Selection of Reference Genes for qRT-PCR Analysis in Lentinula edodes after Hot-Air Drying
Abstract
:1. Introduction
2. Results
2.1. Selection of Candidate Genes with Stable Expression Using RNA-seq Data
2.2. Expression Profiles of Candidate Reference Genes
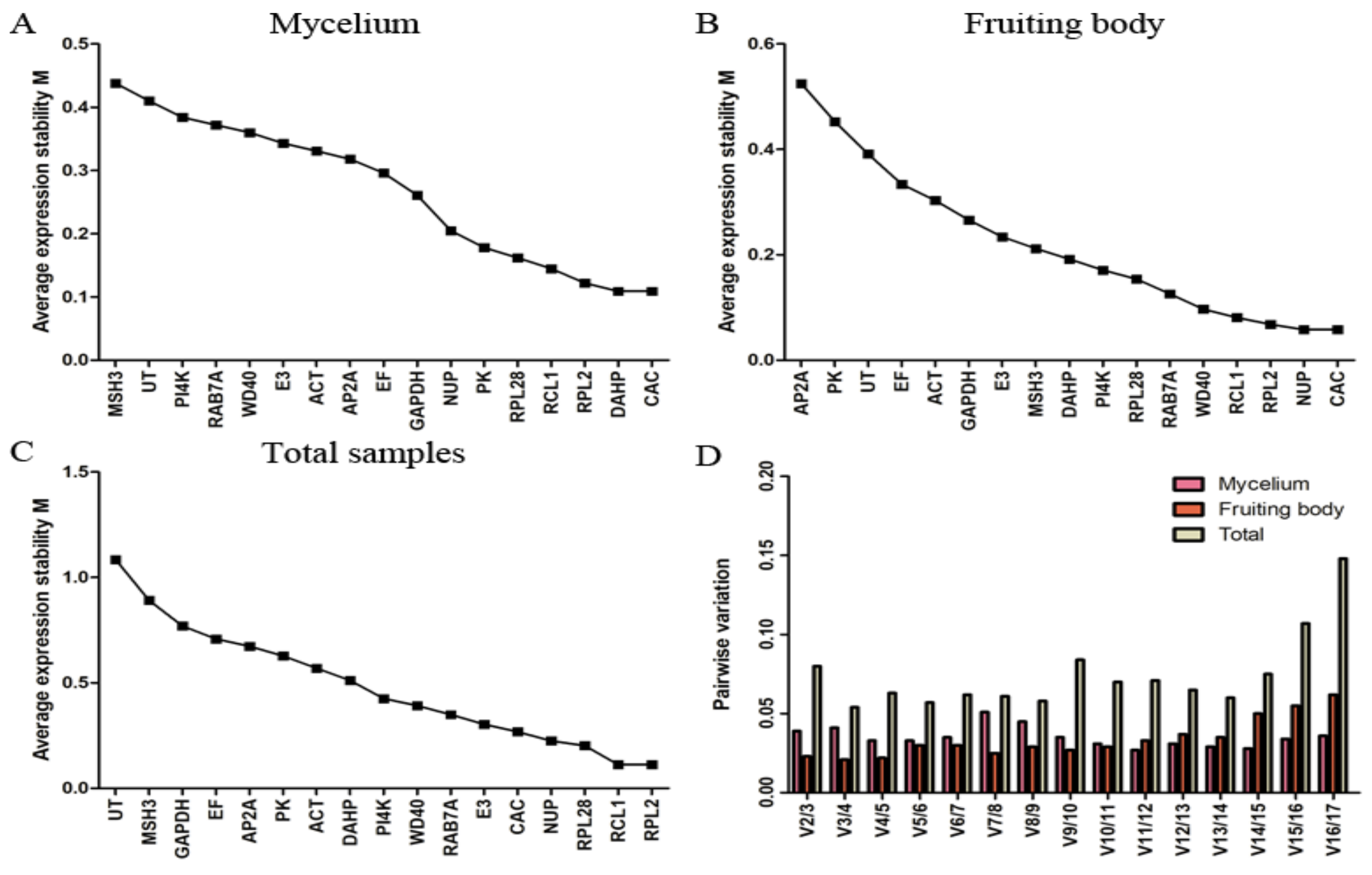
2.3. geNorm Analysis
2.4. NormFinder Analysis
2.5. BestKeeper Analysis
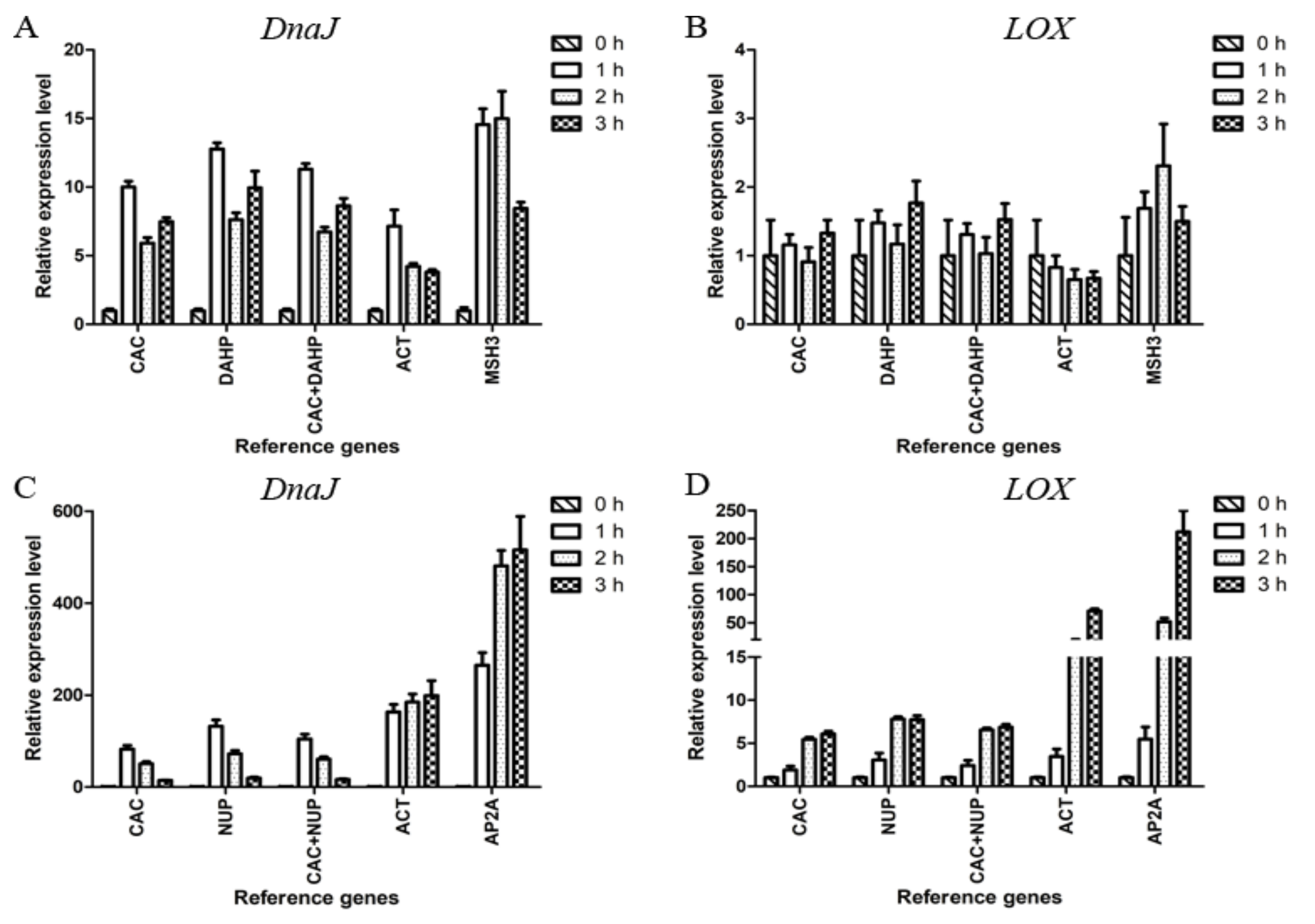
2.6. Validation of Reference Genes
2.7. Application of Selected Reference Genes for qRT-PCR Analysis of Heat-Regulated Genes
3. Discussion
4. Materials and Methods
4.1. Organisms, Growth Conditions, and Sample Harvest
4.2. RNA Isolation, Quality Control, cDNA Synthesis
4.3. Selection of the Reference Genes Using RNA-seq Data and Primer Design
4.4. Evaluation of Amplification Efficiency of Candidate Reference Genes
4.5. qRT-PCR Amplification
4.6. Data Processing and Statistical Analysis
4.7. Validation of Reference Genes
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Philippoussis, A.; Diamantopoulou, P.; Israilides, C. Productivity of agricultural residues used for the cultivation of the medicinal fungus Lentinula edodes. Int. Biodeter. Biodegr. 2007, 59, 216–219. [Google Scholar] [CrossRef]
- Finimundy, T.C.; Dillon, A.J.P.; Henriques, J.A.P.; Ely, M.R. A review on general nutritional compounds and pharmacological properties of the Lentinula edodes mushroom. Food. Nut. Sci. 2014, 5, 1095–1105. [Google Scholar]
- Hiraide, M.; Miyazaki, Y.; Shibata, Y. The smell and odorous components of dried shiitake mushroom, Lentinula edodes I: relationship between sensory evaluations and amounts of odorous components. J. Wood. Sci. 2004, 50, 358–364. [Google Scholar] [CrossRef]
- Jasinghe, V.J.; Perera, C.O. Ultraviolet irradiation: The generator of Vitamin D2 in edible mushrooms. Food. Chem. 2006, 95, 638–643. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, Y.; Huang, J.; Zeng, H.; Zheng, B. Effects of different drying methods on the product quality and volatile compounds of whole shiitake mushrooms. Food. Chem. 2016, 197, 714–722. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Hageleit, M. Validities of mRNA quantification using recombinant RNA and recombinant DNA external calibration curves in real-time RT-PCR. Biotechnol. Lett. 2001, 23, 275–282. [Google Scholar] [CrossRef]
- Engel, H.; Kueppers, C.; Koenig, M.; Loeffert, D. Successful gene expression analysis by multiplex, real-time, one-step RT-PCR, irrespective of the targets amplified. Biotechniques 2007, 43, 230–231. [Google Scholar] [CrossRef] [Green Version]
- Angela Pérez-Novo, C.; Claeys, C.; Speleman, F.; Van Cauwenberge, P.; Bachert, C.; Vandesompele, J. Impact of RNA quality on reference gene expression stability. Biotechniques 2005, 39, 52–56. [Google Scholar] [CrossRef]
- Bustin, S.A. Why the need for qPCR publication guidelines?—The case for MIQE. Methods 2010, 50, 217–226. [Google Scholar] [CrossRef]
- Edmunds, R.C.; McIntyre, J.K.; Luckenbach, J.A.; Baldwin, D.H.; Incardona, J.P. Toward enhanced MIQE compliance: Reference residual normalization of qPCR gene expression data. J. Biomol. Tech. 2014, 25, 54–60. [Google Scholar] [CrossRef]
- Huggett, J.; Dheda, K.; Bustin, S.; Zumla, A. Real-time RT-PCR normalisation; strategies and considerations. Genes. Immun. 2005, 6, 279–284. [Google Scholar] [CrossRef] [Green Version]
- VanGuilder, H.D.; Vrana, K.E.; Freeman, W.M. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques 2008, 44, 619–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obrero, Á.; Die, J.V.; Román, B.; Gómez, P.; Nadal, S.; González-Verdejo, C.I. Selection of reference genes for gene expression studies in zucchini (Cucurbita pepo) using qPCR. J. Agric. Food. Chem. 2011, 59, 5402–5411. [Google Scholar] [CrossRef] [PubMed]
- Dheda, K.; Huggett, J.F.; Chang, J.S.; Kim, L.U.; Bustin, S.A.; Johnson, M.A.; Rook, G.A.W.; Zumla, A. The implications of using an inappropriate reference gene for real-time reverse transcription PCR data normalization. Anal. biochem. 2005, 344, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Czechowski, T.; Stitt, M.; Altmann, T.; Udvardi, M.K.; Scheible, W.R. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant. Physiol. 2005, 139, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Nicot, N.; Hausman, J.F.; Hoffmann, L.; Evers, D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005, 56, 2907–2914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.J.; Zhu, Z.F.; Lu, R. Selection of control genes in transcription analysis of gene expression. Prog. Biochem. Biophys. 2007, 34, 546–550. [Google Scholar]
- Zhao, Y.; Luo, J.; Xu, S.; Wang, W.; Liu, T.; Han, C.; Chen, Y.; Kong, L. Selection of reference genes for gene expression normalization in Peucedanum praeruptorum dunn under abiotic stresses, hormone treatments and different tissues. PLoS ONE 2016, 11, e0152356. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, W.; Cai, Y.; Lan, A.-F.; Bian, Y. Validation of Internal Control Genes for Quantitative Real-Time PCR Gene Expression Analysis in Morchella. Molecules 2018, 23, 2331. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Z.; Zhu, Y.; Luo, H.; Qian, J.; Ji, A.; Hu, Y.; Sun, W.; Wang, B.; Song, J.; et al. Identification and evaluation of reference genes for qRT-PCR normalization in Ganoderma lucidum. Curr. Microbiol. 2014, 68, 120–126. [Google Scholar] [CrossRef]
- Liu, Y.N.; Lu, X.X.; Ren, A.; Shi, L.; Jiang, A.L.; Yu, H.S.; Zhao, M.W. Identification of reference genes and analysis of heat shock protein gene expression in lingzhi or reishi medicinal mushroom, Ganoderma lucidum, after exposure to heat stress. Int. J. Med. Mushrooms. 2017, 19, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, Y.; Zhao, L.; Liu, Y.; Zhao, M. Selection of reliable reference genes for qRT-PCR during methyl jasmonate, salicylic acid and hydrogen peroxide treatments in Ganoderma lucidum. World. J. Microb. Biot. 2018, 34, 92. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Van Peer, A.F.; Huang, Q.; Shao, Y.; Zhang, L.; Xie, B.; Jiang, Y.; Zhu, J.; Xie, B. Identification of novel and robust internal control genes from Volvariella volvacea that are suitable for qRT-PCR in filamentous fungi. Sci. Rep. 2016, 6, 29236. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Gao, Y.; Wáng, Y.; Wu, Y.; Wāng, Y.; Zhao, Y.; Chen, H.; Bao, D.; Xu, J.; Bian, X. Selection and Evaluation of Appropriate Reference Genes for qRT-PCR Normalization of Volvariella volvacea Gene Expression under Different Conditions. Biomed. Res. Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Li, J.; Qin, P.; He, M.; Yu, X.; Zhao, K.; Zhang, X.; Ma, M.; Chen, Q.; Chen, X.; et al. Identification and evaluation of reference genes for qRT-PCR studies in Lentinula edodes. PLoS ONE 2018, 13, e0190226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, H.; Chen, M.; Song, X.; Yu, C.; Zhao, Y.; Wu, Y. Reference gene selection for quantitative real-time PCR of mycelia from Lentinula edodes under high-temperature stress. Biomed. Res. Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Pombo, M.A.; Zheng, Y.; Fei, Z.; Martin, G.B.; Rosli, H.G. Use of RNA-seq data to identify and validate RT-qPCR reference genes for studying the tomato-Pseudomonas pathosystem. Sci. Rep. 2017, 7, 44905. [Google Scholar] [CrossRef]
- Wang, G.Z.; Ma, C.J.; Luo, Y.; Zhou, S.S.; Zhou, Y.; Ma, X.L.; Cai, Y.L.; Yu, J.J.; Bian, Y.B.; Gong, Y.H. Proteome and transcriptome reveal involvement of heat shock proteins and indoleacetic acid metabolism process in Lentinula edodes thermotolerance. Cell. Physiol. Biochem. 2018, 50, 1617–1637. [Google Scholar] [CrossRef]
- Chen, L.F.; Gong, Y.H.; Cai, Y.L.; Liu, W.; Zhou, Y.; Xiao, Y.; Xu, Z.Y.; Liu, Y.; Lei, X.Y.; Wang, G.Z.; et al. Genome sequence of the edible cultivated mushroom Lentinula edodes (Shiitake) reveals insights into lignocellulose degradation. PLoS ONE 2016, 11, e0160336. [Google Scholar] [CrossRef]
- Die, J.V.; Román, B.; Nadal, S.; González-Verdejo, C.I. Evaluation of candidate reference genes for expression studies in Pisum sativum under different experimental conditions. Planta 2010, 232, 145–153. [Google Scholar] [CrossRef]
- Castanera, R.; López-Varas, L.; Pisabarro, A.G.; Ramírez, L. Validation of reference genes for transcriptional analyses in Pleurotus ostreatus by using reverse transcription-quantitative PCR. Appl. Environ. Microbiol. 2015, 81, 4120–4129. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Mizukami-Murata, S.; Ando, A.; Murata, Y.; Takagi, H.; Shima, J. Changes in gene expression of commercial baker’s yeast during an air-drying process that simulates dried yeast production. J. Biosci. Bioeng. 2008, 106, 405–408. [Google Scholar] [CrossRef]
- Langer, S.E.; Oviedo, N.C.; Marina, M.; Burgos, J.L.; Martínez, G.A.; Civello, P.M.; Villarreal, N.M. Effects of heat treatment on enzyme activity and expression of key genes controlling cell wall remodeling in strawberry fruit. Plant Physiol. Bioch. 2018, 130, 334–344. [Google Scholar] [CrossRef]
- Løvdal, T.; Lillo, C. Reference gene selection for quantitative real-time PCR normalization in tomato subjected to nitrogen, cold, and light stress. Anal. Biochem. 2009, 387, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Petriccione, M.; Mastrobuoni, F.; Zampella, L.; Scortichini, M. Reference gene selection for normalization of RT-qPCR gene expression data from Actinidia deliciosa leaves infected with Pseudomonas syringae pv. actinidiae. Sci. Rep. 2015, 5, 16961. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Zhao, Z.; Qian, C.; Sui, Y.; Malik, A.A.; Chen, J. Selection of appropriate reference genes for gene expression studies by quantitative real-time polymerase chain reaction in cucumber. Anal. Biochem. 2010, 399, 257–261. [Google Scholar] [CrossRef]
- Jain, N.; Vergish, S.; Khurana, J.P. Validation of house-keeping genes for normalization of gene expression data during diurnal/circadian studies in rice by RT-qPCR. Sci. Rep. 2018, 8, 3203. [Google Scholar] [CrossRef]
- Thorrez, L.; Deun, K.V.; Tranchevent, L.; Lommel, L.V.; Engelen, K.; Marchal, K.; Moreau, Y.; Mechelen, I.V.; Schuit, F. Using ribosomal protein genes as reference: A tale of caution. PLoS ONE 2008, 3, e1854. [Google Scholar]
- Mitra, T.; Bilic, I.; Hess, M.; Liebhart, D. The 60s ribosomal protein L13 is the most preferable reference gene to investigate gene expression in selected organs from turkeys and chickens, in context of different infection models. Vet. Res. 2016, 47, 105. [Google Scholar] [CrossRef]
- Cruz, F.; Kalaoun, S.; Nobile, P.; Colombo, C.; Almeida, J.; Barros, L.M.G.; Romano, E.; Grossi-de-Sá, M.F.; Vaslin, M.; Alves-Ferreira, M. Evaluation of coffee reference genes for relative expression studies by quantitative real-time RT-PCR. Mol. Breed. 2009, 23, 607–616. [Google Scholar] [CrossRef]
- Chen, L.; Zhong, H.Y.; Kuang, J.F.; Li, J.G.; Lu, W.J.; Chen, J.Y. Validation of reference genes for RT-qPCR studies of gene expression in banana fruit under different experimental conditions. Planta 2011, 234, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Expósitorodríguez, M.; Borges, A.A.; Borgespérez, A.; Pérez, J.A. Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biol. 2008, 8, 1. [Google Scholar]
- Demidenko, N.V.; Logacheva, M.D.; Penin, A.A. Selection and validation of reference genes for quantitative real-time PCR in Buckwheat (Fagopyrum esculentum) based on transcriptome sequence data. PLoS ONE 2011, 6, e19434. [Google Scholar] [CrossRef] [PubMed]
- Chandna, R.; Augustine, R.; Bisht, N.C. Evaluation of candidate reference genes for gene expression normalization in Brassica juncea using real time quantitative RT-PCR. PLoS ONE 2012, 7, e36918. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, N.; Heimhalt, M.; Liuni, P.; To, F.; Wilson, D.J.; Junop, M.S.; Berti, P.J. Potent inhibition of 3-deoxy-D-arabinoheptulosonate-7-phosphate (DAHP) synthase by DAHP oxime, a phosphate group mimic. Biochemistry 2016, 55, 6617–6629. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, M.E.; Caplan, A.J. Structure, function and evolution of DnaJ: Conservation and adaptation of chaperone function. Cell. Stress. Chaperon. 1998, 3, 28–36. [Google Scholar] [CrossRef]
- Combet, E.; Henderson, J.; Eastwood, D.C.; Burton, K.S. Eight-carbon volatiles in mushrooms and fungi: Properties, analysis, and biosynthesis. Mycoscience 2006, 47, 317–326. [Google Scholar] [CrossRef]

| Gene | Symbol | Primer Sequence (5′–3′) | Length (bp) | E (%) | R2 | Min Ct | Max Ct |
|---|---|---|---|---|---|---|---|
| WD40 protein | WD40 | AGTCCGACAAATCCATCAG | 138 | 97.8 | 0.990 | 23.81 | 33.84 |
| CAATAACCCTCAGACACCC | |||||||
| Phosphatidylinositol 4-kinase | PI4K | TATCCGAACGGTTACTGG | 198 | 109.5 | 0.990 | 23.81 | 32.98 |
| AATTGAGGACGACGCTTT | |||||||
| Urea transporter | UT | AGAGGGTAATCAGAATGGAA | 153 | 104.9 | 0.984 | 26.64 | 35.96 |
| TTTGTAGTCGAAGTAGGGTG | |||||||
| MutS Homolog 3 | MSH3 | GAACCGAGGAGTAAGATTGT | 244 | 91.2 | 0.992 | 24.95 | 35.33 |
| TGTTCGTAGCCGAGTGG | |||||||
| Ribosomal protein L28 | RPL28 | CAAATGCGTGGATAGCG | 223 | 95.7 | 0.998 | 22.22 | 32.44 |
| CCAAGCAAGTTCCGATGT | |||||||
| Clathrin adaptor complexes medium subunit | CAC | CACCTTCCCTTTCCACTG | 135 | 93.6 | 0.994 | 22.21 | 32.70 |
| TTTGCTCCTTTACCACCA | |||||||
| Nucleoporin | NUP | AACAGTCTAACTTCGGTGCG | 193 | 99.4 | 0.998 | 23.00 | 32.97 |
| CCTGTCGTTGCCTCCTCA | |||||||
| Ubiquitin–protein ligase E3 | E3 | TAAACGGCGGACAAATGC | 263 | 100.8 | 0.998 | 21.22 | 31.05 |
| ACGACCTACAGGCGAAAT | |||||||
| DAHP synthetase | DAHP | CCTTTGTCTGGACCTTCTG | 113 | 97.0 | 0.999 | 22.12 | 32.25 |
| CGAGCCTTTACTCCTTCAC | |||||||
| Protein kinase | PK | TCGGATTCTTTACCTACTGG | 128 | 104.3 | 0.997 | 22.61 | 32.18 |
| CATGGATGGCACTTCACA | |||||||
| Ras-related protein Rab-7a | RAB7A | ACTCGTTCGCTGTATGCC | 171 | 100.0 | 0.997 | 23.03 | 32.86 |
| GCTGTCCAGACTCCCTATGA | |||||||
| 18S rRNA biogenesis protein RCL1 | RCL1 | TGCCGTTCGTGTAAATC | 176 | 97.1 | 0.987 | 23.74 | 33.78 |
| ACAAAGCAGAGGTGGTAGA | |||||||
| Ribosomal protein L2 | RPL2 | AACGAGGACAAGGAAGCC | 166 | 102.2 | 0.996 | 23.34 | 33.10 |
| CCAGGCAATGTTCTCAGTC | |||||||
| Actin | ACT | CCCATCTTTCCGTCCACT | 242 | 98.8 | 0.998 | 18.22 | 28.25 |
| TTCTGACCCATCCCAACC | |||||||
| Adaptor-related protein complex 2 subunit alpha | AP2A | CGATGAGGATTTGGGAGT | 178 | 101.5 | 0.998 | 23.44 | 33.25 |
| CAGCCAGGGTGAAGGTAC | |||||||
| Elongation factor | EF | TTCCCAGGCTGATTGTG | 176 | 97.8 | 1.000 | 16.46 | 26.54 |
| ATCGGTCCTCGCTCCAT | |||||||
| Glyceraldehyde 3-phosphate ehydrogenase | GAPDH | CATCCCTTCTTCAACTGG | 240 | 96.8 | 0.998 | 17.70 | 27.89 |
| AAATCGGTGGAGACAACA |
| Ranking | Mycelium | Fruiting Body | Total | |||
|---|---|---|---|---|---|---|
| Gene | Stability Value | Gene | Stability Value | Gene | Stability Value | |
| 1 | NUP | 0.066 | RCL1 | 0.059 | RCL1 | 0.039 |
| 2 | CAC | 0.138 | MSH3 | 0.096 | RPL2 | 0.058 |
| 3 | RPL2 | 0.142 | DAHP | 0.100 | NUP | 0.077 |
| 4 | RPL28 | 0.147 | E3 | 0.102 | E3 | 0.082 |
| 5 | RCL1 | 0.158 | NUP | 0.123 | RPL28 | 0.123 |
| 6 | DAHP | 0.159 | RPL2 | 0.136 | CAC | 0.218 |
| 7 | E3 | 0.164 | WD40 | 0.140 | RAB7A | 0.237 |
| 8 | WD40 | 0.179 | CAC | 0.149 | WD40 | 0.332 |
| 9 | PK | 0.202 | GAPDH | 0.153 | PI4K | 0.415 |
| 10 | RAB7A | 0.205 | RAB7A | 0.176 | ACT | 0.486 |
| 11 | AP2A | 0.239 | ACT | 0.251 | DAHP | 0.512 |
| 12 | PI4K | 0.248 | RPL28 | 0.256 | PK | 0.587 |
| 13 | ACT | 0.278 | EF | 0.280 | AP2A | 0.611 |
| 14 | GAPDH | 0.300 | PI4K | 0.281 | EF | 0.686 |
| 15 | EF | 0.321 | PK | 0.555 | GAPDH | 0.946 |
| 16 | UT | 0.358 | UT | 0.591 | MSH3 | 1.068 |
| 17 | MSH3 | 0.415 | AP2A | 0.726 | UT | 1.742 |
| Two best genes | NUP/CAC | RCL1/MSH3 | RCL1/RPL2 | |||
| Ranking | Mycelium | Fruiting Body | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | SD [±Cq] | SD [±x-fold] | r | Gene | SD [±Cq] | SD [±x-fold] | r | Gene | SD [±Cq] | SD [±x-fold] | r | |
| 1 | DAHP | 0.52 | 1.43 | 0.993 | CAC | 0.28 | 1.21 | 1.000 | DAHP | 0.58 | 1.49 | 0.953 |
| 2 | CAC | 0.58 | 1.50 | 0.979 | WD40 | 0.28 | 1.22 | 0.966 | CAC | 0.91 | 1.87 | 0.995 |
| 3 | RPL2 | 0.61 | 1.52 | 0.990 | RPL2 | 0.30 | 1.23 | 0.992 | RPL2 | 1.07 | 2.10 | 0.998 |
| 4 | RPL28 | 0.67 | 1.59 | 0.967 | NUP | 0.33 | 1.26 | 0.995 | RCL1 | 1.14 | 2.21 | 0.997 |
| 5 | RCL1 | 0.68 | 1.60 | 0.987 | RCL1 | 0.34 | 1.27 | 0.996 | E3 | 1.22 | 2.34 | 0.984 |
| 6 | NUP | 0.74 | 1.67 | 0.993 | DAHP | 0.35 | 1.28 | 0.929 | RPL28 | 1.24 | 2.37 | 0.99 |
| 7 | E3 | 0.75 | 1.68 | 0.938 | RAB7A | 0.36 | 1.28 | 0.934 | NUP | 1.28 | 2.43 | 0.999 |
| 8 | WD40 | 0.89 | 1.85 | 0.992 | RPL28 | 0.39 | 1.31 | 0.891 | RAB7A | 1.48 | 2.78 | 0.995 |
| 9 | RAB7A | 0.90 | 1.87 | 0.987 | MSH3 | 0.49 | 1.41 | 0.955 | WD40 | 1.62 | 3.08 | 0.997 |
| 10 | MSH3 | 0.98 | 1.97 | 0.966 | E3 | 0.56 | 1.47 | 0.955 | MSH3 | 2.52 | 5.73 | 0.989 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Wang, G.; Huang, Z.; Lei, X.; Bian, Y.; Liu, Y.; Huang, W. Selection of Reference Genes for qRT-PCR Analysis in Lentinula edodes after Hot-Air Drying. Molecules 2019, 24, 136. https://doi.org/10.3390/molecules24010136
Gao S, Wang G, Huang Z, Lei X, Bian Y, Liu Y, Huang W. Selection of Reference Genes for qRT-PCR Analysis in Lentinula edodes after Hot-Air Drying. Molecules. 2019; 24(1):136. https://doi.org/10.3390/molecules24010136
Chicago/Turabian StyleGao, Shuangshuang, Gangzheng Wang, Zhicheng Huang, Xiaoyu Lei, Yinbing Bian, Ying Liu, and Wen Huang. 2019. "Selection of Reference Genes for qRT-PCR Analysis in Lentinula edodes after Hot-Air Drying" Molecules 24, no. 1: 136. https://doi.org/10.3390/molecules24010136





