Removal Properties of Anionic Dye Eosin by Cetyltrimethylammonium Organo-Clays: The Effect of Counter-Ions and Regeneration Studies
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
2.2. Synthesis of OCs
2.3. Effect of Washing Solution
2.4. Eosin Removal Experiment
2.5. Regeneration/Removal Cycles
2.6. Characterization Methods
3. Results and Discussion
3.1. Elemental Analysis
3.2. Powder XRD Data
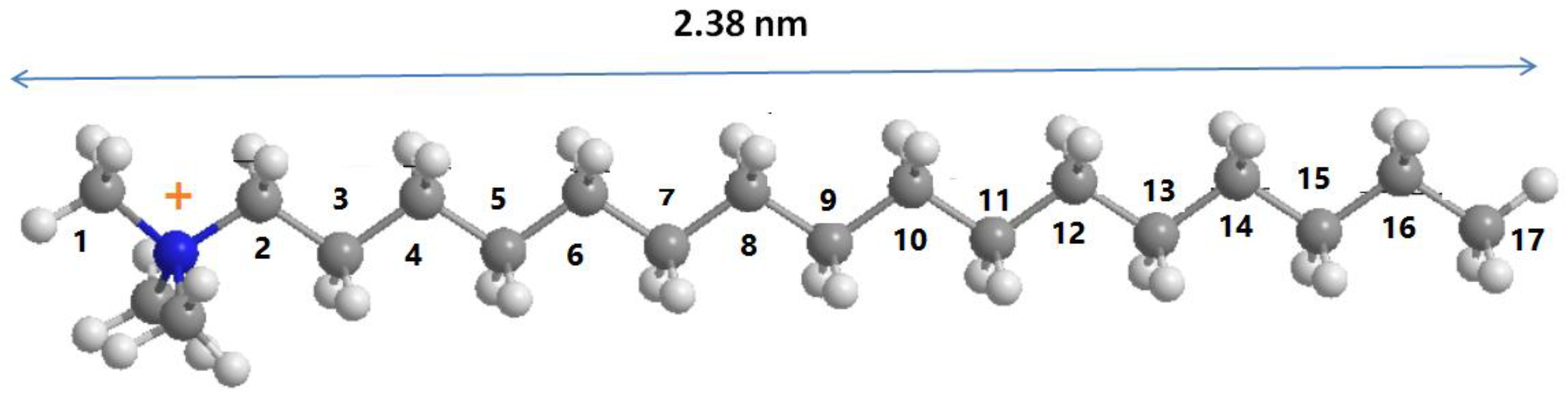
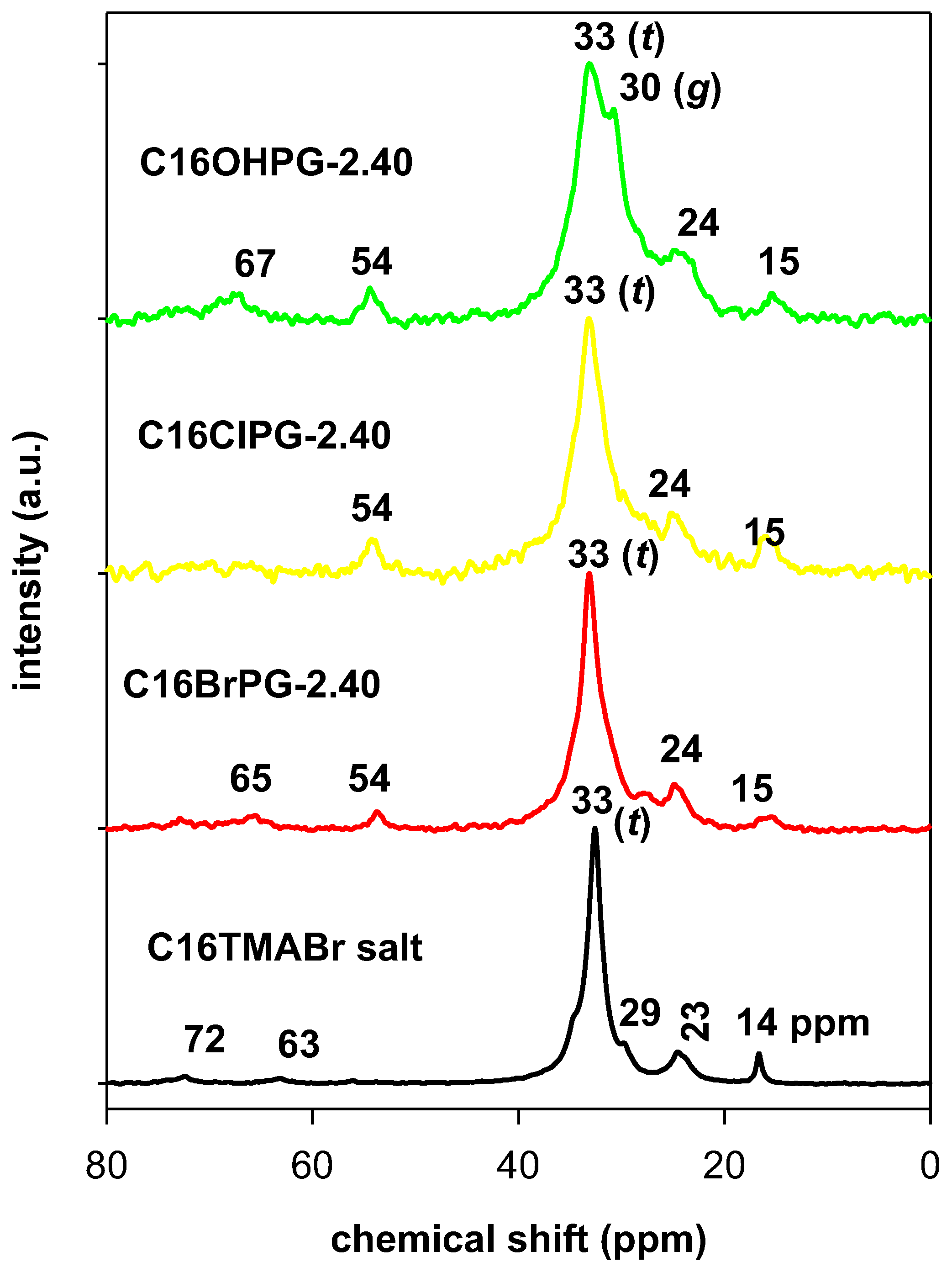
3.3. Solid 13C CP NMR Studies
3.4. Microtextural Properties
3.5. Thermogravimetric Analysis
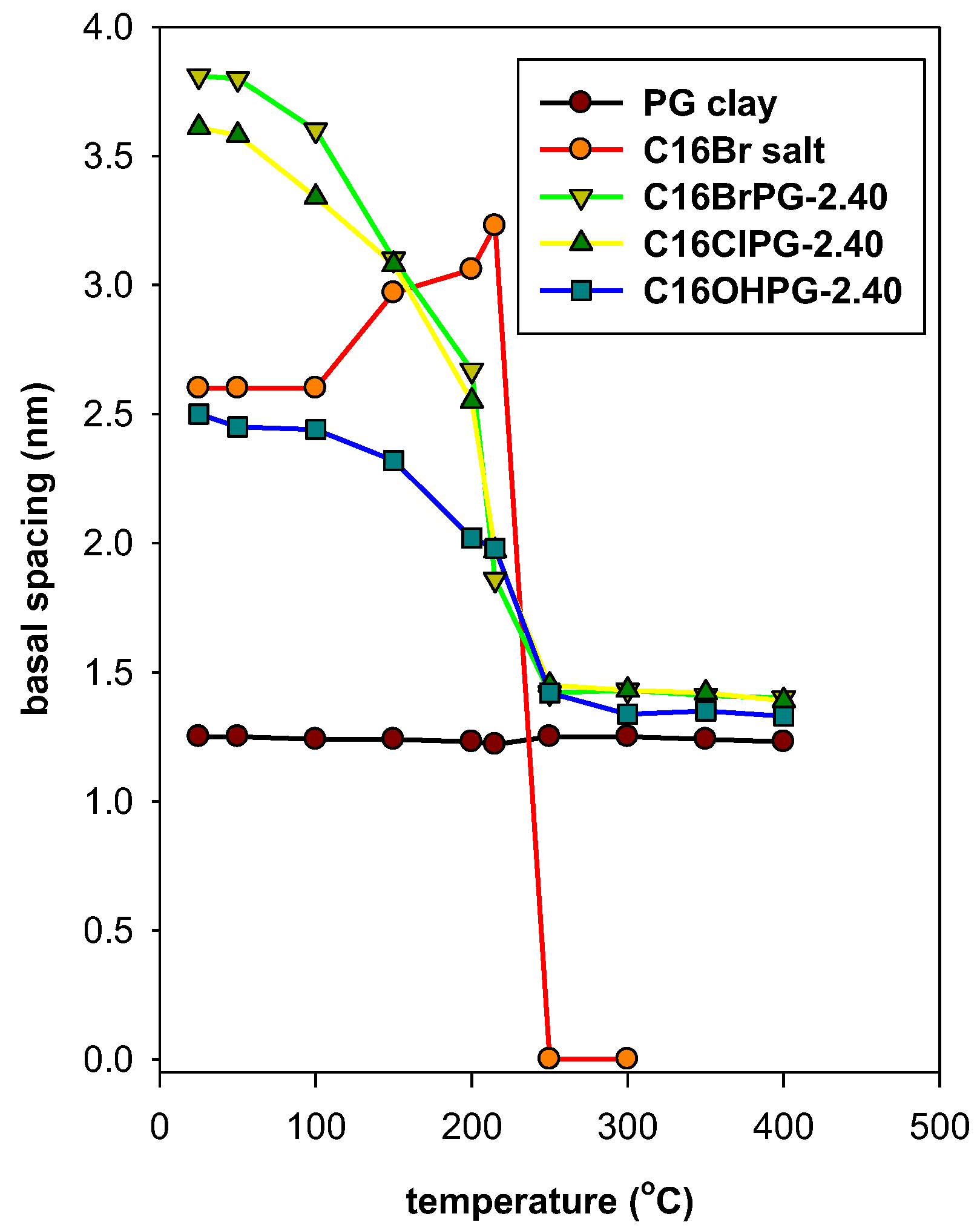
3.6. Thermal Stability Study
3.7. Removal Properties of Eosin Dye
3.7.1. Effect of the Initial Concentration
3.7.2. Effect of C16TMA Contents
3.7.3. Effect of Temperature
3.7.4. Effect of Preheated Treatment of OCs
3.8. Maximum Removal Amount
3.9. Regeneration Cycles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nazir, M.S.; Mohamad Kassim, M.H.; Mohapatra, L.; Gilani, M.A.; Raza, M.R.; Majeed, K. Characteristic Properties of Nanoclays and Characterization of Nanoparticulates and Nanocomposites. In Nanoclay Reinforced Polymer Composites; Jawaid, M., Qaiss, A., Bouhfid, R., Eds.; Engineering Materials; Springer: Singapore, 2016; pp. 33–35. [Google Scholar]
- Uddin, F. Clays, nanoclays, and montmorillonite minerals. Metall. Mater. Trans. A 2008, 39, 2804–2814. [Google Scholar] [CrossRef]
- CalabiFloody, M.; Theng, B.K.G.; Reyes, P.; Mora, M.L. Natural nanoclays: Applications and future trends a Chilean perspective. Clay Min. 2009, 44, 161–176. [Google Scholar] [CrossRef]
- Beall, G.W. The use of OCs in water treatment. Appl. Clay Sci. 2003, 24, 11–20. [Google Scholar] [CrossRef]
- Lee, S.M.; Tiwari, D. Organo and inorgan0-organo-modified clays in the remediation of aqueous solutions: An overview. Appl. Clay Sci. 2012, 59–60, 84–102. [Google Scholar] [CrossRef]
- Pandey, S.; Ramontja, J. Natural bentonite clay and its composites for dye removal: Current state and future potential. Amer. J. Chem. Appl. 2016, 3, 8–19. [Google Scholar]
- Paiva, L.B.; Morales, A.R.; Valenzuela Díaz, F.R. Organoclays: Properties, preparation and applications. Appl. Clay Sci. 2008, 42, 8–24. [Google Scholar] [CrossRef]
- Young, D.A.; Smith, D.E. Simulations of clay mineral swelling and hydration: Dependence upon interlayer ion size and charge. J. Phys. Chem. B 2000, 104, 9163–9170. [Google Scholar] [CrossRef]
- Nigam, C.; Setua, D.K.; Mathu, G.N.; Kar, K.K.J. Epoxy-montmorillonite clay nanocomposites: Synthesis and characterization. Appl. Polym. Sci. 2004, 93, 2201–2210. [Google Scholar] [CrossRef]
- Bezrodna, T.; Puchkovska, G.; Styopkin, V.; Baran, J. Structure of cetyltrimethylammonium bromide films obtained by evaporation-induced precipitation method. Thin Solid Films 2009, 517, 1759–1764. [Google Scholar] [CrossRef]
- Groisman, L.; Rav-Acha, C.; Gerstl, Z.; Mingelrin, U. Sorption and detoxification of toxic compounds by a bifunctional organoclays. J. Environ. Qual. 2004, 33, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Boyd, A. Alternative Model for Cationic Surfactant Adsorption by Layer Silicates. Environ. Sci. Technol. 1995, 29, 3022–3028. [Google Scholar] [CrossRef] [PubMed]
- Komadel, P.; Madejova, J. Handbook of Clay Science; Elsevier: Amesterdam, The Netherlands, 2006. [Google Scholar]
- Heinz, H.; Vaia, R.A.; Krishnamoorti, R.; Farmer, B.L. Self-assembly of alkylammonium chains on montmorillonite: Effect of chain length, head group structure, and cation exchange capacity. Chem. Mater. 2007, 19, 59–68. [Google Scholar] [CrossRef]
- Liu, R.; Frost, R.L.; Martens, W.N.; Yuan, Y. Synthesis, characterization of mono, di and tri alkyl surfactant intercalated Wyoming montmorillonite for the removal of phenol from aqueous systems. J. Colloid Interface Sci. 2008, 32, 287–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baskaralingam, P.; Pulikesi, M.; Elango, D.; Ramamurthi, V.; Sivanesan, S. Adsorption of acid dye onto organobentonite. J. Hazard. Mater. 2006, 128, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, A.C.; Sousa, B.V.; Santana, L.N.L.; Neves, G.A.; Rodrigues, M.G.F. Study of different methods in the preparation of organoclays from the bentonite with application in the petroleum industry. Braz. J. Pet. Gas. 2007, 1, 16–25. [Google Scholar]
- Cifuentes, A.; Bernat, J.L.; Diez-Masa, J.C. Determination of critical micelle concentration values using capillary electrophoresis instrumentation. Anal. Chem. 1997, 69, 4271–4274. [Google Scholar] [CrossRef]
- Magid, L.J.; Han, Z.; Warr, G.C.; Cassidy, M.A.; Butler, P.D.; Hamilton, W.A. Effect of counterion competition on micellar growth horizons for cetyltrimethylammonium micellar surfaces: electrostatics and specific binding. J. Phys. Chem. B 1997, 101, 7919–7927. [Google Scholar] [CrossRef]
- Rashidi-Alavijeh, M.; Javadian, S.; Gharibi, H.; Moradi, M.; Tehrani-Bagha, A.; Shahir, A.A. Intermolecular interactions between a dye and cationic surfactants: Effects of alkyl chain, head group, and counterion. Colloid Surf. A 2011, 380, 119–127. [Google Scholar] [CrossRef]
- Kooli, F. Exfoliation properties of acid-activated montmorillonites and their resulting organoclays. Langmuir 2009, 25, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Kooli, F.; Khimyak, Y.Z.; Alshahateet, S.F.; Chen, F. Effect of the acid activation levels of montmorillonite clay on the cetyltrimethylammonium cations adsorption. Langmuir 2005, 21, 8717–8723. [Google Scholar] [CrossRef] [PubMed]
- Kooli, F.; Yan, L. Thermal stable cetyl trimethylammonium-magadiites: Influence of the surfactant solution type. J. Phys. Chem. C 2009, 113, 1947–1952. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Kooli, F.; Liu, Y.; Hbaieb, K.; Al-Faze, R. Characterization of organo-kenyaites: Thermal stability and their effects on eosin removal characteristics. Clay Miner. 2018, 53, 91–104. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpaa, M. Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment—A review. Chem. Eng. J. 2010, 157, 277–296. [Google Scholar] [CrossRef]
- Su, Y.; Jiao, Y.; Dou, C.; Han, R. Biosorption of methyl orange from aqueous solutions using cationic surfactant-modified wheat straw in batch mode. Desalin. Water Treat. 2014, 52, 6145–6155. [Google Scholar] [CrossRef]
- Hunger, K. Industrial Dyes: Chemsitry, Properties, Applications; Wiley-VCH: Weinhiem, Germany, 2003. [Google Scholar]
- Sabnis, R.W. Handbook of Biological Dyes and Stains: Synthesis and Industrial Applications; John Wiley and Sons: Hoboken, NJ, USA, 2010; p. 173. [Google Scholar]
- Mittal, A.; Jhare, D.; Mittal, J. Adsorption of hazardous dye eosin yellow from aqueous solution onto waste material de-oiled soya: Isotherm, kinetics and bulk removal. J. Mol. Liq. 2013, 179, 133–140. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Alshahateet, S.F.; Messali, M.; Bergaya, F. Reaction of acid activated montmorillonites with hexadecyl trimethylammonium bromide solution. Appl. Clay Sci. 2009, 43, 357–363. [Google Scholar] [CrossRef]
- Al-Faze, R.; Kooli, F. Eosin removal properties of organo-local clay from aqueous solution. Orient. J. Chem. 2014, 30, 675–680. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Al-Faze, R.; Al-Suhaimi, A. Effect of acid activation of Saudi local clay mineral on removal properties of basic blue 41 from an aqueous solution. Appl. Clay Sci. 2015, 116–117, 23–30. [Google Scholar] [CrossRef]
- Kooli, F. Organo-bentonites with improved cetyltrimethylammonium contents. Clay Miner. 2014, 49, 683–692. [Google Scholar] [CrossRef]
- He, H.; Ma, Y.; Zhu, J.; Yuan, P.; Qing, Y. Organoclays prepared from montmorillonites with different cation exchange capacity and surfactant configuration. Appl. Clay Sci. 2010, 48, 67–72. [Google Scholar] [CrossRef]
- Slade, P.G.; Gates, W.P. The swelling of HDTMA smectites as influenced by their preparation and layer charges. Appl. Clay Sci. 2004, 25, 93–101. [Google Scholar] [CrossRef]
- Slade, P.G.; Gates, W.P. The ordering of HDTMA in the interlayers of vermiculite and the influence of solvents. Clays Clay Miner. 2004, 52, 204–210. [Google Scholar] [CrossRef]
- Ferrage, E.; Sakharov, B.A.; Michot, L.J.; Delville, A.; Bauer, A.; Lanson, B.; Grangeon, S.; Frapper, G.; Jimenez-Ruiz, M.; Cuello, G.J. Hydration properties and interlayer organization of water and ions in synthetic Na-smectite with tetrahedral layer charge. part 2. toward a precise coupling between molecular simulations and diffraction data. J. Phys. Chem. C 2011, 115, 1867–1881. [Google Scholar] [CrossRef]
- Huang, W.L.; Bassett, W.A.; Wu, T.C. Dehydration and hydration of montmorillonite at elevated temperatures and pressures monitored using synchrotron radiation. Amer. Mineral. 1994, 79, 683–691. [Google Scholar]
- Kooli, F. Effect of C16TMA contents on the thermal stability of organo-bentonites: In situ X-ray diffraction analysis. Thermochim. Acta 2013, 551, 7–13. [Google Scholar] [CrossRef]
- He, H.; Duchet, J.; Galy, J.; Gerard, J.F. Influence of cationic surfactant removal on the thermal stability of organoclays. J. Colloid Interface Sci. 2006, 295, 202–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lepoitevin, M.; Balme, S.; Jaber, M.; Guégan, R.; Janot, J.M.; Dejardin, P.; Henn, F. BSA and lysozyme adsorption on montmorillonite: Influence of the interlayercation. Appl. Clay Sci. 2014, 95, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Zhu, R.; Zhu, L.; Zhu, J.; Xu, L. Structure of cetyltrimethylammonium intercalated hydrobiotite. Appl. Clay Sci. 2008, 42, 224–231. [Google Scholar] [CrossRef]
- Lan, T.; Kaviratna, P.D.; Pinnavaia, T.J. Mechanism of clay tactoid exfoliation in epoxy-clay nanocomposites. Chem. Mater. 1995, 7, 2144–2150. [Google Scholar] [CrossRef]
- Wang, L.Q.; Liu, J.; Exarhos, G.J.; Flanigan, K.Y.; Bordia, R. Conformation Heterogeneity and Mobility of Surfactant Molecules in Intercalated Clay Minerals Studied by Solid-State NMR. J. Phys. Chem. B 2000, 104, 2810–2816. [Google Scholar] [CrossRef]
- Gerstmans, A.; Urbanczyk, L.; Jérôme, R.; Robert, J.L.; Grandjean, J. XRD and NMR characterization of synthetic hectorites and the corresponding surfactant-exchanged clays. Clays Clay Miner. 2008, 43, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Earl, W.L.; VanderHart, D.L. Observations in solid polyethylenes by Carbon-13 Nuclear Magnetic Resonance with Magic Angle Sample Spinning. Macromolecules 1979, 12, 762–767. [Google Scholar] [CrossRef]
- Tonelli, A.E.; Schilling, F.C. Carbon-13 NMR chemical shifts and the microstructure of polymers. Acc. Chem. Res. 1981, 14, 233–238. [Google Scholar] [CrossRef]
- Mungse, H.P.; Singh, R.; Sugimura, H.; Kumar, N.; Khatri, O.P. Molecular pillar supported graphene oxide framework: Conformational heterogeneity and tunable d-spacing. Phys. Chem. Chem. Phys. 2015, 17, 20822–20829. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Frost, R.L.; Deng, F.; Zhu, J.X.; Wen, X.Y.; Yuan, P. Conformation of Surfactant Molecules in the Interlayer of Montmorillonite Studied by 13C MAS NMR. Clays Clay Miner. 2004, 52, 350–356. [Google Scholar] [CrossRef]
- Naik, V.V.; Vasudevan, S. Effect of Alkyl chain arrangement on conformation and dynamics in a surfactant intercalated layered double hydroxide: Spectroscopic measurements and MD simulations. J. Phys. Chem. C 2011, 115, 8221–8232. [Google Scholar] [CrossRef]
- Simonutti, R.; Comotti, A.; Bracco, S.; Sozzani, P. Surfactant organization in MCM-41 mesoporous materials as studied by 13C and 29Si Solid-State NMR. Mater. Chem. 2001, 13, 771–777. [Google Scholar] [CrossRef]
- Zhao, Q.; Choo, H.; Bhatt, A.; Burns, S.E.; Bate, B. Review of the fundamental geochemical and physical behaviors oforganoclays in barrier applications. Appl. Clay Sci. 2017, 142, 2–20. [Google Scholar] [CrossRef]
- He, H.P.; Frost, R.L.; Bostrom, T.; Yuan, P.; Duong, L.; Yang, D.; Yunfel, X.F.; Kloprogge, J.T. Changes in the morphology of organoclays with HDTMA (+) surfactant loading. Appl. Clay Sci. 2006, 31, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Bate, B.; Choo, H.; Burns, S. Dynamic properties of fine-grained soils engineered with a controlled organic phase. Soil Dyn. Earthq. Eng. 2013, 53, 176–186. [Google Scholar] [CrossRef]
- Burns, S.E.; Bartelt-Hunt, S.L.; Smith, J.A.; Redding, A.Z. Coupled mechanical and chemical behavior of bentonite engineered with a controlled organic phase. J. Geotech. Geoenviron. Eng. 2006, 132, 1404–1412. [Google Scholar] [CrossRef]
- Kooli, F.; Yan, L.; Tan, S.X.; Zheng, J. Organoclays from alkaline-treated acid-activated clays: Properties and thermal stability. J. Therm. Anal. Calorim. 2014, 115, 1465–1475. [Google Scholar] [CrossRef]
- Wolters, F.; Emmerich, K. Thermal reactions of smectites—Relation of dehydroxylation temperature to octahedral structure. Thermochim. Acta 2007, 462, 80–88. [Google Scholar] [CrossRef]
- Xie, W.; Gao, Z.; Liu, K.; Pan, W.P.; Vaia, R.; Hunter, D.; Singh, A. Thermal characterization of organically modified montmorillonite. Thermochim. Acta 2001, 367–368, 339–350. [Google Scholar] [CrossRef]
- Onal, M.; Sarikaya, Y. Thermal analysis of some organoclays. J. Thermal. Anal. Calirom. 2008, 91, 261–265. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, G.; Puckette, J.; Blum, F.D. Effect of Silica on the Structure of Cetyltrimethylammonium Bromide. J. Phys. Chem. C 2012, 116, 11626–11634. [Google Scholar] [CrossRef]
- Tomašić, V.; Popović, S.; Filipović-Vinceković, N. Solid State Transitions of Asymmetric Cationic Surfactants. J. Colloid Interface Sci. 1999, 215, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Kooli, F.; Liu, Y.; Abouddi, M.; Rakass, S.; OudgiriHassani, H.; Ibrahim, S.M.; Al-Faze, R. Application of organo-magadiites for the removal of eosin dye from aqueous solutions: Thermal treatment and regeneration. Molecules 2018, 23, 2280. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z.; Tezer, S. Biosorption of reactive dyes on the green alga vulgaris. Process. Biochem. 2005, 40, 1361–1437. [Google Scholar] [CrossRef]
- Andreo dos Santos, O.A.; Castelli, C.Z.; Oliveira, M.F.; de Almeida Neto, A.F.; da Silva, M.G.C. Adsorption of synthetic orange dye wastewater in organoclay. Chem. Eng. Trans. 2013, 32, 307–312. [Google Scholar]
- Jovic-Jovicic, N.; Milutinovic-Nikolic, A.; Grzetic, I.; Jovanovic, D. Organobentonite as efficient textile dye sorbent. Chem. Eng. Technol. 2008, 31, 567–574. [Google Scholar] [CrossRef]
- Ma, J.; Cui, B.; Li, D. Mechanism of adsorption of anionic dye from aqueous solutions onto organobentonite. J. Hazard. Mater. 2011, 186, 1758–1765. [Google Scholar] [CrossRef] [PubMed]
- Onal, M.; Sarikaya, Y. Some physicochemical properties of partition nanophase formed in sorptive organoclays. Colloid Surf. A 2007, 296, 216–221. [Google Scholar] [CrossRef]
- Kausar, A.; Iqbal, M.; Javed, A.; Aftab, K.; Nazli, Z.; Bhatti, H.N.; Nouren, S. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018. [Google Scholar] [CrossRef]
- Ozcan, A.; Oncu, E.M.; Ozcan, A.S. Adsoprtion of acid blue 193 from aqueous solutions onto DEDMA-sepiolite. J. Hazard. Mater. 2006, 129, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Senturk, H.B.; Ozdes, D.; Duran, C. Biosorption of Rhodamine 6G from aqueous solutions onto almond shell (Prunus dulcis) as a low cost biosorbent. Desalination 2010, 252, 81–87. [Google Scholar] [CrossRef]
- Oyetade, O.A.; Nyamori, V.O.; Martincigh, B.S.; Jonnalagadda, S.B. Effectiveness of carbon nanotube–cobalt ferrite nanocomposites for the adsorption of rhodamine B from aqueous solutions. RSC Adv. 2015, 5, 22724–22739. [Google Scholar] [CrossRef]
- Borisover, M.; Bukhanovsky, N.; Lapides, I.; Yariv, S. Mild pre-heating of organic cation-exchanged clays enhances their interactions with nitrobenzene in aqueous environment. Adsorption 2010, 16, 223–232. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Bello, O.S.; Olusegun, O.A.; Njoku, V.O. Fly ash: An alternative to powdered activated carbon for the removal of eosin dye from aqueous solutions. Bull. Chem. Soc. Ethiop. 2013, 27, 191–204. [Google Scholar] [CrossRef]
- Thabet, M.S.; Ismaiel, A.M. Sol-gel γ-Al2O3 nanoparticles assessment of the removal of eosin yellow using: Adsoprtion, kinetic and thermodynamic parameters. J. Encapsulation Adsorpt. Sci. 2016, 6, 70–90. [Google Scholar] [CrossRef]
- Shahadat, M.M.; Ismail, S. Regeneration performance of clay-based adsorbents for the removal of industrial dyes: A review. RSC Adv. 2018, 8, 24571–24587. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |













| Samples | C% | H% | N% | Up Take Amount (mmol/g) * |
|---|---|---|---|---|
| C16BrPG-2.40 | 39.23 | 5.88 | 2.34 | 1.60 |
| C16ClPG-2.40 | 30.77 | 4.88 | 1.96 | 1.26 |
| C16OHPG-2.40 | 18.91 | 3.60 | 1.69 | 0.93 |
| Samples | C% | H% | N% | Up Take Amount (mmol/g) |
|---|---|---|---|---|
| C16BrPG-2.40 | 39.23 | 5.88 | 2.34 | 1.60 |
| 25% ethanol * | 20.52 | 4.40 | 1.22 | 0.90 |
| 50% ethanol * | 19.55 | 4.87 | 1.06 | 0.85 |
| 75% ethanol * | 18.76 | 4.54 | 1.01 | 0.82 |
| Uploaded Amount | d001 (nm) | Interlayer Spacing | Arrangement |
|---|---|---|---|
| 0.40 (Br) | 1.62 | 0.66 | Lateral monolayer |
| 0.80 (Br) | 2.14 | 1.18 | Lateral bilayer |
| 1.65 (Br) | 2.51 | 1.55 | Pseudo-trilayer |
| 2.40 (Br) | 3.61 | 2.65 | Paraffin bilayer, θ = 34° |
| 3.20 (Br) | 3.61 | 2.65 | Paraffin bilayer, θ = 34° |
| C16BrPG-2.40r | 3.61 | 2.65 | Paraffin bilayer, θ = 34° |
| C16ClPG-2.40 | 3.51 | 2.55 | Paraffin bilayer, θ = 32° |
| C16OHPG-2.40 | 2.42 | 1.46 | paraffin monolayer, θ = 38° or pseudo trilayer |
| C16BrPG-2.40 * | 2.42 | 1.46 | Lateral bilayer |
| Carbon Atom | C16BrPG-2.40 | C16OHPG-2.40 | C16TMABr Solid |
|---|---|---|---|
| C2 | 65 | 67 | 63 (67.05, 64.55) |
| C1 | 54 | 54 | 53 (54.61) |
| C15 | 35 | 35 | 35 (36.40) |
| C3–C17 (trans, t) | 33 | 33 | 33 (34.70) |
| C3–C17 (gauche, g) | 30 | 30 | 29 (30.77–29.19) |
| C2, C16 | 24 | 24 | 23 (23.12) |
| C17 | 15 | 15 | 14 (16.14, 14.12) |
| Samples | SBET (m2/g) | P.V. (cc/g) | A.P.D (nm) | (g) Ratio Conformation |
|---|---|---|---|---|
| PG-clay | 67.0 | 0.147 | 8.79 | - |
| C16BrPG-2.40 | 23.4 | 0.081 | 13.6 | 0.36 |
| C16ClPG-2.40 | 28.4 | 0.078 | 10.9 | 0.24 |
| C16OHPG-2.40 | 11.5 | 0.086 | 19.8 | 0.47 |
| Samples | Water Content (Mass%) | Organic Content * (Mass%) | Residue at 900 °C |
|---|---|---|---|
| PG-clay | 15.54 | 0 | 79.49 |
| C16BrPG-2.40 | 3.51 | 36.13 | 47.52 |
| C16ClPG-2.40 | 4.26 | 35.43 | 53.56 |
| C16OHPG-2.40 | 3.85 | 18.79 | 63.56 |
| Samples | Water Content (wt%) | Organic Content (wt%) | Residue at 900 °C |
|---|---|---|---|
| PG-clay | 15.54 | 0 | 79.49 |
| C16BrPG-0.40 | 7.49 | 6.36 | 77.14 |
| C16BrPG-0.80 | 4.71 | 12.97 | 67.26 |
| C16BrPG-1.65 | 4.22 | 26.17 | 57.94 |
| C16BrPG-2.40 | 3.72 | 34.98 | 49.08 |
| Parameter | Temperature | ||
|---|---|---|---|
| 298 K | 308 K | 323 K | |
| ΔG° (kJ mol−1) | −4.11 | −6.02 | −7.91 |
| Kd | 5.25 | 10.49 | 19.00 |
| ΔS° (kJ mol−1·K) | 0.153 | ||
| ΔH° (kJ mol−1) | 41.22 | ||
| Samples | qmax (mg g−1) | KL (L g−1) | R2 |
|---|---|---|---|
| PG clay | 3.45 | 0.00054 | 0.9675 |
| C16BrPG-2.40 | 90.90 | 0.0263 | 0.9934 |
| C16ClPG-2.40 | 74.07 | 0.0154 | 0.9921 |
| C16OHPG-2.40 | 60.60 | 0.0117 | 0.9886 |
| C16BrPG-0.40 | 30.76 | 0.0223 | 0.9974 |
| C16BrPG-0.80 | 46.20 | 0.0242 | 0.9873 |
| C16BrPG-1.65 | 54.64 | 0.0653 | 0.9885 |
| C16BrPG-3.20 | 94.20 | 0.1234 | 0.9921 |
| C16BrPG-2.40 (100) * | 75.11 | 0.0096 | 0.9914 |
| C16BrPG-2.40 (150) * | 75.11 | 0.0096 | 0.9934 |
| C16BrPG-2.40 (200) * | 64.47 | 0.0063 | 0.9827 |
| C16BrPG-2.40 (215) * | 53.76 | 0.0039 | 0.9854 |
| C16BrPG-2.40 (250) * | 38.09 | 0.0014 | 0.9875 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kooli, F.; Liu, Y.; Abboudi, M.; Rakass, S.; Oudghiri Hassani, H.; Ibrahim, S.M.; Al-Faze, R. Removal Properties of Anionic Dye Eosin by Cetyltrimethylammonium Organo-Clays: The Effect of Counter-Ions and Regeneration Studies. Molecules 2018, 23, 2364. https://doi.org/10.3390/molecules23092364
Kooli F, Liu Y, Abboudi M, Rakass S, Oudghiri Hassani H, Ibrahim SM, Al-Faze R. Removal Properties of Anionic Dye Eosin by Cetyltrimethylammonium Organo-Clays: The Effect of Counter-Ions and Regeneration Studies. Molecules. 2018; 23(9):2364. https://doi.org/10.3390/molecules23092364
Chicago/Turabian StyleKooli, Fethi, Yan Liu, Mostafa Abboudi, Souad Rakass, Hicham Oudghiri Hassani, Sheikh Muhammad Ibrahim, and Rawan Al-Faze. 2018. "Removal Properties of Anionic Dye Eosin by Cetyltrimethylammonium Organo-Clays: The Effect of Counter-Ions and Regeneration Studies" Molecules 23, no. 9: 2364. https://doi.org/10.3390/molecules23092364






