The Anti-Candida albicans Agent 4-AN Inhibits Multiple Protein Kinases
Abstract
:1. Introduction
2. Results and Discussion
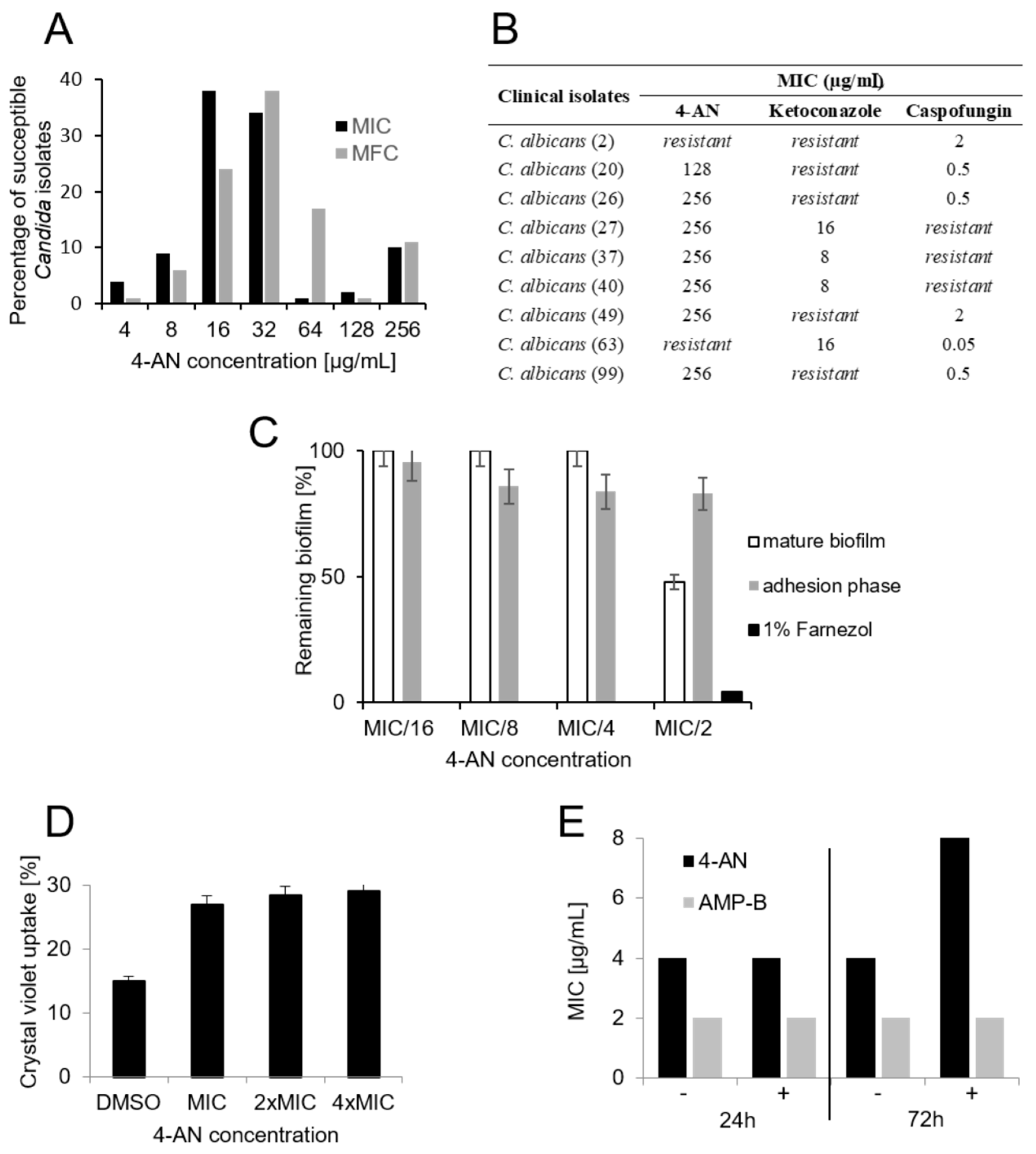
2.1. Activity of 4-AN Against Clinical Isolates Of Candida and Its Influence on Biofilm and Membrane Permeability
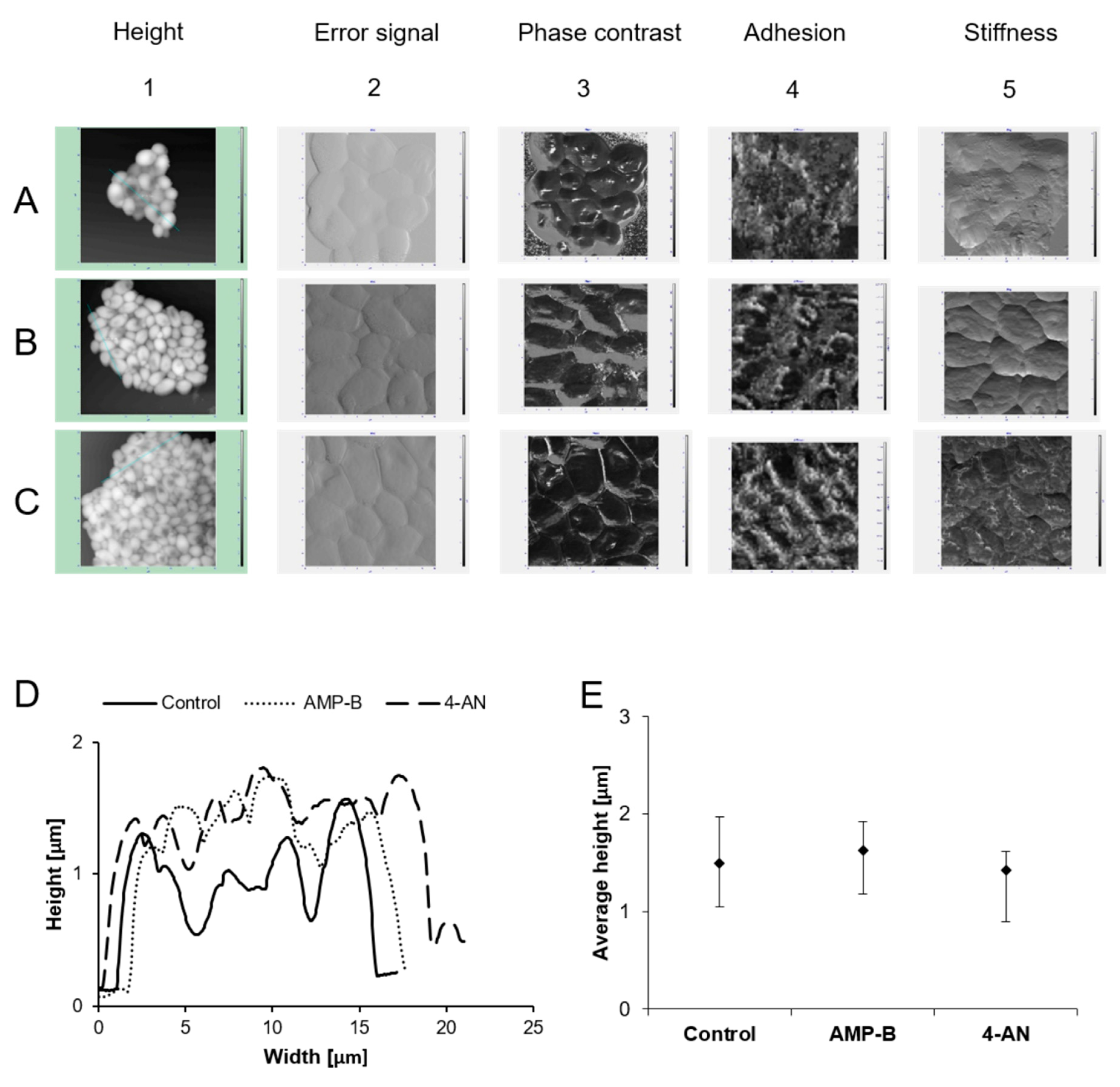
2.2. Atomic Force Microscopy Studies
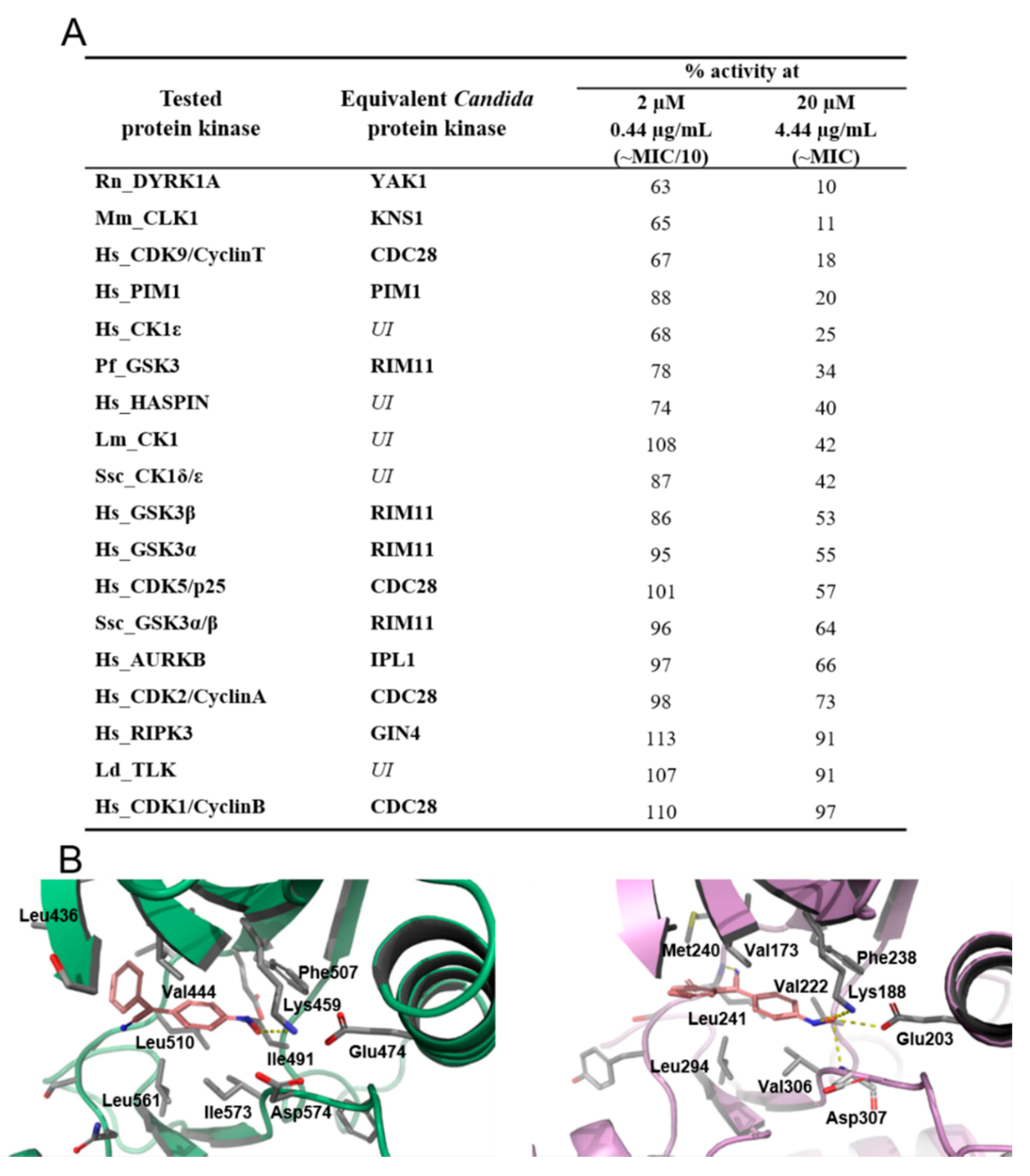
2.3. 4-AN-Mediated Inhibition of Protein Kinases
3. Materials and Methods
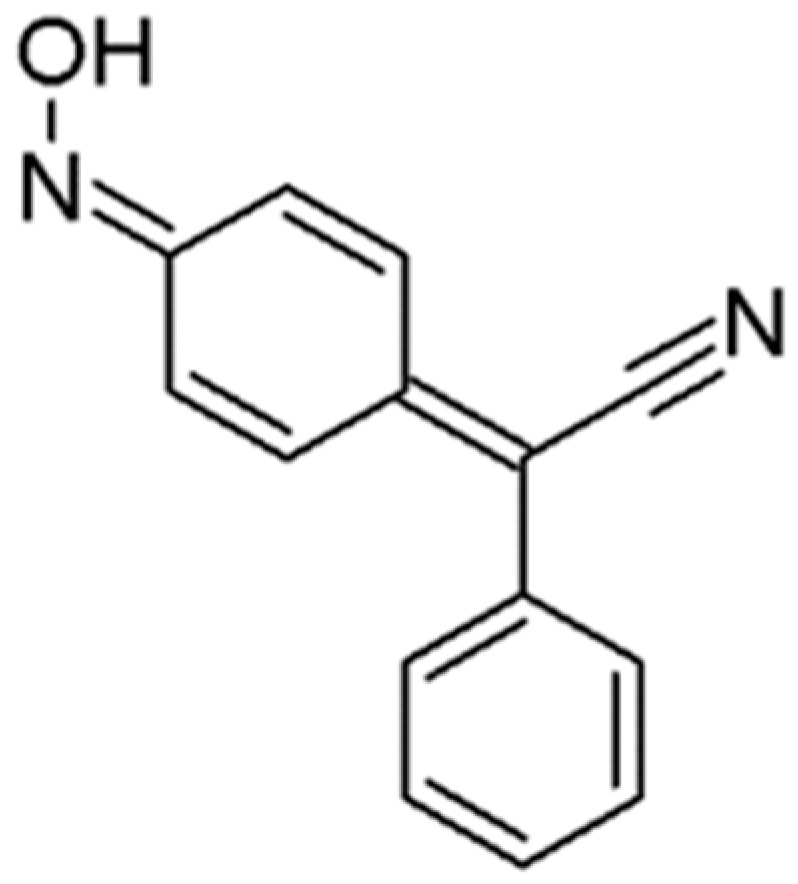
3.1. 4-(Hydroxyimino)cyclohexa-2,5-dien-1-ylidene](phenyl)ethanenitrile (4-AN)
3.2. Microbial Strains
3.3. Determination of Minimal Inhibitory Concentrations
3.4. In Vitro Biofilm Formation Assay
3.5. The Effect of 4-AN on Membrane Permeability
3.6. Sorbitol Protection Assay
3.7. Atomic Force Microscopy (AFM) Analysis of C. albicans Cells Treated with 4-AN
3.8. Protein Kinase Profiling Assay
3.9. Protein Kinase Yak1 3D Model Construction
3.10. Molecular Docking Studies
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Pfaller, M.A.; Andes, D.R.; Diekema, D.J.; Horn, D.L.; Reboli, A.C.; Rotstein, C.; Franks, B.; Azie, N.E. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: Data from the Prospective Antifungal Therapy (PATH) registry 2004–2008. PLoS ONE 2014, 9, e101510. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.H.; Sobel, J.D.; Powderly, W.G. Cansida species. In Antimicrobial therapy and vaccines; Williams & Wilkins Inc.: Baltimore, MD, USA, 1999; pp. 1054–1076. [Google Scholar]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Santos, G.C.; Vasconcelos, C.C.; Lopes, A.J.O.; de Sousa Cartágenes, M.D.S.; Filho, A.K.D.B.; do Nascimento, F.R.F.; Ramos, R.M.; Pires, E.R.R.B.; de Andrade, M.S.; Rocha, F.M.G.; et al. Candida Infections and Therapeutic Strategies: Mechanisms of Action for Traditional and Alternative Agents. Front. Microbiol. 2018, 9, 1351. [Google Scholar] [CrossRef] [PubMed]
- Sritrairat, N.; Nukul, N.; Inthasame, P.; Sansuk, A.; Prasirt, J.; Leewatthanakorn, T.; Piamsawad, U.; Dejrudee, A.; Panichayupakaranant, P.; Pangsomboon, K.; et al. Antifungal activity of lawsone methyl ether in comparison with chlorhexidine. J. Oral Pathol. Med. 2011, 40, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Janeczko, M.; Kubiński, K.; Martyna, A.; Muzyczka, A.; Boguszewska-Czubara, A.; Czernik, S.; Tokarska-Rodak, M.; Chwedczuk, M.; Demchuk, O.M.; Golczyk, H.; et al. 1,4-Naphthoquinone derivatives potently suppress Candida albicans growth, inhibit formation of hyphae and show no toxicity toward zebrafish embryos. J. Med. Microbiol. 2018, 67, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, M.; Thanusu, J.; Kanagarajan, V. A facile solid-state synthesis and in vitro antimicrobial activities of some 2,6-diarylpiperidin/tetrahydrothiopyran and tetrahydropyran-4-one oximes. J. Enzyme Inhib. Med. Chem. 2009, 24, 669–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozłowska, J.; Potaniec, B.; Żarowska, B.; Anioł, M. Synthesis and biological activity of novel o-alkyl derivatives of naringenin and their oximes. Molecules 2017, 22, 1485. [Google Scholar] [CrossRef]
- Masłyk, M.; Janeczko, M.; Demchuk, O.M.; Boguszewska-Czubara, A.; Golczyk, H.; Sierosławska, A.; Rymuszka, A.; Martyna, A.; Kubiński, K. A representative of arylcyanomethylenequinone oximes effectively inhibits growth and formation of hyphae in vitro. Saudi Pharm. J. 2018, 26, 244–252. [Google Scholar] [CrossRef]
- Cohen, P. The origins of protein phosphorylation. Nat. Cell Biol. 2002, 4, E127–E130. [Google Scholar] [CrossRef]
- Caballero-Lima, D.; Sudbery, P.E. In Candida albicans, phosphorylation of Exo84 by Cdk1-Hgc1 is necessary for efficient hyphal extension. Mol. Biol. Cell 2014, 25, 1097–1110. [Google Scholar] [CrossRef]
- Huang, Z.X.; Zhao, P.; Zeng, G.S.; Wang, Y.M.; Sudbery, I.; Wang, Y. Phosphoregulation of Nap1 plays a role in septin ring dynamics and morphogenesis in Candida albicans. MBio 2014, 5, e00915-13. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Gamito, A.M.; Tangarife-Castaño, V.; Zapata, B.; Miguel del Corral, J.M.; Mesa-Arango, A.C.; Betancur-Galvis, L.; San Feliciano, A. Synthesis and antifungal activity of terpenyl-1,4-naphthoquinone and 1,4-anthracenedione derivatives. Eur. J. Med. Chem. 2013, 67, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, J.P.; Baker, C.; Kawasaki, Y.; Subedi, Y.P.; Vincent de Paul, N.N.; Takemoto, J.Y.; Chang, C.T. Synthesis and bioactivity investigation of quinone-based dimeric cationic triazolium amphiphiles selective against resistant fungal and bacterial pathogens. Eur. J. Med. Chem. 2017, 126, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Desnos-Ollivier, M.; Robert, V.; Raoux-Barbot, D.; Groenewald, M.; Dromer, F. Antifungal susceptibility profiles of 1698 yeast reference strains revealing potential emerging human pathogens. PLoS ONE 2012, 7, e32278. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef]
- Al-Fattani, M.A.; Douglas, L.J. Penetration of Candida biofilms by antifungal agents. Antimicrob. Agents Chemother. 2004, 48, 3291–3297. [Google Scholar] [CrossRef]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of Zeta potential and membrane permeability in bacteria: A study with cationic agents. SpringerPlus 2015, 4, 672. [Google Scholar] [CrossRef]
- Frost, D.J.; Brandt, K.D.; Cugier, D.; Goldman, R. A whole-cell Candida albicans assay for the detection of inhibitors towards fungal cell wall synthesis and assembly. J. Antibiot. 1995, 48, 306–310. [Google Scholar] [CrossRef]
- Dufrêne, Y.F. Atomic force microscopy in microbiology: New structural and functional insights into the microbial cell surface. MBio 2014, 5, e01363-14. [Google Scholar]
- Formosa, C.; Schiavone, M.; Boisrame, A.; Richard, M.L.; Duval, R.E.; Dague, E. Multiparametric imaging of adhesive nanodomains at the surface of Candida albicans by atomic force microscopy. Nanomedicine 2015, 11, 57–65. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. In situ SEM, TEM and AFM studies of the antimicrobial activity of lemon grass oil in liquid and vapour phase against Candida albicans. Micron 2010, 41, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Quilès, F.; Accoceberry, I.; Couzigou, C.; Francius, G.; Noël, T.; El-Kirat-Chatel, S. AFM combined to ATR-FTIR reveals Candida cell wall changes under caspofungin treatment. Nanoscale 2017, 9, 13731–13738. [Google Scholar] [CrossRef] [PubMed]
- Janeczko, M.; Masłyk, M.; Kubiński, K.; Golczyk, H. Emodin, a natural inhibitor of protein kinase CK2, suppresses growth, hyphal development, and biofilm formation of Candida albicans. Yeast 2017, 34, 253–265. [Google Scholar] [CrossRef]
- Ferguson, A.D.; Sheth, P.R.; Basso, A.D.; Paliwal, S.; Gray, K.; Fischmann, T.O.; Le, H.V. Structural basis of CX-4945 binding to human protein kinase CK2. FEBS Lett. 2011, 585, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.E.; Lepak, A.J.; Marchillo, K.; Andes, D.R. Time course global gene expression analysis of an in vivo Candida biofilm. J. Infect. Dis. 2009, 200, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Fox, E.P.; Nett, J.E.; Sorrells, T.R.; Mitrovich, Q.M.; Hernday, A.D.; Tuch, B.B.; Andes, D.R.; Johnson, A.D. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 2012, 148, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Goyard, S.; Knechtle, P.; Chauvel, M.; Mallet, A.; Prévost, M.C.; Proux, C.; Coppée, J.Y.; Schwarz, P.; Schwartz, P.; Dromer, F.; et al. The Yak1 kinase is involved in the initiation and maintenance of hyphal growth in Candida albicans. Mol. Biol. Cell 2008, 19, 2251–2266. [Google Scholar] [CrossRef]
- Davis, R.B.; Pizzini, L.C.; Bara, E.J. The Condensation of Aromatic Nitro Compounds with Arylacetonitriles. 111. Some ortho- and meta-Substituted Nitrobenzenes. J. Org. Chem. 1961, 26, 4270–4274. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed.; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Moreira, C.S.; Silva, A.C.; Novais, J.S.; Sá Figueiredo, A.M.; Ferreira, V.F.; da Rocha, D.R.; Castro, H.C. Searching for a potential antibacterial lead structure against bacterial biofilms among new naphthoquinone compounds. J. Appl. Microbiol. 2017, 122, 651–662. [Google Scholar] [CrossRef]
- Ramage, G.; Saville, S.P.; Wickes, B.L.; López-Ribot, J.L. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 2002, 68, 5459–5463. [Google Scholar] [CrossRef]
- Vaara, M.; Vaara, T. Outer membrane permeability barrier disruption by polymyxin in polymyxin-susceptible and -resistant Salmonella typhimurium. Antimicrob. Agents. Chemother. 1981, 19, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, Y. Antifungal Activity of Salvia miltiorrhiza Against Candida albicans Is Associated with the Alteration of Membrane Permeability and (1,3)-β-d-Glucan Synthase Activity. J. Microbiol. Biotechnol. 2016, 26, 610–617. [Google Scholar] [CrossRef]
- Remmert, M.; Biegert, A.; Hauser, A.; Soding, J. HHblits: Lightning-fast iterative protein sequence searching by HMM-HMM alignment. Nat. Methods 2012, 9, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Guex, N.; Peitsch, M.C.; Schwede, T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: A historical perspective. Electrophoresis 2009, 30 (Suppl. S1), S162–S173. [Google Scholar] [CrossRef] [PubMed]
- Benkert, P.; Biasini, M.; Schwede, T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 2011, 27, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, M.; Kiefer, F.; Biasini, M.; Bordoli, L.; Schwede, T. Modeling protein quaternary structure of homo- and hetero-oligomers beyond binary interactions by homology. Sci. Rep. 2017, 7, 10480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krieger, E.; Joo, K.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins 2009, 77 (Suppl. S9), 114–122. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masłyk, M.; Janeczko, M.; Martyna, A.; Czernik, S.; Tokarska-Rodak, M.; Chwedczuk, M.; Foll-Josselin, B.; Ruchaud, S.; Bach, S.; Demchuk, O.M.; et al. The Anti-Candida albicans Agent 4-AN Inhibits Multiple Protein Kinases. Molecules 2019, 24, 153. https://doi.org/10.3390/molecules24010153
Masłyk M, Janeczko M, Martyna A, Czernik S, Tokarska-Rodak M, Chwedczuk M, Foll-Josselin B, Ruchaud S, Bach S, Demchuk OM, et al. The Anti-Candida albicans Agent 4-AN Inhibits Multiple Protein Kinases. Molecules. 2019; 24(1):153. https://doi.org/10.3390/molecules24010153
Chicago/Turabian StyleMasłyk, Maciej, Monika Janeczko, Aleksandra Martyna, Sławomir Czernik, Małgorzata Tokarska-Rodak, Marta Chwedczuk, Béatrice Foll-Josselin, Sandrine Ruchaud, Stéphane Bach, Oleg M. Demchuk, and et al. 2019. "The Anti-Candida albicans Agent 4-AN Inhibits Multiple Protein Kinases" Molecules 24, no. 1: 153. https://doi.org/10.3390/molecules24010153





