonly used drugs in hematologic disorders, Neurologic Aspects of Systemic Disease Part II. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1125–1139. [Google Scholar]
- Xie, B.H.; Guan, Z.; He, Y.H. Promiscuous enzyme-catalyzed Michael addition: Synthesis of warfarin and derivatives. J. Chem. Technol. Biotechnol. 2012, 87, 1709–1714. [Google Scholar] [CrossRef]
- Galvao, J.; Davis, B.; Tilley, M.; Normando, E.; Duchen, M.R.; Cordeiro, M.F. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 2014, 28, 1317–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raut, D.G.; Sundman, O.; Su, W.; Virtanen, P.; Sugano, Y.; Kordas, K.; Mikkola, J.P. A morpholinium ionic liquid for cellulose dissolution. Carbohydr. Polym. 2015, 130, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Vijaykumar, B.V.D.; Premkumar, B.; Jang, K.; Choi, B.I.; Falck, J.R.; Sheldrake, G.N.; Shin, D.S. Environmentally benign perfluorooctanesulfonate alternatives using a Zn/CuI mediated Michael-type addition in imidazolium ionic liquids. Green Chem. 2014, 16, 2406–2410. [Google Scholar] [CrossRef]
- Schutt, T.C.; Hegde, G.A.; Bharadwaj, V.S.; Johns, A.J.; Maupin, C.M. Impact of water-dilution on the solvation properties of the ionic liquid 1-methyltriethoxy-3-ethylimidazolium acetate for model biomass molecules. J. Phys. Chem. B 2017, 121, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T. Ionic liquids as tool to improve enzymatic organic synthesis. Chem. Rev. 2017, 117, 10567–10607. [Google Scholar] [CrossRef] [PubMed]
- Lozano, P.; Bernal, J.M.; Garcia-Verdugo, E.; Sanchez-Gomez, G.; Vaultier, M.; Burguete, M.I.; Luis, S.V. Sponge-like ionic liquids: A new platform for green biocatalytic chemical processes. Green Chem. 2015, 17, 3706–3717. [Google Scholar] [CrossRef]
- Xu, P.; Zheng, G.W.; Du, P.X.; Zong, M.H.; Lou, W.Y. Whole-cell biocatalytic processes with ionic liquids. ACS Sustain. Chem. Eng. 2016, 4, 371–386. [Google Scholar] [CrossRef]
- Burney, P.R.; Nordwald, E.M.; Hickman, K.; Kaar, J.L.; Pfaendtner, J. Molecular dynamics investigation of the ionic liquid/enzyme interface: Application to engineering enzyme surface charge. Proteins Struct. Funct. Bioinform. 2015, 83, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.G.; Wu, Y.; Lu, X.Y.; Ren, Y.P.; Wang, Q.; Zhu, C.M.; Yu, D.; Wang, H. Lipase-catalyzed esterification of ferulic acid with lauryl alcohol in ionic liquids and antibacterial properties in vitro against three food-related bacteria. Food Chem. 2017, 220, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.Y.; Zhao, Y.J.; Shi, Y.G.; Xia, C.G.; Li, S.B. Lipase-catalyzed naproxen methyl ester hydrolysis in water-saturated ionic liquid: Significantly enhanced enantioselectivity and stability. World J. Microb. Biotechnol. 2005, 21, 193–199. [Google Scholar] [CrossRef]
- Su, F.; Peng, C.; Li, G.L.; Xu, L.; Yan, Y.J. Biodiesel production from woody oil catalyzed by Candida rugosa lipase in ionic liquid. Renew. Energy 2016, 90, 329–335. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, D.; Yan, Y.; Peng, C.; Xu, L. Biodiesel synthesis and conformation of lipase from Burkholderia cepacia in room temperature ionic liquids and organic solvents. Bioresour. Technol. 2011, 102, 10414–10418. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.; Happe, M.; Emery, J.; Fornage, A.; Schütz, R. Enzymatic synthesis of 6- and 6′-O-linoleyl-α-d-maltose: From solvent-free to binary ionic liquid reaction media. J. Mol. Catal. B 2013, 90, 98–106. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, Z.; Xu, X. Ionic liquid-assisted solubilization for improved enzymatic esterification of phenolic acids. J. Am. Oil Chem. Soc. 2012, 89, 1049–1055. [Google Scholar] [CrossRef]
- Freire, M.G.; Neves, C.M.; Marrucho, I.M.; Coutinho, J.A.; Fernandes, A.M. Hydrolysis of tetrafluoroborate and hexafluorophosphate counter ions in imidazolium-based ionic liquids. J. Phys. Chem. A 2010, 114, 3744–3749. [Google Scholar] [CrossRef] [PubMed]
- Sowmiah, S.; Srinivasadesikan, V.; Tseng, M.C.; Chu, Y.H. On the chemical stabilities of ionic liquids. Molecules 2009, 14, 3780–3813. [Google Scholar] [CrossRef] [PubMed]
- Somers, A.E.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. A review of ionic liquid lubricants. Lubricants 2013, 1, 3–21. [Google Scholar] [CrossRef]
- Dong, X.; Fan, Y.; Yang, P.; Kong, J.; Li, D.; Miao, J.; Hua, S.; Hu, C. Ultraviolet-visible (UV-Vis) and fluorescence spectroscopic investigation of the interactions of ionic liquids and catalase. Appl. Spectrosc. 2016, 70, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.A.; Tzani, A.; Alivertis, D.; Katsoura, M.H.; Polydera, A.C.; Detsi, A.; Stamatis, H. Hydroxyl ammonium ionic liquids as media for biocatalytic oxidations. Green Chem. 2016, 18, 1147–1158. [Google Scholar] [CrossRef]
- Hernández-Fernández, F.J.; de los Ríos, A.P.; Tomás-Alonso, F.; Gómez, D.; Víllora, G. Stability of hydrolase enzymes in ionic liquids. Can. J. Chem. Eng. 2009, 87, 910–914. [Google Scholar] [CrossRef]
- Zhao, H. Methods for stabilizing and activating enzymes in ionic liquids—A review. J. Chem. Technol. Biotechnol. 2010, 85, 891–907. [Google Scholar] [CrossRef]
- Klähn, M.; Lim, G.S.; Seduraman, A.; Wu, P. On the different roles of anions and cations in the solvation of enzymes in ionic liquids. Phys. Chem. Chem. Phys. 2011, 13, 1649–1662. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Q.; Li, Z.; Lü, Y.H.; Yang, Z. Specific ion effects of ionic liquids on enzyme activity and stability. Green Chem. 2011, 13, 1860–1868. [Google Scholar] [CrossRef]
- Zhao, H.; Baker, G.A.; Holmes, S. New eutectic ionic liquids for lipase activation and enzymatic preparation of biodiesel. Org. Biomol. Chem. 2011, 9, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.P.M.; Santos, L.D.F.; Saraiva, J.A.; Coutinho, J.A.P. Concentration effect of hydrophilic ionic liquids on the enzymatic activity of Candida antarctica lipase B. World J. Microb. Biotechnol. 2012, 28, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Hegde, G.A.; Bharadwaj, V.S.; Kinsinger, C.L.; Schutt, T.C.; Pisierra, N.R.; Maupin, C.M. Impact of water dilution and cation tail length on ionic liquid characteristics: Interplay between polar and non-polar interactions. J. Chem. Phys. 2016, 145, 064504. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, H.; Pan, H.; Liu, X.; Yang, K.; Huang, S.; Yang, S. Efficient production of biodiesel with promising fuel properties from Koelreuteria integrifoliola oil using a magnetically recyclable acidic ionic liquid. Energy Convers. Manag. 2017, 138, 45–53. [Google Scholar] [CrossRef]
- Yang, J.; Feng, Y.; Zeng, T.; Guo, X.; Li, L.; Hong, R.; Qiu, T. Synthesis of biodiesel via transesterification of tung oil catalyzed by new Brönsted acidic ionic liquid. Chem. Eng. Res. Des. 2017, 117, 584–592. [Google Scholar] [CrossRef]
- Zhang, K.P.; Lai, J.Q.; Huang, Z.L.; Yang, Z. Penicillium expansum lipase-catalyzed production of biodiesel in ionic liquids. Bioresour. Technol. 2011, 102, 2767–2772. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.D.; Santos, A.G.; Marrucho, I.M. Biocatalysis in ionic liquids. In White Biotechnology for Sustainable Chemistry; Royal Society of Chemistry: Cambridge, UK, 2015. [Google Scholar]
- Aghababaie, M.; Beheshti, M.; Razmjou, A.; Bordbar, A.K. Enzymatic biodiesel production from crude Eruca sativa oil using Candida rugosa lipase in a solvent-free system using response surface methodology. Biofuels 2017, 14, 1–7. [Google Scholar] [CrossRef]
- Bhonoah, Y. Warfarin: Rat Poison or Wonder Drug? Available online: http://www.ch.ic.ac.uk/local/projects/bhonoah/characterisation.html (accessed on 17 August 2018).
- Takahashi, H.; Echizen, H. Pharmacogenetics of warfarin elimination and its clinical implications. Clin. Pharmacokinet. 2001, 40, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Dong, X.; Li, Y.; Zhong, Y.; Miao, J.; Hua, S.; Sun, Y. Extraction of L-tryptophan by hydroxyl-functionalized ionic liquids. Ind. Eng. Chem. Res. 2015, 54, 12966–12973. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, S.B. Octanol/water partition coefficients of ionic liquids. J. Chem. Technol. Biotechnol. 2009, 84, 202–207. [Google Scholar] [CrossRef]
- Ropel, L.; Belvèze, L.S.; Aki, S.N.V.K.; Stadtherr, M.A.; Brennecke, J.F. Octanol–water partition coefficients of imidazolium-based ionic liquids. Green Chem. 2005, 7, 83–90. [Google Scholar] [CrossRef]
- Jamalzadeh, L.; Ghafoori, H.; Sariri, R.; Rabuti, H.; Nasirzade, J.; Hasani, H.; Aghamaali, M.R. Cytotoxic effects of some common organic solvents on MCF-7, RAW-264.7 and human umbilical vein endothelial cells. Avicenna J. Med. Biochem. 2016, 4, e33453. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |






| Name | Abbreviation |
|---|---|
| 1-Butyl-3-methylimidazolium tetrafluoroborate | [C1C4im]BF4 |
| 1-Butyl-3-methylimidazolium hexafluorophosphate | [C1C4im]PF6 |
| 1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide | [C1C4im]NTf2 |
| 1-Methyl-3-(6-hydroxyhexyl) imidazolium bis(trifluoromethylsulfonyl)imide | [C1C6OHim]NTf2 |
| 1-Butyl-3-(3-hydroxypropyl) imidazolium bis(trifluoromethylsulfonyl)imide | [C4C3OHim]NTf2 |
| 1-Methyl-3-(3-hydroxypropyl) imidazolium bis(trifluoromethylsulfonyl)imide | [C1C3OHim]NTf2 |
| 1-Butyl-3-(11-hydroxyundecyl) imidazolium bis(trifluoromethylsulfonyl)imide | [C4C11OHim]NTf2 |
| 1-Methyl-3-(11-hydroxyundecyl) imidazolium bis(trifluoromethylsulfonyl)imide | [C1C11OHim]NTf2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Cai, D.; Wang, X.; Yang, L. Ionic Liquids: Efficient Media for the Lipase-Catalyzed Michael Addition. Molecules 2018, 23, 2154. https://doi.org/10.3390/molecules23092154
Fan Y, Cai D, Wang X, Yang L. Ionic Liquids: Efficient Media for the Lipase-Catalyzed Michael Addition. Molecules. 2018; 23(9):2154. https://doi.org/10.3390/molecules23092154
Chicago/Turabian StyleFan, Yunchang, Dongxu Cai, Xin Wang, and Lei Yang. 2018. "Ionic Liquids: Efficient Media for the Lipase-Catalyzed Michael Addition" Molecules 23, no. 9: 2154. https://doi.org/10.3390/molecules23092154
APA StyleFan, Y., Cai, D., Wang, X., & Yang, L. (2018). Ionic Liquids: Efficient Media for the Lipase-Catalyzed Michael Addition. Molecules, 23(9), 2154. https://doi.org/10.3390/molecules23092154