Design, Synthesis and Biological Evaluation of Ligustrazine-Flavonoid Derivatives as Potential Anti-Tumor Agents
Abstract
:1. Introduction
2. Results
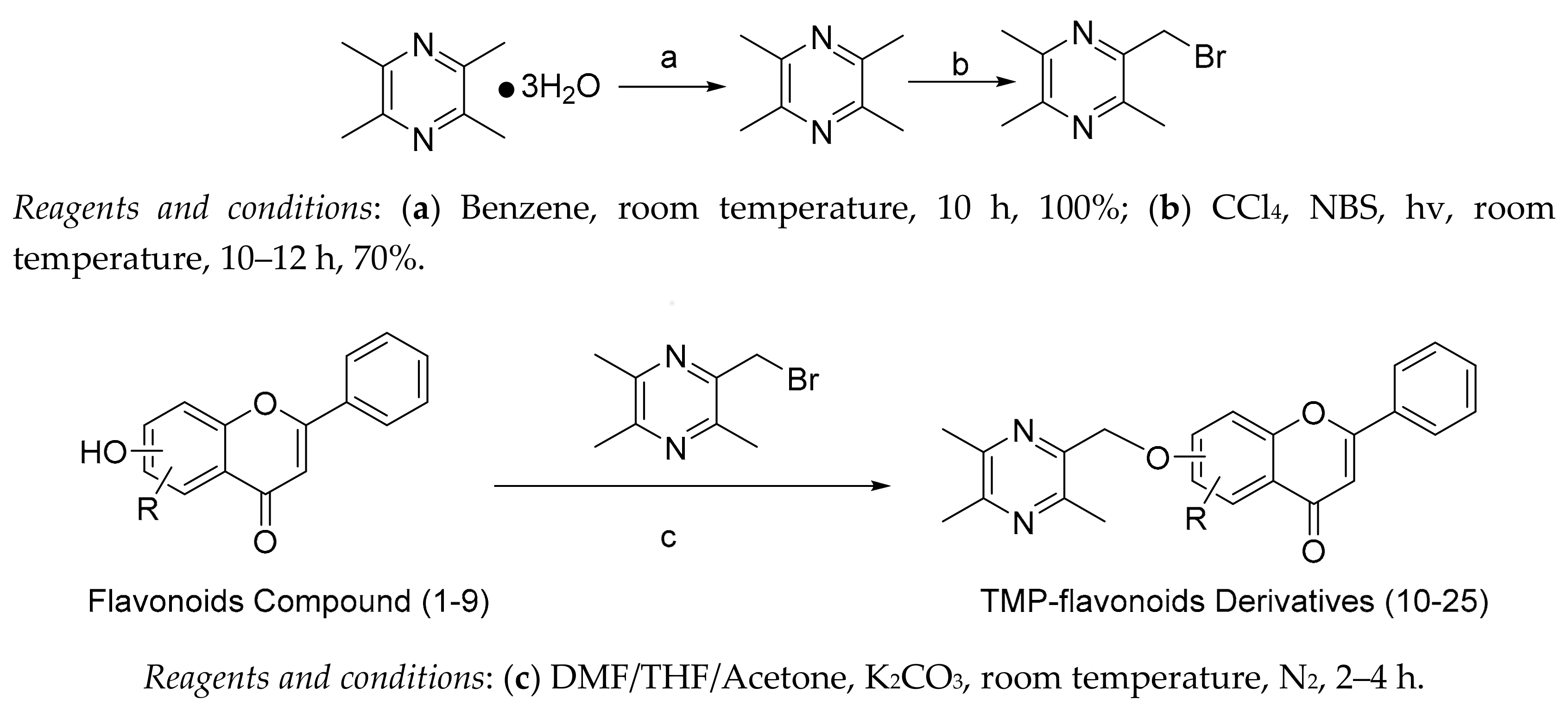
2.1. Chemical Synthesis
2.2. Biological Activities
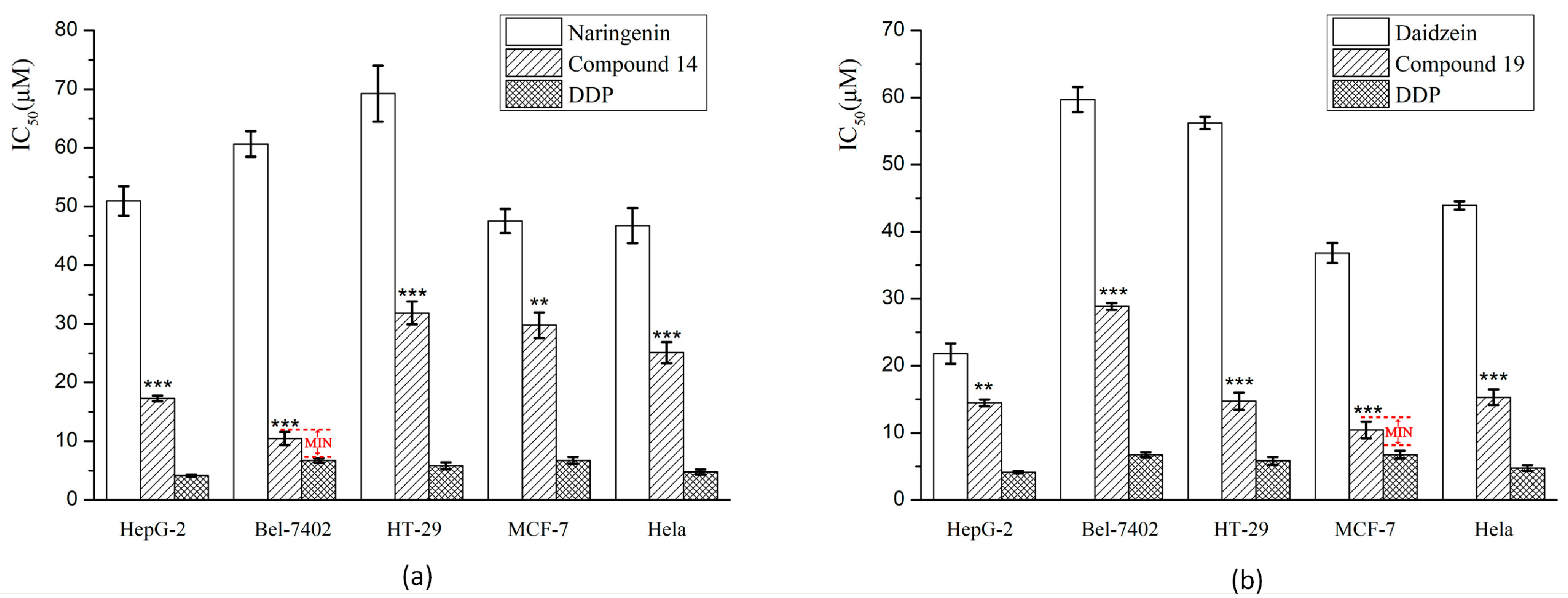
2.2.1. Cytotoxicity Assay Using Five Tumor Cell Lines
2.2.2. Cytotoxicity Assay Using HUVEC-12 Cell
2.2.3. Morphological Analysis Using Giemsa and DAPI Staining
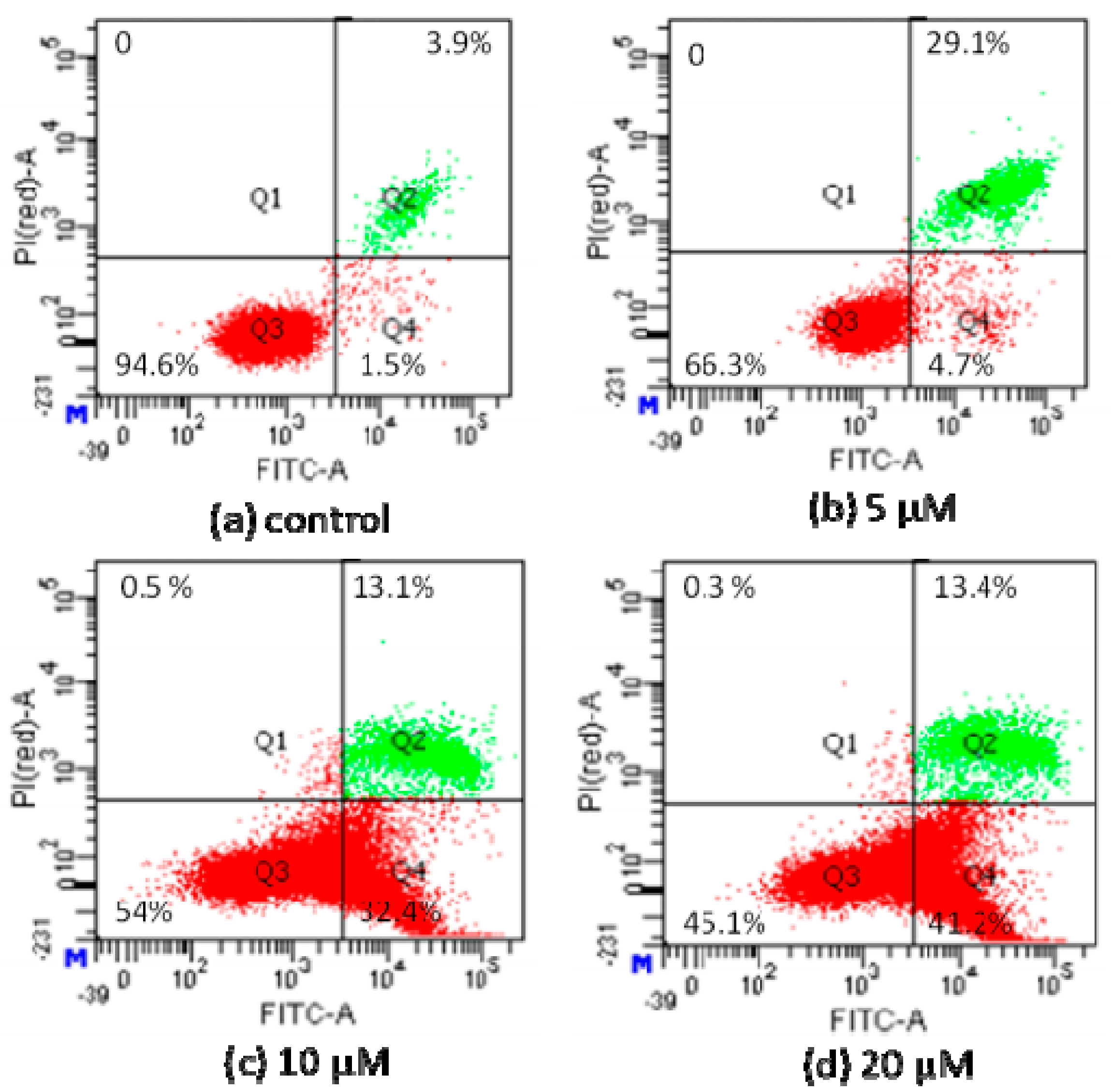
2.2.4. Apoptosis Analysis by Flow Cytometric Using Annexin V-FITC/Propidium Iodide (PI) Staining
3. Discussion
4. Materials and Methods
4.1. Materials and Instruments
4.2. Chemical Syntheses
4.3. Bio-Evaluation Methods
4.3.1. Drugs
4.3.2. Cell Culture
4.3.3. Cytotoxicity Assay Using Five Tumor Cell Lines
4.3.4. Cytotoxicity Assay Using HUVEC-12 Cell
4.3.5. Morphological Analysis Using Giemsa and DAPI Staining
4.3.6. Apoptosis Analysis by Flow Cytometric Using Annexin V-FITC/Propidium Iodide (PI) Staining
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.W.; Zeng, H.M.; Bray, F.; Jemal, A.; Yu, X.Q. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.J.; Ward, Z.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 65, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.Y.; Ma, X.J.; Liu, Q.; Yin, H.J.; Shi, D.Z. Effect of chinese herbal drug-containing serum for activating blood, activating blood and dispelling toxin on tnf-alpha-induced adherence between endothelial cells and neutrophils and the expression of mapk pathway. Chin. J. Integr. Med. 2015, 35, 204–209. [Google Scholar]
- Tao, L.; Wang, S.; Zhao, Y.; Wang, A.Y.; Zhang, L.; Ruan, J.S.; Fan, F.T.; Liu, Y.P.; Li, Y.; Yue, Z.Q.; et al. Pleiotropic effects of herbs characterized with blood-activating and stasis-resolving functions on angiogenesis. Chin. J. Integr. Med. 2016, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007, 6, 273. [Google Scholar] [CrossRef] [PubMed]
- Groselj, A.; Kranjc, S.; Bosnjak, M.; Krzan, M.; Kosjek, T.; Prevc, A.; Cemazar, M.; Sersa, G. Vascularization of the tumours affects the pharmacokinetics of bleomycin and the effectiveness of electrochemotherapy. Basic Clin. Pharmacol. Toxicol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Keedy, V.L.; Sandler, A.B. Inhibition of angiogenesis in the treatment of non-small cell lung cancer. Cancer Sci. 2007, 98, 1825–1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saif, M.W.; Elfiky, A.; Salem, R.R. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann. Surg. Oncol. 2007, 14, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Papetti, M.; Herman, I.M. Mechanisms of normal and tumor-derived angiogenesis. Am. J. Physiol. Cell. Physiol. 2002, 282, 947–970. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Maishi, N.; Ohga, N.; Hida, Y.; Ohba, Y.; Alam, M.T.; Taisuke, K.; Hitomi, O.; Kenji, Y.; Chisaho, T.; et al. Inhibition of multidrug transporter in tumor endothelial cells enhances antiangiogenic effects of low-dose metronomic paclitaxel. Am. J. Pathol. 2015, 185, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Maishi, N.; Torii, C.; Hida, Y. Tumor angiogenesis—Characteristics of tumor endothelial cells. Int. J. Clin. Oncol. 2016, 21, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Petrik, J.; Lawler, J.; Kortenaar, S.T. Anti-angiogenic therapy and induction of blood vessel normalization in the treatment of ovarian cancer. Curr. Ang. 2014, 3, 203–214. [Google Scholar]
- Ren, S.Q.; Yang, J.Z.; Yang, S.M.; Song, Y.Z.; Cao, D.Y. Bacteriostatic activity of combined application of galla chinensis, scutellaria baicalensis and rhizoma coptidis against mrsa in vitro. Chin. Pharm. 2010, 21, 198–199. [Google Scholar]
- Zhang, C.Z.; Zhao, R.; Yan, W.Q.; Wang, H.; Jia, M.L.; Zhu, N.L.; Zhu, Y.D.; Zhang, Y.Z.; Wang, P.L.; Lei, H.M. Compositions, formation mechanism, and neuroprotective effect of compound precipitation from the traditional chinese prescription huang-lian-jie-du-tang. Molecules 2016, 21, 1094. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.M.; Wu, H.Y.; Wu, Y.J.; Liu, L.; Cai, R.P.; Xu, Y.J. Effects of baicalin and ligustrazine on airway inflammation and remodeling and underlying mechanism in asthmatic rats. Adv. Biosci. Biotechnol. 2012, 3, 585–591. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X. Study on the effects of tetramethylpyrazine on tumor cells: Survey and prospects. Chin. J. Integr. Med. 2003, 28, 295–298. [Google Scholar]
- Wang, X.B.; Wang, S.S.; Zhang, Q.F.; Liu, M.; Li, H.L.; Liu, Y.; Wang, J.N.; Zheng, F.; Guo, L.Y.; Xiang, J.Z. Inhibition of tetramethylpyrazine on P-gp, MRP2, MRP3 and MRP5 in multidrug resistant human hepatocellular carcinoma cells. Oncol. Rep. 2010, 23, 211–215. [Google Scholar] [PubMed]
- Yang, X.G.; Jiang, C. Ligustrazine as a salvage agent for patients with relapsed or refractory non-hodgkin’s lymphoma. Chin. Med. J. 2010, 123, 3206–3211. [Google Scholar] [PubMed]
- Cui, C.; Wang, R.T.; Zhang, Y.H.; Zhi, G.C.; Wang, Z.H.; Deng, Y.Q.; Zhang, Z.Z. Study on the down-regulatory effects of ligustrazine hydrochloride on tumor-induced immunosuppression by Colon26 tumor cells in vitro. Chin. J. Immunol. 2009, 25, 413–416. [Google Scholar]
- Zou, J.; Gao, P.; Hao, X.; Xu, H.; Zhan, P.; Liu, X. Recent progress in the structural modification and pharmacological activities of ligustrazine derivatives. Eur. J. Med. Chem. 2018, 147, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Yong, A.; Zhu, B.; Ren, C.; Kang, F.H.; Li, J.L.; Huang, Z.J.; Lai, Y.S.; Peng, S.X.; Ding, K.; Tian, J.; et al. Discovery of new monocarbonyl ligustrazine-curcumin hybrids for intervention of drug-sensitive and drug-resistant lung cancer. J. Med. Chem. 2016, 59, 1747–1760. [Google Scholar]
- Qin, H.L.; Leng, J.; Zhang, C.P.; Jantan, I.; Amjad, M.W.; Sher, M.; Naeem-Ul-Hassan, M.; Hussain, M.A.; Bukhari, S.N. Synthesis of α, β-unsaturated carbonyl-based compounds, oxime and oxime ether analogs as potential anticancer agents for overcoming cancer multidrug resistance by modulation of efflux pumps in tumor cells. J. Med. Chem. 2016, 59, 3549. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Xie, Q.; Zhang, Q.Y.; Peng, X.L.; Zhu, J.D.; Mi, M.T. Flavonoids, flavonoid subclasses and breast cancer risk: A meta-analysis of epidemiologic studies. PLoS ONE 2013, 8, 54318. [Google Scholar]
- Guerrero, L.; Castillo, J.; Quiñones, M.; Garcia-Vallvé, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of Angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. PLoS ONE 2012, 7, 49493. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, F.; Darbon, J.M. Effects of structurally related flavonoids on cell cycle progression of human melanoma cells: Regulation of cyclin-dependent kinases CDK2 and CDK1. Biochem. Pharmacol. 2001, 61, 1205–1215. [Google Scholar] [CrossRef]
- Shi, X.F.; Liu, D.Y.; Zhang, J.M.; Hu, P.B.; Shen, W.; Fan, B.; Ma, Q.H.; Wang, X.D. Extraction and purification of total flavonoids from pine needles of cedrus deodara contribute to anti-tumor in vitro. BMC Complement. Altern. Med. 2016, 16, 245. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Zhu, S.Q.; Xie, Y. Mechanism of flavonoids against p-glycoprotein mediated tumor multidrug resistance: Research advances. J. Int. Pharm. Res. 2016, 43, 818–823. [Google Scholar]
- Haddad, A.Q.; Venkateswaran, V.; Viswanathan, L.; Teahan, S.J.; Fleshner, N.E.; Klotz, L.H. Novel antiproliferative flavonoids induce cell cycle arrest in human prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2006, 9, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, K.; Jiang, K.; Tao, S.S.; Li, Y.X.; Chen, W.W.; Kou, S.M.; Gu, C.R.; Li, Z.R.; Guo, L.S.; et al. A review of flavonoids from cassia species and their biological activity. Curr. Pharm. Biotechnol. 2016, 17, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shao, J.; Sun, C.; Song, Y.; Li, Q.; Lu, L.; Hu, Y.F.; Gui, C.; Zhang, H.; Ju, J.H. Borrelidins F–I, cytotoxic and cell migration inhibiting agents from mangrove-derived streptomyces rochei SCSIO ZJ89. Bioorg. Med. Chem. 2018, 26, 8. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, A.; Izumi, Y.; Yamagishi, M. Spatial and temporal expression of chalcone synthase and dihydroflavonol 4-reductase genes in the asiatic hybrid lily. Plant Sci. 2003, 165, 759–767. [Google Scholar] [CrossRef]
- Shi, R. Anti-Tumor Mechanisms of Luteolin, a Major Flavonoid of Chrysanthemum Morifolium. Ph.D. Thesis, National University of Singapore, Singapore, 2006. [Google Scholar]
- Poór, M.; Zrínyi, Z.; Kőszegi, T. Structure related effects of flavonoid aglycones on cell cycle progression of HepG2 cells: Metabolic activation of fisetin and quercetin by catechol-O-methyltransferase (COMT). Biomed. Pharm. 2016, 83, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.H.; Xu, X.; Li, G.L.; Gu, S.; Xu, K.; Gong, Y.; Xu, B.; Wang, M.N.; Zhang, H.Z.; Zhang, Y.Z.; et al. Amino acid derivatives of ligustrazine-oleanolic acid as new cytotoxic agents. Molecules 2014, 19, 18215–18231. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.B.; Yan, M.N.; Xu, B.; Chu, F.H.; Wang, W.; Zhang, C.Z.; Jia, X.H.; Han, Y.T.; Xiang, H.J.; Zhang, Y.Z.; et al. Design, synthesis, and biological evaluation of the novel glycyrrhetinic acid-cinnamoyl hybrids as anti-tumor agents. Chem Cent. J. 2016, 10, 78. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, A.C.; Wang, M.S.; Peng, X. Detection of apoptosis induced bynew type gosling viral enteritis virus in vitro through fluorescein annexin VFITC/PI double labeling. World J. Gastroenterol. 2008, 14, 2174–2178. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 10–25 are available from the authors. |

| Classification | Flavonoids’ Structure | Flavonoid Compounds (1–9) | |
|---|---|---|---|
| Flavone | 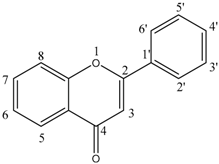 | Luteolin (1) | 5, 7, 3′, 4′—OH |
| Baicalein (2) | 5, 6, 7—OH | ||
| Apigenin (3) | 5, 7 ,3′—OH | ||
| Chrysin (4) | 5, 7—OH | ||
| Flavonol | 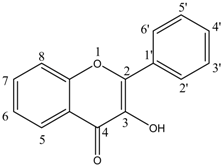 | Quercetin (5) | 5, 7, 3′, 4′—OH |
| Fisetin (6) | 7, 3′, 4′—OH | ||
| Isoflavone | 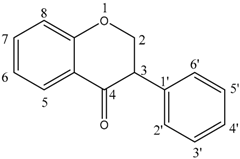 | Genistein (7) | 5, 7, 4′—OH |
| Daidzein (8) | 7, 4′—OH | ||
| Flavanone | 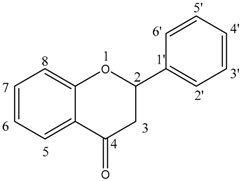 | Naringenin (9) | 5, 7, 4′—OH |
| No. | Structure | No. | Structure |
|---|---|---|---|
| 10 |  | 11 |  |
| 12 | 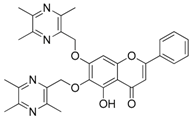 | 13 | 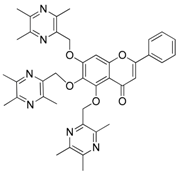 |
| 14 | 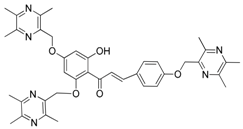 | 15 | 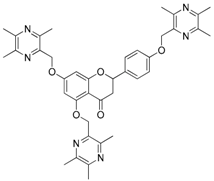 |
| 16 | 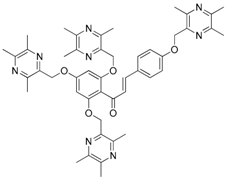 | 17 | 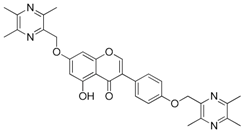 |
| 18 | 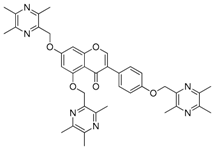 | 19 | 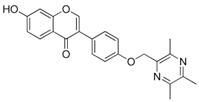 |
| 20 |  | 21 | 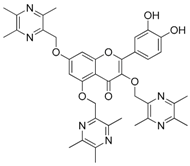 |
| 22 |  | 23 |  |
| 24 | 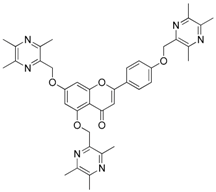 | 25 | 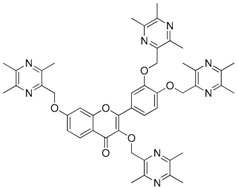 |
| Compound | IC50 (μM) | ||||
|---|---|---|---|---|---|
| HepG-2 | Bel-7402 | HT-29 | MCF-7 | HeLa | |
| Luteolin | 11.83 ± 0.44 | 14.51 ± 0.68 | 19.24 ± 1.17 | 16.99 ± 0.31 | 21.28 ± 1.12 |
| Baicalein | 20.35 ± 2.07 | 21.59 ± 3.68 | 19.20 ± 2.72 | 37.88 ± 1.52 | 22.76 ± 3.54 |
| Apigenin | 16.11 ± 1.52 | 28.22 ± 0.38 | 23.44 ± 1.89 | 29.71 ± 1.43 | 30.58 ± 0.71 |
| Chrysin | 15.56 ± 0.30 | 35.00 ± 1.46 | 30.31 ± 1.96 | 32.06 ± 2.78 | >40 |
| Quercetin | 13.57 ± 0.73 | 30.39 ± 2.51 | 27.00 ± 0.47 | >40 | 20.83 ± 4.04 |
| Fisetin | 17.39 ± 1.04 | 25.46 ± 1.54 | 23.88 ± 0.81 | 38.10 ± 1.83 | 20.88 ± 1.65 |
| Genistein | 19.76 ± 1.31 | 34.66 ± 0.63 | 31.13 ± 0.26 | 35.62 ± 1.38 | 34.61 ± 2.52 |
| Daidzein | 21.81 ± 1.53 | >40 | >40 | 36.82 ± 1.48 | >40 |
| 10 | >40 | >40 | >40 | >40 | 26.67±0.34 |
| 13 | 13.37 ± 2.50 | 26.13 ± 0.11 | 22.42 ± 0.35 | >40 | 17.31 ± 1.07 |
| 14 | 17.31 ± 0.47 | 10.74 ± 1.12 | 31.88 ± 1.96 | 29.79 ± 2.18 | 25.11 ± 1.80 |
| 16 | 20.62 ± 1.39 | >40 | 34.39 ± 2.32 | 16.54 ± 0.45 | 30.50 ± 2.62 |
| 17 | >40 | >40 | 10.67 ± 1.35 | >40 | >40 |
| 19 | 14.49 ± 0.48 | 28.87 ± 0.49 | 11.72 ± 1.29 | 10.43 ± 1.23 | 14.31 ± 1.17 |
| 20 | >40 | 25.16 ± 1.72 | 10.90 ± 2.30 | >40 | 16.48 ± 1.79 |
| DDP | 4.14 ± 0.16 | 6.73 ± 0.37 | 5.83 ± 0.59 | 6.75 ± 0.57 | 4.76 ± 0.41 |
| Compound | Proliferation Rate (%) | IC50 (μM) | ||||
|---|---|---|---|---|---|---|
| 2.5 μM | 5 μM | 10 μM | 20 μM | 40 μM | ||
| 14 | 1.62 ± 0.75 | 1.99 ± 0.23 | 14.84 ± 1.41 | 16.71 ± 0.29 | −2.32 ± 1.37 | >40 |
| 19 | −8.75 ± 0.38 | −19.35 ± 0.35 | −19.49 ± 2.17 | −24.22 ± 3.19 | −25.07 ± 1.02 | >40 |
| DDP | −23.93 ± 0.75 | −31.38 ± 1.46 | −58.24 ± 0.82 | −66.58 ± 1.75 | −82.69 ± 2.19 | 9.11 ± 0.54 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, W.; Cheng, Y.; Zhang, X.; Xue, N.; Wu, G.; Chen, M.; Fang, K.; Guo, W.; Zhou, F.; et al. Design, Synthesis and Biological Evaluation of Ligustrazine-Flavonoid Derivatives as Potential Anti-Tumor Agents. Molecules 2018, 23, 2187. https://doi.org/10.3390/molecules23092187
Wang H, Zhang W, Cheng Y, Zhang X, Xue N, Wu G, Chen M, Fang K, Guo W, Zhou F, et al. Design, Synthesis and Biological Evaluation of Ligustrazine-Flavonoid Derivatives as Potential Anti-Tumor Agents. Molecules. 2018; 23(9):2187. https://doi.org/10.3390/molecules23092187
Chicago/Turabian StyleWang, Hui, Wenxi Zhang, Yatao Cheng, Xinyu Zhang, Nannan Xue, Gaorong Wu, Meng Chen, Kang Fang, Wenbo Guo, Fei Zhou, and et al. 2018. "Design, Synthesis and Biological Evaluation of Ligustrazine-Flavonoid Derivatives as Potential Anti-Tumor Agents" Molecules 23, no. 9: 2187. https://doi.org/10.3390/molecules23092187






