Effect of A Polyphenol-Rich Canarium album Extract on the Composition of the Gut Microbiota of Mice Fed a High-Fat Diet
Abstract
:1. Introduction
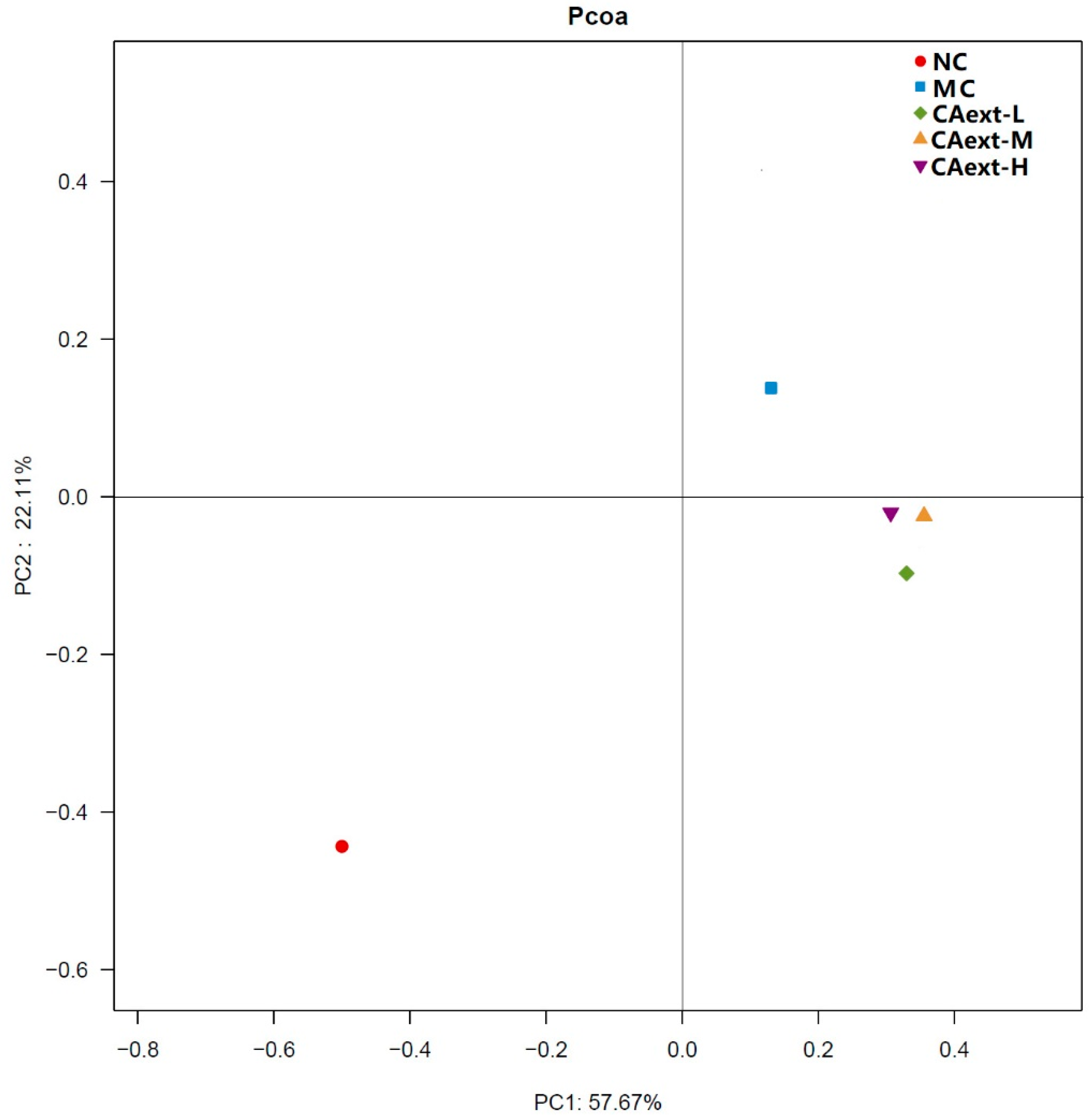
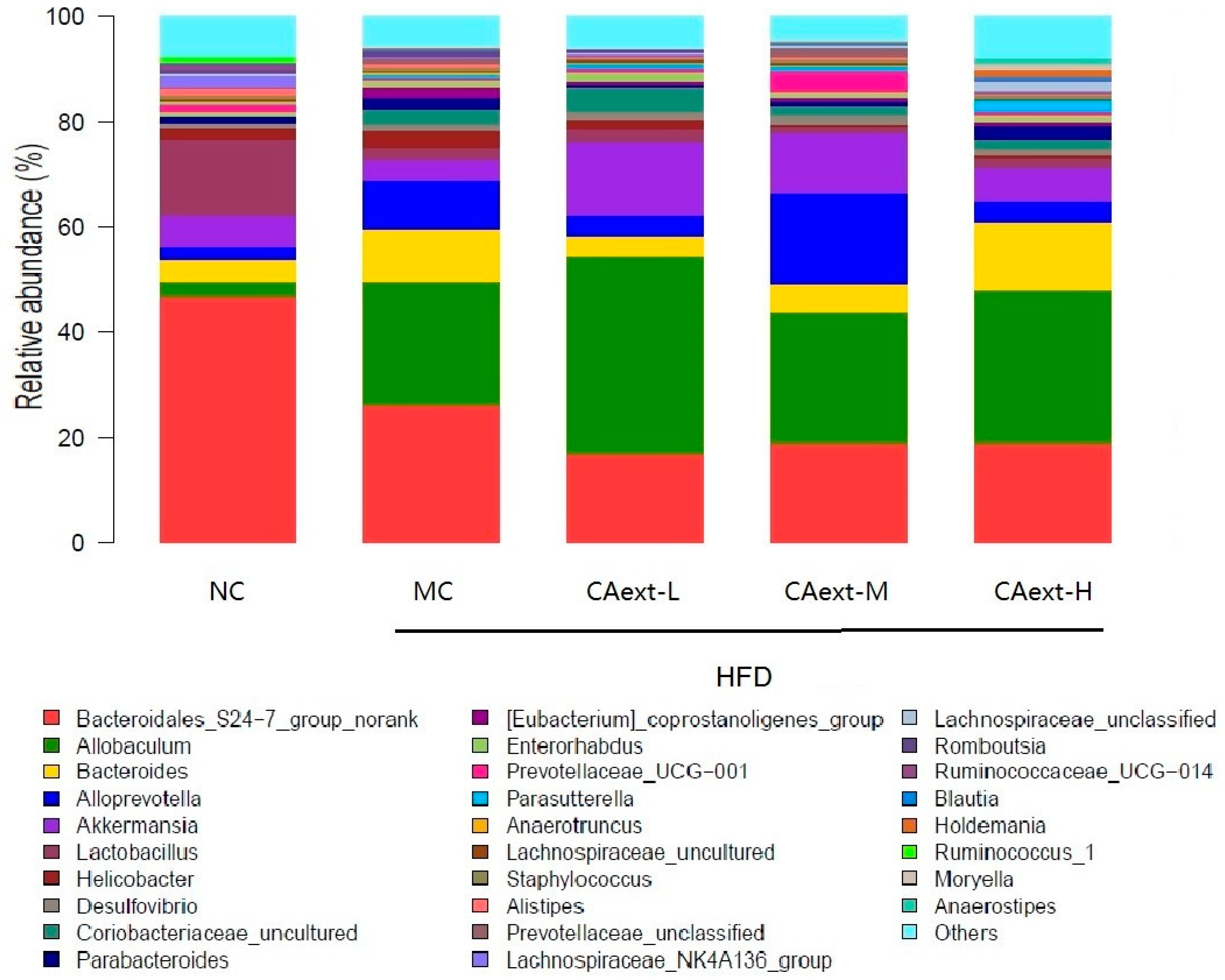
2. Results
3. Discussion
4. Materials and Methods
4.1. Preparation of CAext
4.2. Animal Experiments
4.3. Fecal DNA Extraction
4.4. Fecal Microbiota Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, Y.; Wu, J.F.; Li, J.V.; Zhou, N.Y.; Tang, H.R.; Wang, Y.L. Gut microbiota composition modifies fecal metabolic profiles in mice. J. Proteome Res. 2013, 12, 2987–2999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, M.; Wang, S.; Han, R.; Cao, Y.; Hua, W.; Mao, Y.; Zhang, X.; Pang, X.; Wei, C.; et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010, 4, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.; Valdés, L.; Salazar, N.; de los Reyes-Gavilán, C.G.; Ruas-Madiedo, P.; Gueimonde, M.; González, S. Pilot study of diet and microbiota: Interactive associations of fibers and polyphenols with human intestinal bacteria. J. Agric. Food Chem. 2014, 62, 5330–5336. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.; Arboleya, S.; Gueimonde, M.; González, S. Microbiota modulation by diet in humans. Prebiotics, fibres and other compouds. Agro Food Ind. Hi-Tech. 2012, 23, 23–26. [Google Scholar]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill-Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009, 137, 1716–1724. [Google Scholar] [CrossRef] [PubMed]
- Serino, M.; Luche, E.; Gres, S.; Baylac, A.; Bergé, M.; Cenac, C.; Waget, A.; Klopp, P.; Iacovoni, J.; Klopp, C.; et al. Metaboic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 2012, 61, 543–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; Francois, P.; de Vos, W.M.; et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Magistrelli, D.; Zanchi, R.; Malagutti, L.; Galassi, G.; Canzi, E.; Rosi, F. Effects of cocoa husk feeding on the composition of swine intestinal microbiota. J. Agric. Food Chem. 2016, 64, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Fernandez-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martinez, J.A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Luceri, C.; Vivoli, E.; Pagliuca, C.; Lodovici, M.; Moneti, G.; Dolara, P. Polyphenol metabolites from colonic microbiota exert anti-inflammatory activity on different inflammation models. Mol. Nutr. Food Res. 2009, 53, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Su, M.H.; Chen, Q.X.; Zeng, B.Y.; Li, H.H.; Wang, W. Physicochemical properties and antioxidant capacity of Chinese olive (Canarium album L.) cultivars. J. Food Sci. 2017, 82, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Shimura, H.; Watanabe, N.; Tamai, M.; Hanada, K.; Takahashi, A.; Tanaka, Y.; Arai, K.; Zhang, P.L.; Chang, R. Hepatoprotective compounds from Canarium album and Euphorbia nematocypha. Chem. Pharm. Bull. 1990, 38, 2201–2203. [Google Scholar] [PubMed]
- Liu, H.; Qiu, N.; Ding, H.; Yao, R. Polyphenols contents and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses. Food Res. Int. 2008, 41, 363–370. [Google Scholar] [CrossRef]
- Santino, A.; Scarano, A.; Santis, S.; Benedictis, M.; Giovinazzo, G.; Chieppa, M. Gut microbiota modulation and anti-inflammatory properties of dietary polyphenols in IBD: New and consolidated perspectives. Curr. Pharm. Des. 2017, 23, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Tan, S.; Chen, J.; Liu, S.; Jiang, S.; Xiang, H.; Xie, Y. Isolation of anti-HIV components from Canarium album fruits by high-speed counter-current chromatography. Anal. Lett. 2013, 46, 1057–1068. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, M.; Zheng, M.; Chen, N.; Zheng, X.; Zeng, S.; Zheng, B. Canarium album extract restrains lipid excessive accumulation in hepatocarcinoma cells. Int. J. Clin. Exp. Med. 2016, 9, 17509–17518. [Google Scholar]
- Dong, W.W.; Xuan, F.L.; Zhong, F.L.; Jiang, J.; Wu, S.; Li, D.; Li, D. Comparative analysis of the rats’ gut microbiota composition in animals with different ginsenosides metabolizing activity. J. Agric. Food Chem. 2017, 65, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Sun, J.; Ding, Y.; Le, G.; Shi, Y. Alterations of the gut microbiota in high-fat diet mice is strongly linked to oxidative stress. Appl. Microbiol. Biotechnol. 2013, 97, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Li, G.; Ding, Y.; Ren, T.; Zheng, J.; Kan, J. Effect of whole grain Qingke (Tibetan Hordeum vulgare L. Zangqing 320) on the serum lipid levels and intestinal microbiota of rats under high-fat diet. J. Agric. Food Chem. 2017, 65, 2686–2693. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Anhe, F.F.; Roy, D.; Pilon, G.; Dudonne, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Queipo-Ortuno, M.I.; Boto-Ordonez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andres-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axling, U.; Olsson, C.; Xu, J.; Fernandez, C.; Larsson, S.; Ström, K.; Ahrné, S.; Holm, C.; Molin, G.; Berger, K. Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr. Metab. 2012, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Kemperman, R.A.; Gross, G.; Mondot, S.; Possemiers, S.; Marzorati, M.; Van de Wiele, T.; Doré, J.; Vaughan, E.E. Impact of polyphenols from black tea and red wine/grape juice on a gut model microbiome. Food Res. Int. 2013, 53, 659–669. [Google Scholar] [CrossRef]
- Belzer, C.; de Vos, W.M. Microbes inside--from diversity to function: The case of Akkermansia. ISME J. 2012, 6, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Gérard, P.; Rabot, S.; Bruneau, A.; El Aidy, S.; Derrien, M.; Kleerebezem, M.; Zoetendal, E.G.; Simdt, H.; Verstraete, W.; et al. Arabinoxylans and inulin differentially modulate the mucosal and luminal gut microbiota and mucin-degradation in humanized rats. Environ. Microbiol. 2011, 13, 2667–2680. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; de Vos, W.M. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microbiol. 2008, 74, 1646–1648. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Zhang, X.; Wang, Z.; Gao, Z.; Liu, G.; Hu, H.; Zou, L.; Li, X. Insulin sensitivity-enhancing activity of phlorizin is associated with lipopolysaccharide decrease and gut microbiota changes in obese and type 2 diabetes (db/db) Mice. J. Agric. Food Chem. 2016, 64, 7502–7511. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Shen, M.; Zhao, X.; Zhu, H.; Yang, Y.; Lu, S.; Tan, Y.; Li, G.; Li, M.; Wang, J.; et al. Anti-obesity effect of capsaicin in mice fed with high-fat diet is associated with an increase in population of the gut bacterium Akkermansia muciniphila. Front. Microbiol. 2017, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; West, L.; Moussy, Y.; Moussy, F. Transcutaneous implantation methods for improving the long-term performance of glucose sensors in rats. IEEE Sens. J. 2008, 8, 97–103. [Google Scholar] [CrossRef]
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue engineering approaches in the design of healthy and pathological in vitro tissue models. Front. Bioeng. Biotechnol. 2017, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Yeung, A.C.; Ikeno, F. The representative porcine model for human cardiovascular disease. J. Biomed. Biotechnol. 2015, 2011, 195483. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Gonzàlez-Paramàs, A.M.; Santos-Buelga, C.; Quiles, J.L.; Bompadre, S.; Mezzetti, B.; et al. Polyphenol-rich strawberry extract protects human dermal fibroblasts against hydrogen peroxide oxidative damage and improves mitochondrial functionality. Molecules 2014, 19, 7798–7816. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, D.Y. Study on the effect of olive oil on hyperlipidemia in model rats. China Trop. Med. 2007, 7, 2136–2137. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |


| NC | HFD | ||||
|---|---|---|---|---|---|
| MC | CAext-L | CAext-M | CAext-H | ||
| Ace | 298.38 ± 11.63 a | 266.80 ± 11.07 b,c | 272.74 ± 14.05 b | 251.69 ± 13.70 c | 222.70 ± 10.44 d |
| Chao1 | 304.65 ± 19.48 a | 269.87 ± 15.92 b | 279.94 ± 22.79 a,b | 254.45 ± 18.83 b | 223.33 ± 13.17 c |
| Shannon | 4.19 ± 0.02 a | 3.73 ± 0.02 b | 3.07 ± 0.02 c | 3.20 ± 0.02 d | 3.54 ± 0.02 e |
| Ingredients (g/100 g) | Normal Standard Chow Diet | HFD |
|---|---|---|
| Corn starch | 30 | 26.3 |
| Wheat bran | 25 | 21.9 |
| Fish meal | 5 | 4.4 |
| Soy bean flour | 20 | 17.5 |
| Wheat flour | 16 | 14.0 |
| Yeast powder | 1 | 0.9 |
| Bone meal | 2 | 1.8 |
| Salt | 1 | 0.9 |
| Cholesterol | - | 2 |
| Lard oil | - | 10 |
| Sodium deoxycholate | - | 0.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.-N.; Guo, W.-H.; Hu, H.; Zhou, A.-R.; Liu, Q.-P.; Zheng, B.-D.; Zeng, S.-X. Effect of A Polyphenol-Rich Canarium album Extract on the Composition of the Gut Microbiota of Mice Fed a High-Fat Diet. Molecules 2018, 23, 2188. https://doi.org/10.3390/molecules23092188
Zhang N-N, Guo W-H, Hu H, Zhou A-R, Liu Q-P, Zheng B-D, Zeng S-X. Effect of A Polyphenol-Rich Canarium album Extract on the Composition of the Gut Microbiota of Mice Fed a High-Fat Diet. Molecules. 2018; 23(9):2188. https://doi.org/10.3390/molecules23092188
Chicago/Turabian StyleZhang, Ning-Ning, Wen-Hui Guo, Han Hu, A-Rong Zhou, Qing-Pei Liu, Bao-Dong Zheng, and Shao-Xiao Zeng. 2018. "Effect of A Polyphenol-Rich Canarium album Extract on the Composition of the Gut Microbiota of Mice Fed a High-Fat Diet" Molecules 23, no. 9: 2188. https://doi.org/10.3390/molecules23092188





