Treatment Strategies for Infected Wounds
Abstract
:1. Introduction
2. Wound Healing Process and Skin Wound Microbiology
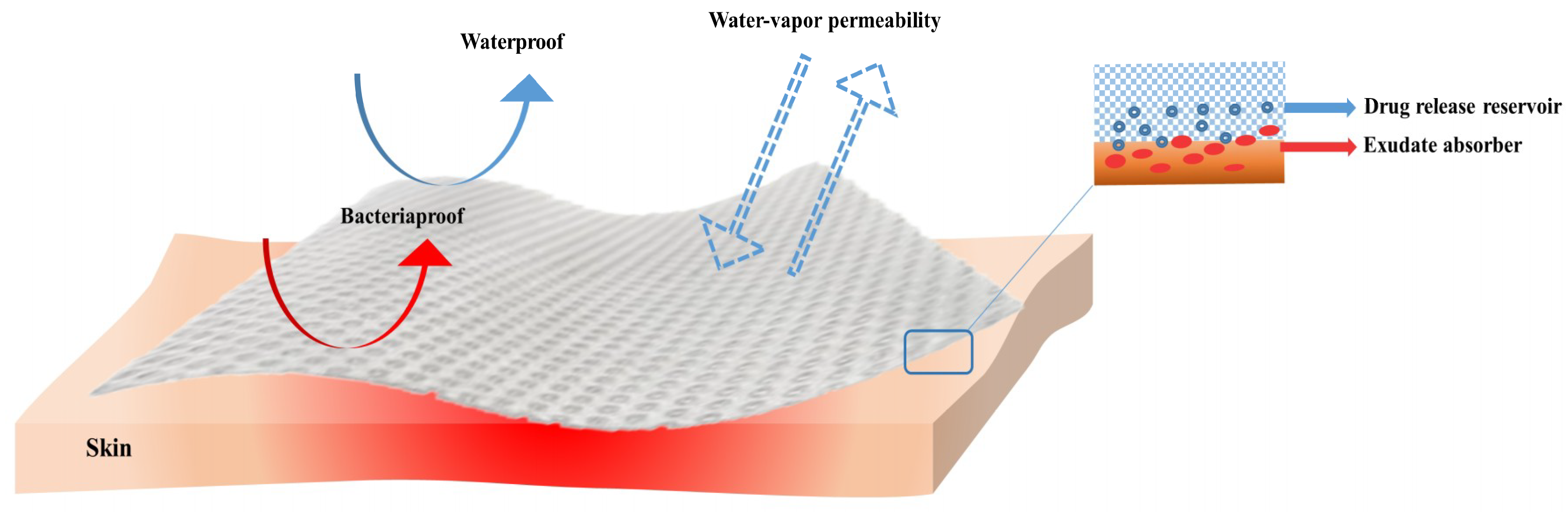
3. Ideal Properties of Wound Dressings
4. Antibacterial Agents in Wound Dressings
4.1. Antibiotics
4.2. Natural Antimicrobials for Wound Infections
4.2.1. Essential Oils
4.2.2. Honey
- -
- It’s acidic pH (regularly in the range of 3.4–6.1). It has been found that the acidic character of honey may encourage macrophages to eradicate bacteria and inhibit microbial biofilm establishment [128].
- -
- The osmotic pressure applied by sugars found in its chemical composition. The high osmolality obstructs microbial development [129].
- -
- The presence of antibacterial components such as hydrogen peroxide, antioxidants, lysozyme, phenolic acids, flavonoids, methylglyoxal and bee peptides (such as defensin-1) [130,131]. The production of hydrogen peroxide is a crucial component for the inhibition of bacterial development. In particular, hydrogen peroxide is gradually released/formed when the wound exudate interrelates with glucose oxidation, triggering the oxidative damage to pathogens’ macromolecules; hydrogen peroxide can react with the bacterial cell wall, as well as with intracellular lipids, proteins and nucleic acids [132].
4.3. Nanoparticles
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- van Koppen, C.J.; Hartmann, R.W. Advances in the treatment of chronic wounds: A patent review. Expert Opin. Ther. Pat. 2015, 25, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Who Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://tinyurl.com/kmva5da (accessed on 26 May 2018).
- Cardona, A.F.; Wilson, S.E. Skin and soft-tissue infections: A critical review and the role of telavancin in their treatment. Clin. Infect. Dis. 2015, 61, S69–S78. [Google Scholar] [CrossRef] [PubMed]
- Vyas, K.; Vasconez, H.C. Wound healing: Biologics, skin substitutes, biomembranes and scaffolds. Healthcare 2014, 2, 356–400. [Google Scholar] [CrossRef] [PubMed]
- Kopecki, Z.; Cowin, A.J. Fighting chronic wound infection—One model at a time. Wound Pract. Res. J. Aust. Wound Manag. Assoc. 2017, 25, 6–13. [Google Scholar]
- Jones, V.; Grey, J.E.; Harding, K.G. Wound dressings. BMJ 2006, 332, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef]
- Ye, S.; Jiang, L.; Wu, J.; Su, C.; Huang, C.; Liu, X.; Shao, W. Flexible amoxicillin-grafted bacterial cellulose sponges for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2018, 10, 5862–5870. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Arora, A.; Alam, M.S.; Gupta, B. Development of antimicrobial and scar preventive chitosan hydrogel wound dressings. Int. J. Pharm. 2016, 508, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Sim, C.H.; Ng, S.F. Hydrogels Containing Antibiofilm and Antimicrobial Agents Beneficial for Biofilm-Associated Wound Infection: Formulation Characterizations and In vitro Study. AAPS PharmSciTech 2018, 19, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Rădulescu, M.; Holban, A.M.; Mogoantă, L.; Bălşeanu, T.A.; Mogoșanu, G.D.; Savu, D.; Popescu, R.C.; Fufă, O.; Grumezescu, A.M.; Bezirtzoglou, E.; et al. Fabrication, Characterization, and Evaluation of Bionanocomposites Based on Natural Polymers and Antibiotics for Wound Healing Applications. Molecules 2016, 21, 761. [Google Scholar] [CrossRef] [PubMed]
- Low, W.L.; Kenward, K.; Britland, S.T.; Amin, M.C.I.M.; Martin, C. Essential oils and metal ions as alternative antimicrobial agents: A focus on tea tree oil and silver. Int. Wound J. 2017, 14, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Saporito, F.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Boselli, C.; Icaro Cornaglia, A.; Mannucci, B.; Grisoli, P.; Vigani, B.; Ferrari, F. Essential oil-loaded lipid nanoparticles for wound healing. Int. J. Nanomed. 2017, 2018, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Ramasubbu, D.A.; Smith, V.; Hayden, F.; Cronin, P. Systemic antibiotics for treating malignant wounds. Cochrane Database Syst. Rev. 2017, 8, CD011609. [Google Scholar] [CrossRef] [PubMed]
- Everts, R. How to Treat Wound Infection. Prevention and Treatment. 2016. Available online: https://www.acc.co.nz/assets/provider/treating-wound-infections.pdf (accessed on 26 May 2018).
- Das, P.; Horton, R. Antibiotics: Achieving the balance between access and excess. Lancet 2016, 387, 102–104. [Google Scholar] [CrossRef]
- Aumeeruddy-Elalfi, Z.; Mahomoodally, M. Chapter: Extraction techniques and pharmacological potential of essential oils from medicinal and aromatic plants of Mauritius. In Essential Oils: Historical Significance, Chemical Composition and Medicinal Uses and Benefits; Peters, M., Ed.; Nova Publisher: Hauppauge, NY, USA, 2016; pp. 51–80. ISBN 978-1-63484-367-6. [Google Scholar]
- Aumeeruddy-Elalfi, Z.; Gurib-Fakim, A.; Mahomoodally, M. Chemical composition, antimicrobial and antibiotic potentiating activity of essential oils from 10 tropical medicinal plants from Mauritius. J. Herb. Med. 2016, 6, 88–95. [Google Scholar] [CrossRef]
- Scagnelli, A.M. Therapeutic review: Manuka honey. J. Exot. Pet Med. 2016, 25, 168–171. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Moghaddam, A.S.; Manouchehri, S.; Atoufi, Z.; Amiri, A.; Amirkhani, M.A.; Nilforoushzadeh, M.A.; Saeb, M.R.; Hamblin, M.R.; Mozafari, M. Can regenerative medicine and nanotechnology combine to heal wounds? The search for the ideal wound dressing. Nanomedicine 2017, 12, 2403–2422. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.; Tofail, S.A.M. Nanoparticles in biomedical applications. Adv. Phys. X 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Zewde, B.; Ambaye, A.; Stubbs, J., III; Raghavan, D. A review of stabilized silver nanoparticles—Synthesis, biological properties, characterization, and potential areas of applications. JSM Nanotechnol. Nanomed. 2016, 4, 1043. [Google Scholar]
- Cabuzu, D.; Cirja, A.; Puiu, R.; Grumezescu, A.M. Biomedical applications of gold nanoparticles. Curr. Top. Med. Chem. 2015, 15, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qin, Z.; Zeng, W.; Yang, T.; Cao, Y.; Mei, C.; Kuang, Y. Toxicity assessment of nanoparticles in various systems and organs. Nanotechnol. Rev. 2017, 6, 279–289. [Google Scholar] [CrossRef]
- Chua, A.W.C.; Tan, B.K.; Foo, C.L.; Tan, K.C.; Chong, S.J.; Khoo, Y.C. Skin tissue engineering advances in severe burns: Review and therapeutic applications. Burns Trauma 2016, 4, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Walsh, C.; Yue, D.; Dardik, A.; Cheema, U. Current Advancements and Strategies in Tissue Engineering for Wound Healing: A Comprehensive Review. Adv. Wound Care 2017, 6, 191–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivier, G.; Wael, N.H.; Gamal, B. Wound healing: Time to look for intelligent, ‘natural’ immunological approaches? BMC Immunol. 2017, 18 (Suppl. 1), 23. [Google Scholar] [CrossRef]
- Enoch, S.; Leaper, D.J. Basic science of wound healing. Surgery 2008, 26, 31–37. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization during Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L. Cellular mechanisms of skin repair in humans and other mammals. J. Cell Commun. Signal. 2016, 10, 103–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanon, S.; Hart, D.A.; Tredget, E.E. Molecular and cellular biology of wound healing and skin regeneration. In Skin Tissue Engineering and Regenerative Medicine; Albanna, M.Z., Holmes, J.H., Eds.; Elsevier Inc.: New York, NY, USA, 2016; pp. 19–47. ISBN 9780128016541. [Google Scholar]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demidova-Rice, T.N.; Durham, J.T.; Herman, I.M. Wound healing angio genesis: Innovations and challenges in acute and chronic wound healing. Adv. Wound Care 2012, 1, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Gould, L.J.; Fulton, A.T. Wound Healing in Older Adults. R. I. Med. J. 2016, 99, 34–36. [Google Scholar]
- Boateng, S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- van Rensburg, J.J.; Lin, H.; Gao, X.; Toh, E.; Fortney, K.R.; Ellinger, S.; Zwickl, B.; Janowicz, D.M.; Katz, B.P.; Nelson, D.E.; et al. The human skin microbiome associates with the outcome of and is influenced by bacterial infection. mBio 2015, 6, e01315-15. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A. The skin microbiome: Potential for novel diagnostic and therapeutic approaches to cutaneous disease. Semin. Cutan. Med. Surg. 2014, 33, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Sarheed, O.; Ahmed, A.; Shouqair, D.; Boateng, J. Antimicrobial dressings for improving wound healing. In Wound Healing-New Insights into Ancient Challenges; Alexandrescu, V., Ed.; InTech: London, UK, 2016; pp. 373–398. ISBN 978-953-51-2679-9. [Google Scholar]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti-Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Ortines, R.V.; Cheng, L.; Cohen, T.S.; Gami, A.; Dillen, C.A.; Ashbaugh, A.G.; Miller, R.J.; Wang, Y.; Tkaczyk, C.; Sellman, B.R.; et al. Anti-alpha-toxin immunoprohylaxis reduces disease severity against a Staphylococcus aureus full-thickness skin wound infection in immunocompetent and diabetic mice. J. Immunol. 2017, 198 (Suppl. 1), 77.20. Available online: http://www.jimmunol.org/content/198/1_Supplement/77.20 (accessed on 26 May 2018).
- Peerayeh, S.N.; Moghadas, A.J.; Behmanesh, M. Prevalence of Virulence-Related Determinants in Clinical Isolates of Staphylococcus epidermidis. Jundishapur J. Microbiol. 2016, 9, e30593. [Google Scholar] [CrossRef]
- Regev, A.; Weinberger, M.; Fishman, M.; Samra, Z.; Pitlik, S.D. Necrotizing fasciitis caused by Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yang, M.; Zhan, M.; Xu, X.; Yue, J.; Xu, T. Antibiotics for treating infected burn wounds. Cochrane Database Syst. Rev. 2016, 2, CD012084. [Google Scholar] [CrossRef]
- Church, D.; Lloyd, T.; Peirano, G.; Pitout, J. Antimicrobial susceptibility and combination testing of invasive Stenotrophomonas maltophilia isolates. Scand. J. Infect. Dis. 2013, 45, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Brooke, J.S. Stenotrophomonas maltophilia: An emerging global opportunistic pathogen. Clin. Microbiol. Rev. 2012, 25, 2–41. [Google Scholar] [CrossRef] [PubMed]
- Moet, G.J.; Jonesab, R.N.; Biedenbach, D.J.; Stilwell, M.G.; Fritsche, T.R. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: Report from the SENTRY Antimicrobial Surveillance Program (1998–2004). Diagn. Microbiol. Infect. Dis. 2007, 57, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Kishore, J. Isolation, identification & characterization of Proteus penneri—A missed rare pathogen. Indian J. Med. Res. 2012, 135, 341–345. [Google Scholar] [PubMed]
- Mihai, M.M.; Holban, A.M.; Giurcăneanu, C.; Popa, L.G.; Buzea, M.; Filipov, M.; Lazăr, V.; Chifiriuc, M.C.; Popa, M.I. Identification and phenotypic characterization of the most frequent bacterial etiologies in chronic skin ulcers. Rom. J. Morphol. Embryol. 2014, 55, 1401–1408. [Google Scholar] [PubMed]
- Lee, M.J.; Pottinger, P.S.; Butler-Wu, S.; Bumgarner, R.E.; Russ, S.M.; Matsen, F.A. Propionibacterium persists in the skin despite standard surgical preparation. J. Bone Jt. Surg. Am. 2014, 96, 1447–1450. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.; O’Donoghue, M.; Feeney, A.; Sleator, R.D. Acinetobacter baumannii: An emerging opportunistic pathogen. Virulence 2012, 3, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, O.D.; Vittorini, T.; Kao, J.; McBurney, M.I.; Nasmith, P.E.; Grinstein, S. A soluble Bacteroides by-product impairs phagocytic killing of Escherichia coli by neutrophils. Infect. Immun. 1989, 57, 745–753. [Google Scholar] [PubMed]
- Cutting, K.F.; White, R.J. Criteria for identifying wound infection revisited. Ostomy Wound Manag. 2005, 51, 28–34. [Google Scholar]
- Felk, A.; Kretschmar, M.; Albrecht, A.; Schaller, M.; Beinhauer, S.; Nichterlein, T.; Sanglard, D.; Korting, H.C.; Schäfer, W.; Hube, B. Candida albicans hyphal formation and the expression of the Efg1-regulated proteinases Sap4 to Sap6 are required for the invasion of parenchymal organs. Infect. Immun. 2002, 70, 3689–3700. [Google Scholar] [CrossRef] [PubMed]
- Katakura, T.; Yoshida, T.; Kobayashi, M.; Herndon, D.N.; Suzuki, F. Immunological control of methicillin-resistant Staphylococcus aureus (MRSA) infection in an immunodeficient murine model of thermal injuries. Clin. Exp. Immunol. 2005, 142, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Shirtliff, M.E.; Peters, B.M.; Jabra-Rizk, M.A. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol. Lett. 2009, 299, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beele, H.; Meuleneire, F.; Nahuys, M.; Percival, S.L. A prospective randomised open label study to evaluate the potential of a new silver alginate/carboxymethylcellulose antimicrobial wound dressing to promote wound healing. Int. Wound J. 2010, 7, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Bowler MPhil, P.; Woods, E.J. Assessing the effect of an antimicrobial wound dressing on biofilms. Wound Repair Regen. 2008, 16, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.; Brand, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Dutra, J.A.P.; Carvalho, S.G.; Zampirolli, A.C.D.; Daltoé, R.D.; Teixeira, R.M.; Careta, F.P.; Cotrim, M.A.P.; Oréfice, R.L.; Villanova, J.C.O. Papain wound dressings obtained from poly (vinyl alcohol)/calcium alginate blends as new pharmaceutical dosage form: Preparation and preliminary evaluation. Eur. J. Pharm. Biopharm. 2017, 113, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, L.; Dhurai, B. Preparation and Analysis of Chitosan-Honey Films for Wound Dressing Application. World Acad. Sci. Eng. Technol. Int. J. Mater. Text. Eng. 2018, 12, 54. Available online: urn:dai:10.1999/1307-6892/75464 (accessed on 26 May 2018).
- Ahmed, A.; Boateng, J. Calcium alginate-based antimicrobial film dressings for potential healing of infected foot ulcers. Ther. Deliv. 2018, 9, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Capanema, N.S.V.; Mansur, A.A.P.; Carvalho, S.M.; Mansur, L.L.; Ramos, C.P.; Lage, A.P.; Mansur, H.S. Physicochemical properties and antimicrobial activity of biocompatible carboxymethylcellulose-silver nanoparticle hybrids for wound dressing and epidermal repair. J. Appl. Polym. Sci. 2018, 135, 45812. [Google Scholar] [CrossRef]
- Yao, C.H.; Lee, C.Y.; Huang, C.H.; Chen, Y.S.; Chen, K.Y. Novel bilayer wound dressing based on electrospun gelatin/keratin nanofibrous mats for skin wound repair. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 79, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Cerchiara, T.; Abruzzo, A.; Ñahui Palomino, R.A.; De Rose, B.V.R.; Chidichimo, G.; Ceseracciu, L.; Athanassiou, A.; Saladini, B.; Dalena, F.; Bigucci, F.; et al. Spanish Broom (Spartium junceum L.) fibers impregnated with vancomycin-loaded chitosan nanoparticles as new antibacterial wound dressing: Preparation, characterization and antibacterial activity. Eur. J. Pharm. Sci. 2017, 99, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Swenty, C.F. Principles to Guide Your Dressing Choice. J. Nurse Pract. 2016, 12, e125–e127. [Google Scholar] [CrossRef]
- Asfaw, T.; Jackson, J.C.; Lu, Z.; Zhai, X.; Shums, S.; Hirt, T.; Hu, X.; René, C.R. In-Situ Forming Hydrogel Wound Dressings Containing Antimicrobial Agents. U.S. Patent US923280B2, 12 January 2016. [Google Scholar]
- Ousey, K.; Cutting, K.; Rogers, A.A.; Rippon, M. The importance of hydration in wound healing: Reinvigorating the clinical perspective. J. Wound Care 2016, 25, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. Biomedicine 2015, 5, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Qiu, L.; Cui, J.; Wei, Q. Bacterial cellulose and bacterial cellulose-vaccarin membranes for wound healing. Mater. Sci. Eng. C 2016, 59, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a wound dressing based on common wound characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Ramos-e-Silva, M.; de Castro, M.C.R. New dressings, including tissue-engineered living skin. Clin. Dermatol. 2002, 20, 715–723. [Google Scholar] [CrossRef]
- Flores, C.; Lopez, M.; Tabary, N.; Neut, C.; Chai, F.; Betbeder, D.; Herkt, C.; Cazaux, F.; Gaucher, V.; Martel, B. Preparation and characterization of novel chitosan and β-cyclodextrin polymer sponges for wound dressing applications. Carbohydr. Polym. 2017, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Pott, F.S.; Meier, M.J.; Stocco, J.G.D.; Crozeta, K.; Ribas, J.D. The effectiveness of hydrocolloid dressings versus other dressings in the healing of pressure ulcers in adults and older adults: A systematic review and meta-analysis. Rev. Lat.-Am. Enferm. 2014, 22, 511–529. [Google Scholar] [CrossRef]
- Das, S.; Baker, A. Biomaterials and Nanotherapeutics for Enhancing Skin Wound Healing. Front. Bioeng. Biotechnol. 2016, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Woundcarehandbook. Available online: http://www.woundcarehandbook.com (accessed on 30 June 2018).
- Sweeney, I.R.; Miraftab, M.; Collyer, G. A critical review of modern and emerging absorbent dressings used to treat exuding wounds. Int. Wound J. 2012, 9, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T.; Glick, G.D. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calo, E.; Ballamy, L.; Khutoryanskiy, V.V. Hydrogels in Wound Management. In Hydrogels: Design, Synthesis and Application in Drug Delivery and Regenerative Medicine; Singh, T.R.R., Leverty, G., Donelly, R., Eds.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Vermeulen, H.; Ubbink, D.T.; Goossens, A.; de Vos, R.; Legemate, D.A. Systematic review of dressings and topical agents for surgical wounds healing by secondary intention. Br. J. Surg. 2005, 92, 665–672. [Google Scholar] [CrossRef] [PubMed]
- International Wound Infection Institute (IWII). Wound Infection in Clinical Practice; Terry Swanson, N.P.W.M., Ed.; Wounds International: London, UK, 2016; Available online: https://tinyurl.com/y8skcrnd (accessed on 25 July 2018).
- Liu, X.; Nielsen, L.H.; Kłodzińska, S.N.; Nielsen, H.M.; Quc, H.; Christensen, L.P.; Rantanen, J.; Yangad, M. Ciprofloxacin-loaded sodium alginate/poly(lactic-co-glycolic acid) electrospun fibrous mats for wound healing. Eur. J. Pharm. Biopharm. 2018, 123, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Contardi, M.; Heredia-Guerrero, J.A.; Perotto, G.; Valentini, P.; Pompa, P.P.; Spanò, R.; Goldonic, L.; Bertorelli, R.; Athanassiou, A.; Bayera, I.S. Transparent ciprofloxacin-povidone antibiotic films and nanofiber mats as potential skin and wound care dressings. Eur. J. Pharm. Sci. 2017, 104, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Williams, G.R.; Wang, J.W.H.; Sun, X.; Zhu, L.M. Poly(N-isopropylacrylamide)/poly(l-lactic acid-co-ɛ-caprolactone) fibers loaded with ciprofloxacin as wound dressing materials. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Pamfil, D.; Vasile, C.; Tarţău, L.; Vereştiuc, L.; Poiată, A. pH-Responsive 2-hydroxyethyl methacrylate/citraconic anhydride–modified collagen hydrogels as ciprofloxacin carriers for wound dressings. J. Bioact. Compat. Polym. 2017, 32, 355–381. [Google Scholar] [CrossRef]
- Khampieng, T.; Wnek, G.E.; Supaphol, P. Electrospun DOXY-h loaded-poly(acrylic acid) nanofiber mats: In vitro drug release and antibacterial properties investigation. J. Biomater. Sci.-Polym. Ed. 2014, 25, 1292–1305. [Google Scholar] [CrossRef] [PubMed]
- Michalska-Sionkowska, M.; Kaczmarek, B.; Walczak, M.; Sionkowska, A. Antimicrobial activity of new materials based on the blends of collagen/chitosan/hyaluronic acid with gentamicin sulfate addition. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 86, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Robertson, D.D.; van Reenen, A.J.; Dicks, L.M.T. Polyethylene oxide (PEO)-hyaluronic acid (HA) nanofibers with kanamycin inhibits the growth of Listeria monocytogenes. Biomed. Pharmacother. 2017, 86, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Etebu, E.; Arikekpar, I. Antibiotics: Classification and mechanisms of action with emphasis on molecular perspectives. Int. J. Appl. Microbiol. Biotechnol. Res. 2016, 4, 90–101. [Google Scholar]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Kon, K.; Gade, A.; Ingle, A.; Nagaonkar, D.; Paralikar, P.; da Silva, S.S. Chapter 6—Antibiotic Resistance: Can Nanoparticles Tackle the Problem? In Antibiotic Resistance. Mechanisms and New Antimicrobial Approaches; Elsevier Science: New York, NY, USA, 2016; pp. 121–143. [Google Scholar] [CrossRef]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Pîrvănescu, H.; Bălăşoiu, M.; Ciurea, M.E.; Bălăşoiu, A.T.; Mănescu, R. Wound infections with multi-drug resistant bacteria. Chirurgia 2014, 109, 73–79. [Google Scholar] [PubMed]
- Chávez-González, M.L.; Rodríguez-Herrera, R.; Aguilar, C.N. Chapter 11—Essential Oils: A Natural Alternative to Combat Antibiotics Resistance. In Antibiotic Resistance. Mechanisms and New Antimicrobial Approaches; Elsevier Science: New York, NY, USA, 2016; pp. 227–237. [Google Scholar] [CrossRef]
- Shrestha, G.; Raphael, J.; Leavitt, S.D.; St Clair, L.L. In vitro evaluation of the antibacterial activity of extracts from 34 species of North American lichens. Pharm. Biol. 2014, 52, 1262–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segev-Zarko, L.; Saar-Dover, R.; Brumfeld, V.; Mangoni, M.L.; Shai, Y. Mechanisms of biofilm inhibition and degradation by antimicrobial peptides. Biochem. J. 2015, 468, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.-G. Plant essential oils as active antimicrobialagents. Crit. Rev. Food Sci. Nutr. 2014, 54, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Agyare, C.; Duah, Y.; Oppong, E.; Hensel, A.; Oteng, S.; Appiah, T. Review: African medicinal plants with wound healing properties. J. Ethnopharmacol. 2016, 177, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Semeniuc, C.A.; Popa, C.R.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations againts Gram-positive and Gram-negative bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Kavoosi, G.; Dadfar, S.M.M.; Purfard, A.M.; Mehrabi, R. Antioxidant and Antibacterial Properties of Gelatin Films Incorporated with Carvacrol. J. Food Saf. 2013, 33, 423–432. [Google Scholar] [CrossRef]
- Altiok, D.; Altiok, E.; Tihminlioglu, F. Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. J. Mater. Sci. Mater. Med. 2010, 21, 2227–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, S.E.; Maillard, J.-Y.; Russell, A.D.; Catrenich, C.E.; Charbonneau, D.L.; Bartolo, R.G. Development of bacterial resistance to several biocides and effects on antibiotic susceptibility. J. Hosp. Infect. 2003, 55, 98–107. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Głowacka, A.; Kowalczyk, E.; Wiktorowska-Owczarek, A.; Jóźwiak-Bębenista, M.; Łysakowska, M. The Biological Activities of Cinnamon, Geranium and Lavender Essential Oils. Molecules 2014, 19, 20929–20940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zenati, F.; Benbelaid, F.; Khadir, A.; Bellahsene, C.; Bendahou, M. Antimicrobial effects of three essential oils on multidrug resistant bacteria responsible for urinary infections. J. Appl. Pharm. Sci. 2014, 4, 15–18. [Google Scholar] [CrossRef]
- Liakos, I.; Rizzello, L.; Scurr, D.J.; Pompa, P.P.; Bayer, I.S.; Athanassiou, A. All-natural composite wound dressing films of essential oils encapsulated in sodium alginate with antimicrobial properties. Int. J. Pharm. 2014, 463, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.; Rizzello, L.; Hajiali, H.; Brunetti, V.; Carzino, R.; Pompa, P.; Athanassiou, A.; Mele, E. Fibrous wound dressings encapsulating essential oils as natural antimicrobial agents. J. Mater. Chem. B 2015, 3, 1583–1589. [Google Scholar] [CrossRef]
- Rosa, J.M.; Bicudo Bonato, L.; Bragine Mancuso, C.; Martinelli, L.; Okura, M.H.; Malpass, G.R.P.; Granato, A.C. Antimicrobial wound dressing films containing essential oils and oleoresins of pepper encapsulated in sodium alginate films. Cienc. Rural 2018, 48, e20170740. [Google Scholar] [CrossRef]
- Nogueira, M.N.M.; Aquino, S.G.; Rossa Junior, C.; Spolidorio, D.M.P. Terpinen-4-ol and alpha-terpineol (tea tree oil components) inhibit the production of IL-1b, IL-6 and IL-10 on human macrophages. Inflamm. Res. 2014, 63, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.-Y.; Chou, T.-C.; Tsai, J.-C.; Yu, W.-C. The effect of active ingredient-containing chitosan/polycaprolactone nonwoven mat on wound healing: In vitro and in vivo studies. J. Biomed. Mater. Res. Part A 2014, 102, 2324–2333. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Ge, M. Sustained broad-spectrum antimicrobial and haemostatic chitosan-based film with immerged tea tree oil droplets. Fibers Polym. 2015, 16, 308–318. [Google Scholar] [CrossRef]
- Edmondson, M.; Newall, N.; Carville, K.; Smith, J.; Riley, T.V.; Carson, C.F. Uncontrolled, open-label, pilot study of tea tree (Melaleuca alternifolia) oil solution in the decolonisation of methicillin-resistant Staphylococcus aureus positive wounds and its influence on wound healing. Int. Wound J. 2011, 8, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Leung, P.; Wong, T. A randomized controlled trial of topical tea tree preparation for MRSA colonized wounds. Int. J. Nurs. Sci. 2014, 1, 7–14. [Google Scholar] [CrossRef]
- Cuttle, L.; Kempf, M.; Kravchuk, O.; George, N.; Liu, P.Y.; Chang, H.E.; Mill, J.; Wang, X.Q.; Kimble, R.M. The efficacy of Aloe vera, tea tree oil and saliva as first aid treatment for partial thickness burn injuries. Burns 2008, 34, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Saddiqe, Z.; Naeem, I.; Maimoona, A. A review of the antibacterial activity of Hypericum perforatum L. J. Ethnopharmacol. 2010, 131, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Güneş, S.; Tıhmınlıoğlu, F. Hypericum perforatum incorporated chitosan films as potential bioactive wound dressing material. Int. J. Biol. Macromol. 2017, 102, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Evandri, M.G.; Battinelli, L.; Daniele, C.; Mastrangelo, S.; Bolle, P.; Mazzanti, G. The antimutagenic activity of Lavandula angustifolia (lavender) essential oil in the bacterial reverse mutation assay. Food Chem. Toxicol. 2005, 43, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological activities of lavender essentials oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Imane, M.M.; Houda, F.; Amal, A.H.S.; Kaotar, N.; Mohammed, T.; Imane, R.; Farid, H. Phytochemical Composition and Antibacterial Activity of Moroccan Lavandula angustifolia Mill. J. Essent. Oil Bear. Plants 2017, 20, 1074–1082. [Google Scholar] [CrossRef]
- Mori, H.; Kawanami, H.; Kawahata, H.; Aoki, M. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement. Altern. Med. 2016, 16, 144. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Zengin, G.; Oskay, M.; Uysal, S.; Ceylan, R.; Aktumsek, A. Composition, antioxidant, antimicrobial and enzyme inhibition activities of two Origanum vulgare subspecies (subsp. vulgare and subsp. hirtum) essential oils. Ind. Crops Prod. 2015, 70, 178–184. [Google Scholar] [CrossRef]
- Nostro, A.; Blanco, A.R.; Cannatelli, M.A.; Enea, V.; Flamini, G.; Morelli, I.; Sudano Roccaro, A.; Alonzo, V. Susceptibility of methicillin-resistant staphylococci to oregano essential oil, carvacrol and thymol. FEMS Microbiol. Lett. 2004, 230, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Liakos, I.L.; Holban, A.M.; Carzino, R.; Lauciello, S.; Grumezescu, A.M. Electrospun Fiber Pads of Cellulose Acetate and Essential Oils with Antimicrobial Activity. Nanomaterials 2017, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S. The Bee Products: The Wonders of the Bee Hexagon, Bee Product Science. Available online: www.bee-hexagon.net (accessed on 1 August 2018).
- Kwakman, P.H.; te Velde, A.A.; de Boer, L.; Speijer, D.; Vandenbroucke-Grauls, C.M.; Zaat, S.A. How honey kills bacteria. FASEB J. 2010, 24, 2576–2582. [Google Scholar] [CrossRef] [PubMed]
- Molan, P.C. The evidence supporting the use of honey as a wound dressing. Int. J. Lower Extrem. Wounds 2006, 5, 40–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israili, Z.H. Antimicrobial properties of honey. Am. J. Ther. 2014, 21, 304–423. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.; Diunase, K.N. Comparing the antibacterial and functional properties of cameroonian and manuka honeys for potential wound healing—Have we come full cycle in dealing with antibiotic resistance? Molecules 2015, 20, 16068–16084. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Traynor, K.; Santos, K.; Blaser, G.; Bode, U.; Molan, P. Medical honey for wound care—Still the ‘latest resort’? Evid.-Based Complement. Altern. Med. 2009, 6, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Kuś, P.M.; Szweda, P.; Jerković, I.; Tuberoso, C.I. Activity of Polish unifloral honeys against pathogenic bacteria and its correlation with colour, phenolic content, antioxidant capacity and other parameters. Lett. Appl. Microbiol. 2016, 62, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, O.; Dolan, A.; Athman, R.; Power, A.; Gethin, G.; Cowman, S.; Humphreys, H. Comparison of the antimicrobial activity of Ulmo honey from Chile and Manuka honey against methicillin-resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2010, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.J.; Lim, M.S. Anti-staphylococcal activity of melaleuca honey. Southeast Asian J. Trop. Med. Public Health 2015, 46, 472–479. [Google Scholar] [PubMed]
- Jantakee, K.; Tragoolpua, Y. Activities of different types of Thai honey on pathogenic bacteria causing skin diseases, tyrosinase enzyme and generating free radicals. Biol. Res. 2015, 48, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Packer, J.M.; Irish, J.; Herbert, B.R.; Hill, C.; Padula, M.; Blair, S.E.; Carter, D.A.; Harry, E.J. Specific non-peroxide antibacterial effect of manuka honey on the Staphylococcus aureus proteome. Int. J. Antimicrob. Agents 2012, 40, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R. Honey as an effective antimicrobial treatment for chronic wounds: Is there a place for it in modern medicine? Chronic Wound Care Manag. Res. 2014, 1, 15–22. [Google Scholar] [CrossRef]
- Bulman, S.E.; Tronci, G.; Goswami, P.; Carr, C.; Russell, S.J. Antibacterial properties of nonwoven wound dressings coated with Manuka honey or methylglyoxal. Materials 2017, 10, 954. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Turnbull, L.; Burke, C.M.; Liu, M.; Carter, D.A.; Schlothauer, R.C.; Whitchurch, C.B.; Harry, E.J. Manuka-type honeys can eradicate biofilms produced by Staphylococcus aureus strains with different biofilm-forming abilities. PeerJ 2014, 2, e326. [Google Scholar] [CrossRef] [PubMed]
- Minden-Birkenmaier, B.A.; Neuhalfen, R.M.; Janowiak, B.E.; Sell, S.A. Preliminary Investigation and Characterization of Electrospun Polycaprolactone and Manuka Honey Scaffolds for Dermal Repair. J. Eng. Fiber Fabr. 2015, 10, 126–138. [Google Scholar]
- Yang, X.; Fan, L.; Ma, L.; Wang, Y.; Lin, S.; Yu, F.; Pan, X.; Luo, G.; Zhang, D.; Wang, H. Green electrospun Manuka honey/silk fibroin fibrous matrices as potential wound dressing. Mater. Des. 2017, 119, 76–84. [Google Scholar] [CrossRef]
- Tavakoli, J.; Tang, Y. Honey/PVA hybrid wound dressings with controlled release of antibiotics: Structural, physico-mechanical and in-vitro biomedical studies. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Saikaly, S.K.; Khachemoune, A. Honey and Wound Healing: An Update. Am. J. Clin. Dermatol. 2017, 18, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Curtis, A.; Hoskins, C. Application of Nanoparticle Technologies in the Combat against Anti-Microbial Resistance. Pharmaceutics 2018, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Kandi, V.; Kandi, S. Antimicrobial properties of nanomolecules: Potential candidates as antibiotics in the era of multi-drug resistance. Epidemiol. Health 2015, 37, e2015020. [Google Scholar] [CrossRef] [PubMed]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.W.; An, Y.J. Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci. Total Environ. 2011, 409, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Ashkarran, A.A.; Ghavami, M.; Aghaverdi, H.; Stroeve, P.; Mahmoudi, M. Bacterial effects and protein corona evaluations: Crucial ignored factors for prediction of bio-efficacy of various forms of silver nanoparticles. Chem. Res. Toxicol. 2012, 25, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Brauer, M.J.; Botstein, D. Slow growth induces heat-shock resistance in normal and respiratory-deficient yeast. Mol. Biol. Cell 2009, 20, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Laha, D.; Bhattacharya, D.; Pramanik, P.; Karmakar, P. A novel study of antibacterial activity of copper iodide nanoparticle mediated by DNA and membrane damage. Colloids Surf. B 2012, 96, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles, the powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Anisha, B.S.; Biswas, R.; Chennazhi, K.P.; Jayakumar, R. Chitosan-hyaluronic acid/nano silver composite sponges for drug resistant bacteria infected diabetic wounds. Int. J. Biol. Macromol. 2013, 62, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.; Rangasamy, S.; Purushothaman, B.; Song, J.M. The application of bactericidal silver nanoparticles in wound treatment. Nanomater. Nanotechnol. 2015, 5, 23–37. [Google Scholar] [CrossRef]
- Verma, J.; Kanoujia, J.; Parashar, P.; Tripathi, C.B.; Saraf, S.A. Wound healing applications of sericin/chitosan-capped silver nanoparticles incorporated hydrogel. Drug Deliv. Transl. Res. 2017, 7, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Kaba, S.I.; Egorova, E.M. In vitro studies of the toxic effects of silver nanoparticles on HeLa and U937 cells. Nanotechnol. Sci. Appl. 2015, 8, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, R.; He, T.; Xu, K.; Du, D.; Zhao, N.; Cheng, X.; Yang, J.; Shi, H.; Lin, Y. Biomedical potential of ultrafine Ag/AgCl nanoparticles coated on graphene with special reference to antimicrobial performances and burn wound healing. ACS Appl. Mater. Interfaces 2016, 8, 15067–15075. [Google Scholar] [CrossRef] [PubMed]
- Frankova, J.; Pivodova, V.; Vagnerova, H.; Juranova, J.; Ulrichova, J. Effects of silver nanoparticles on primary cell cultures of fibroblasts and keratinocytes in a wound-healing model. J. Appl. Biomater. Funct. Mater. 2016, 14, 137–142. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.Y.; Gohar, Y.M.; Sorour, M.A.; Waheeb, M.G. Hydrogel dressing with a nano-formula against methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa diabetic foot bacteria. J. Microbiol. Biotechnol. 2016, 26, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Koul, V.; Dinda, A.K. In vitro and in vivo investigational studies of a nanocomposite-hydrogel-based dressing with a silver-coated chitosan wafer for full-thickness skin wounds. J. Appl. Polym. Sci. 2016, 133, 43472. [Google Scholar] [CrossRef]
- Nešović, K.; Kojić, V.; Rhee, K.Y.; Mišković-Stanković, V. Electrochemical synthesis and characterization of silver doped poly(vinyl alcohol)/chitosan hydrogels. Corrosion 2017, 73, 1437–1447. [Google Scholar] [CrossRef]
- Hanif, M.; Juluri, R.R.; Fojan, P.; Popok, V.N. Polymer films with size-selected silver nanoparticles as plasmon resonance-based transducers for protein sensing. Biointerface Res. Appl. Chem. 2016, 6, 1564–1568. [Google Scholar]
- Higa, A.M.; Mambrini, G.P.; Hausen, M.; Strixino, F.T.; Leite, F.L. Ag-nanoparticle-based nano-immunosensor for anti-glutathione s-transferase detection. Biointerface Res. Appl. Chem. 2016, 6, 1053–1058. [Google Scholar]
- Wu, J.; Zheng, Y.; Song, W.; Luan, J.; Wen, X.; Wu, Z.; Chen, X.; Wang, Q.; Guo, S. In situ synthesis of silver-nanoparticles/bacterial cellulose composites for slow-released antimicrobial wound dressing. Carbohydr. Polym. 2014, 102, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, M.; Andronescu, E.; Dolete, G.; Popescu, R.C.; Fufă, O.; Chifiriuc, M.C.; Mogoantă, L.; Bălşeanu, T.A.; Mogoşanu, G.D.; Grumezescu, A.M.; et al. Silver Nanocoatings for Reducing the Exogenous Microbial Colonization of Wound Dressings. Materials 2016, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafiee, M.R.M.; Kargar, M. Synthesis of 3,4,5-substituted furan-2(5h)-ones using zno nanostructure as an efficient catalyst. Biointerface Res. Appl. Chem. 2017, 7, 2170–2173. [Google Scholar]
- Applerot, G.; Lellouche, J.; Perkas, N.; Nitzan, Y.; Gedanken, A.; Banin, E. ZnO nanoparticle-coated surfaces inhibit bacterial biofilm formation and increase antibiotic susceptibility. RSC Adv. 2012, 2, 2314–2321. [Google Scholar] [CrossRef]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Khosravian, P.; Ghashang, M.; Ghayoor, H. Zinc oxide/natural-zeolite composite nano-powders: Efficient catalyst for the amoxicillin removal from wastewater. Biointerface Res. Appl. Chem. 2016, 6, 1538–1540. [Google Scholar]
- Nair, S.; Sasidharan, A.; Rani, V.V.D.; Menon, D.; Nair, S.; Manzoor, K.; Raina, S. Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J. Mater. Sci. Mater. Med. 2009, 20, S235–S241. [Google Scholar] [CrossRef] [PubMed]
- Păunica-Panea, G.; Ficai, A.; Marin, M.M.; Marin, Ș.; Albu, M.G.; Constantin, V.D.; Dinu-Pîrvu, C.; Vuluga, Z.; Corobea, M.C.; Ghica, M.V. New collagen-dextran-zinc oxide composites for wound dressing. J. Nanomater. 2016, 14, 7–11. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Moghaddam, A.; Shamsi, H.; Mansoori Moghadam, Z. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol. 2018, 114, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Rădulescu, M.; Andronescu, E.; Cirja, A.; Holban, A.M.; Mogoantă, L.; Bălşeanu, T.A.; Bogdan, C.; Neagu, T.P.; Lascăr, I.; Florea, D.A.; et al. Antimicrobial coatings based on zinc oxide and orange oil for improved bioactive wound dressings and other applications. Rom. J. Morphol. Embryol. 2016, 57, 107–114. [Google Scholar] [PubMed]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Wound Healing Bionanocomposites Based on Castor Oil Polymeric Films Reinforced with Chitosan-Modified ZnO Nanoparticles. Biomacromolecules 2015, 16, 2631–2644. [Google Scholar] [CrossRef] [PubMed]
- Geraldo, D.A.; Needham, P.; Chandia, N.; Arratia-Perez, R.; Mora, G.C.; Villagra, N.A. Green synthesis of polysaccharides-based gold and silver nanoparticles and their promissory biological activity. Biointerface Res. Appl. Chem. 2016, 6, 1263–1271. [Google Scholar]
- Khashayar, P.; Amoabediny, G.; Larijani, B.; Hosseini, M.; Verplancke, R.; Schaubroeck, D.; De Keersmaecker, M.; Adriaens, A.; Vanfleteren, J. Characterization of gold nanoparticle layer deposited on gold electrode by various techniques for improved sensing abilities. Biointerface Res. Appl. Chem. 2016, 6, 1380–1390. [Google Scholar]
- Mikalauskaite, A.; Karabanovas, V.; Karpicz, R.; Rotomskis, R.; Jagminas, A. Green synthesis of red-fluorescent gold nanoclusters: Characterization and application for breast cancer detection. Biointerface Res. Appl. Chem. 2016, 6, 1702–1709. [Google Scholar]
- Nicol, J.R.; Dixon, D.; Coulter, J.A. Gold nanoparticle surface functionalization: A necessary requirement in the development of novel nanotherapeutics. Nanomedicine 2015, 10, 1315–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akturk, O.; Kismet, K.; Yasti, A.C.; Kuru, S.; Duymus, M.E.; Kaya, F.; Caydere, M.; Hucumenoglu, S.; Keskin, D. Collagen/gold nanoparticle nanocomposites: A potential skin wound healing biomaterial. J. Biomater. Appl. 2016, 21, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Regiel-Futyra, A.; Kus-Liśkiewicz, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Stochel, G.; Kyzioł, A. Development of non cytotoxic chitosan-gold nanocomposites as efficient antibacterial materials. ACS Appl. Mater. Interfaces 2015, 7, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Volkova, N.; Yukhta, M.; Pavlovich, O.; Goltsev, A. Application of cryopreserved fibroblast culture with au nanoparticles to treat burns. Nanoscale Res. Lett. 2016, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.F.; Facchi, S.P.; Monteiro, J.P.; Nocchi, S.R.; Silva, C.T.P.; Nakamura, C.V.; Girotto, E.M.; Rubira, A.F.; Muniz, E.C. Preparation and cytotoxicity of N,N,N-trimethyl chitosan/alginate beads containing gold nanoparticles. Int. J. Biol. Macromol. 2015, 72, 466–471. [Google Scholar] [CrossRef] [PubMed]


| Species | Shape | Metabolism | Incidence | Ref. |
|---|---|---|---|---|
| S. aureus | Cocci | Facultatively anaerobic | Chronic wounds | [44] |
| S. epidermidis | Acute wounds | [45] | ||
| Streptococcus pyogenes | Aerobic | Chronic wounds | [46] | |
| P. aeruginosa | Bacilli | Aerobic | Chronic wounds | [47] |
| Stenotrophomonas maltophilia | [48,49] | |||
| E. coli | Facultatively anaerobic | [50] | ||
| Proteus sp. | [51,52] | |||
| Klebsiella sp. | [5] | |||
| Propionibacterium acnes | Aerotolerant anaerobic | Acute wounds | [53] | |
| Acinetobacter baumannii | Coccobacilli | Aerobic | Chronic wounds | [48,54] |
| Type of Dressing | Formulation | Advantages (A)/Disadvantages (D) | Some Commercially Available Products | Ref. |
|---|---|---|---|---|
| Inert/passive | Gauzes | (A): Are manufactured in forms of bandages, sponges, plasters and stockings. Display a massive porosity, make available thermal isolation, and sustain a humid background at the wound site. Sponges can be applied directly to the surface of suppurating wounds (D): Can stick to wounds, disrupt the wound bed when removed, are suitable mostly for minor wounds; Sponges suffer from lack of mechanical resistance and they are not fitted for third-degree burns management or wounds with desiccated eschar | Multisorb, Urgotul SSD/S.Ag, Curity, Vaseline Gauze, Xeroform | [76,77] |
| Bioactive | Hydrocolloids | (A): Semi-permeable in the form of solid wafers, can enclose hydroactive particles that swell with exudates or form a gel, can be detached from wounds without difficulty by saline or sterilized water, and are usually considered as painless dressings (highly recommended for pediatrics wound care management). (D): Can be applied in wounds with light to heavy exudate (such as eschars, minor burn wounds and traumatic wounds, sloughing, or granulating wounds), can be cytotoxic, can possess a disagreeable odor, sustain an acid pH at the application site and present a low mechanically strength | DouDERM, Granuflex, Comfeel, Tegasorb | [78,79] |
| Alginates | (A): Highly absorbent, hemostatic, applicable for exudating wounds, helpful in debridement of sloughing wounds. (D): Limited use on low exudating wounds, causing dryness and scabbing, should be changed daily | Kaltostat, Algisite, Kaltostat, Sorbsan, Tegagen, SeaSorb, PolyMem | [80,81] | |
| Collagens | (A): They are in the form of pads, gels or particles and encourage the formation and setting of new- formed collagen in wounds, they absorb exudates, offer a humid environment to wounds; They are easy to apply, non-immunogenic, non-pyrogenic, (D): Not recommended to application to wounds with necrosis and third-degree burns; require a secondary dressing | Puracol Plus, Triple Helix Collagen, Cutimed Epiona Sterile, BIOSTEP | [67,82] | |
| Hydrofibers | (A): Soft nonwoven pad or ribbon dressings that absorb exudates and provide a moist environment in a deep wound together with a reduced risk of skin maceration (D): A certain degree of absorption of fluid is required for pH control, however, the absorption of an excessive amount can cause an undesirable swelling of the wound dressing, leading to distension and possible loss of adhesion; | Aquacel | [67] | |
| Interactive | Hydrogels | (A): Rehydrates dry wounds, easy removal/changes, high capacity to accumulate/absorb large volumes of water inside their 3D polymeric network, moist-absorbent wound dressings, permeable to metabolites, non-irritant, and non-reactive with biological tissues (D): May cause over-hydration, weak mechanical properties, consequently necessitating a secondary dressing | Carrasyn, Curagel, Nu-Gel, Purilon, Restore, SAF-gel, XCell | [62,83] |
| Semi-permeable films | (A): Semi-permeable, transparent for allowing wound check, highly elastic, and can follow any contour and do not have need of extra patter; waterproof and permeable to oxygen (D): Mostly suitable for superficial wounds with little exudates and for wound epithelialization, used as an additional layer for hydrogels and foams | Opsite, Tegaderm, Biooclusive, Polyskin | [72] | |
| Semi-permeable foams | (A): Soft, open cell, hydrophobic, usually made from polyurethane sheets; large amounts of exudates. (D): Can cause dryness and scabbing when applied to low exudating wounds and dry scars | Allevyn | [84] |
| Class | Name | Wound Dressing Material | Tested Strains | Ref. |
|---|---|---|---|---|
| Quinolones | Amoxicillin | Flexible sponges from bacterial cellulose | E. coli, C. albicans S. aureus | [10] |
| Ciprofloxacin | Calcium alginate films | E. coli S. aureus P. aeruginosa | [86] | |
| Films and nanofiber mats of Povidone | E. coli Bacillus subtilis | [87] | ||
| Electrospun fibers based on thermoresponsive polymer poly(N-isopropylacrylamide), poly(l–lactic acid–co-ɛ-caprolactone) | E. coli S. aureus | [88] | ||
| Hydrogels from 2-hydroxyethyl methacrylate/citraconic anhydride–modified collagen | S. aureus | [89] | ||
| Tetracyclines | Tetracycline | Cotton fabric coated with chitosan-Poly(vinyl pyrrolidone)–PEG | E. coli S. aureus | [11] |
| Doxycycline | Poly(acrylic acid) nanofiber mats | S. aureus Streptococcus agalactiae | [90] | |
| Aminoglycosides | Gentamicin | Thin films made from collagen, chitosan and hyaluronic acid | E. coli S. aureus P. aeruginosa | [91] |
| Sodium carboxymethyl cellulose loaded with antibiofilm agents (xylitol and ethylenediaminetetraacetic acid) | S. aureus Bacillus subtilis P. aeruginosa E. coli | [12] | ||
| Kanamycin | Nanofibers prepared with a combination of polyethylene oxide and hyaluronic acid | Listeria monocytogenes P. aeruginosa | [92] | |
| Cephalosporins | Cefuroxime and Cefepime | Biocompatible nanostructured composite based on naturally derived biopolymers (chitin and sodium alginate) | E. coli S. aureus | [13] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. https://doi.org/10.3390/molecules23092392
Negut I, Grumezescu V, Grumezescu AM. Treatment Strategies for Infected Wounds. Molecules. 2018; 23(9):2392. https://doi.org/10.3390/molecules23092392
Chicago/Turabian StyleNegut, Irina, Valentina Grumezescu, and Alexandru Mihai Grumezescu. 2018. "Treatment Strategies for Infected Wounds" Molecules 23, no. 9: 2392. https://doi.org/10.3390/molecules23092392
APA StyleNegut, I., Grumezescu, V., & Grumezescu, A. M. (2018). Treatment Strategies for Infected Wounds. Molecules, 23(9), 2392. https://doi.org/10.3390/molecules23092392






