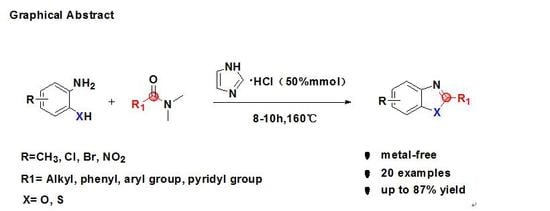
Eco-Friendly Syntheses of 2-Substituted Benzoxazoles and 2-Substituted Benzothiazoles from 2-Aminophenols, 2-Aminothiophenols and DMF Derivatives in the Presence of Imidazolium Chloride
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Experimental
4.1. General Information
4.2. General Procedure for the Synthesis of Benzoxazole Derivatives 2a–2d
4.3. General Procedure for the Synthesis of Benzoxazole Derivatives 2a, 2c, 2g and 2h and Benzothiazole Derivatives 4a–4d
4.4. General Procedure for the Synthesis of Benzoxazole Derivatives 2b, 2d, 2e, 2f, 2i and Benzothiazole Derivatives 4e–4k
4.5. Product Characterization Data
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Viirre, R.D.; Ghotas, E.; Batey, R.A. Copper-catalyzed domino annulation approaches to the synthesis of benzoxazoles under microwave-accelerated and conventional thermal conditions. J. Org. Chem. 2008, 39, 3452–3459. [Google Scholar] [CrossRef] [PubMed]
- Kaplancikli, Z.A.; Gülhan, T.Z.; Revial, G.; Guven, K. Synthesis and study of antibacterial and antifungal activities of novel 2-[[(benzoxazole/benzimidazole-2-yl)sulfanyl] acetylamino]thiazoles. Arch. Pharm. Res. 2004, 27, 1081. [Google Scholar] [CrossRef] [PubMed]
- Carayon, C.; Fery-Forgues, S. 2-Phenylbenzoxazole derivatives: A family of robust emitters of solid-state fluorescence. Photochem. Photobiol. Sci. 2017, 16, 1020–1035. [Google Scholar] [CrossRef] [PubMed]
- Benelhadj, K.; Muzuzu, W.; Massue, J.; Retailleau, P.; Charaf-Eddin, A.; Laurent, A.D.; Jacquemin, D.; Ulrich, G.; Ziessel, R. White emitters by tuning the excited-state intramolecular proton-transfer fluorescence emission in 2-(2′-hydroxybenzofuran)benzoxazole dyes. Chemistry 2014, 20, 12843–12857. [Google Scholar] [CrossRef] [PubMed]
- Aiello, S.; Wells, G.; Stone, E.L.; Kadri, H.; Bazzi, R.; Bell, D.R.; Stevens, M.F.; Matthews, C.S.; Bradshaw, T.D.; Westwell, A.D. Synthesis and biological properties of benzothiazole, benzoxazole, and chromen-4-one analogues of the potent antitumor agent 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (PMX 610, NSC 721648). J. Med. Chem. 2008, 51, 5135–5139. [Google Scholar] [CrossRef] [PubMed]
- Mylari, B.L.; Beyer, T.A.; Scott, P.J.; Aldinger, C.E.; Dee, M.F.; Siegel, T.W.; Zembrowski, W.J. Potent, orally active aldose reductase inhibitors related to zopolrestat: Surrogates for benzothiazole side chain. J. Med. Chem. 1992, 35, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Ian, H.; Sharon, A.J.B.; Rao, V.; Andrew, D.W.; Malcolm, F.G.S. Antitumor benzothiazoles. 16. Synthesis and pharmaceutical properties of antitumor 2-(4-aminophenyl)benzothiazole amino acid prodrugs. J. Med. Chem. 2002, 45, 744–747. [Google Scholar]
- Zhang, Y.; Bao, J.; Deng, X.X.; He, W.; Fan, J.J.; Jiang, F.Q.; Fu, L. Synthesis, biological evaluation and molecular docking of 2-phenyl-benzo[d]oxazole-7-carboxamide derivatives as potential Staphylococcus aureus Sortase A inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 4081–4085. [Google Scholar] [CrossRef]
- Kaur, A.; Pathak, D.P.; Sharma, V.; Wakode, S. Synthesis, biological evaluation and docking study of a new series of di-substituted benzoxazole derivatives as selective COX-2 inhibitors and anti-inflammatory agents. Bioorg. Med. Chem. 2018, 26, 891–902. [Google Scholar] [CrossRef]
- Satyendra, R.V.; Vishnumurthy, K.A.; Vagdevi, H.M.; Dhananjaya, B.L.; Shruthi, A. Synthesis, in vitro anthelmintic, and molecular docking studies of novel 5-nitro benzoxazole derivatives. Med. Chem. Res. 2015, 24, 1342–1350. [Google Scholar] [CrossRef]
- Suvendu, S.; Sudipto, D.; Papu, B. Photocatalysis by 3,6-Disubstituted-s-Tetrazine: Visible-Light Driven Metal-Free Green Synthesis of 2-Substituted Benzimidazole and Benzothiazole. J. Org. Chem. 2014, 45, 11184–11193. [Google Scholar]
- Wen, X.; Bakali, J.E.; Deprez-Poulain, R.; Deprez, B. Efficient propylphosphonic anhydride (®T3P) mediated synthesis of benzothiazoles, benzoxazoles and benzimidazoles. Tetrahedron Lett. 2012, 53, 2440–2443. [Google Scholar] [CrossRef]
- Kozlov, N.S.; Kiselev, B.I. Catalytic synthesis of 2-alkylbenzoxazoles. Chem. Heterocycl. Compd. 1967, 2, 248–249. [Google Scholar] [CrossRef]
- Pottorf, R.S.; Chadha, N.K.; Katkevics, M.; Ozola, V.; Suna, E.; Ghane, H.; Regberg, T.; Player, M.R. Parallel synthesis of benzoxazoles via microwave-assisted dielectric heating. Tetrahedron Lett. 2003, 44, 175–178. [Google Scholar] [CrossRef]
- Yadong, S.; Huanfeng, J.; Wanqing, W.; Wei, Z.; Xia, W. Copper-catalyzed synthesis of substituted benzothiazoles via condensation of 2-aminobenzenethiols with nitriles. Org. Lett. 2013, 15, 1598–1601. [Google Scholar]
- Matsushita, H.; Lee, S.-H.; Joung, M.; Clapham, B.; Janda, K.D. Smart cleavage reactions: The synthesis of benzimidazoles and benzothiazoles from polymer-bound esters. Tetrahedron Lett. 2004, 45, 313–316. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Khatun, N.; Gogoi, A.; Deb, A.; Patel, B.K. Cu(ii) catalysed chemoselective oxidative transformation of thiourea to thioamidoguanidine/2-aminobenzothiazole. RSC Adv. 2013, 3, 438–446. [Google Scholar] [CrossRef]
- Liu, K.; Jia, F.; Xi, H.; Li, Y.; Zheng, X.; Guo, Q.; Shen, B.; Li, Z. Direct benzothiophene formation via oxygen-triggered intermolecular cyclization of thiophenols and alkynes assisted by manganese/PhCOOH. Org. Lett. 2013, 15, 2026–2029. [Google Scholar] [CrossRef]
- Itoh, T.; Mase, T. A novel practical synthesis of benzothiazoles via Pd-catalyzed thiol cross-coupling. Org. Lett. 2007, 9, 3687. [Google Scholar] [CrossRef]
- Evindar, G.; Batey, R.A. Parallel synthesis of a library of benzoxazoles and benzothiazoles using ligand-accelerated copper-catalyzed cyclizations of ortho-halobenzanilides. J. Org. Chem. 2010, 37, 1802–1808. [Google Scholar] [CrossRef]
- Sung, G.H.; Lee, I.-H.; Kim, B.R.; Shin, D.-S.; Kim, J.-J.; Lee, S.-G.; Yoon, Y.-J. Eco-friendly atom-economical synthesis of 2-substituted-benzo[d]thiazoles and 2-substituted-benzo[d]oxazoles using 2-acylpyridazin-3(2H)-ones. Tetrahedron 2013, 69, 3530–3535. [Google Scholar] [CrossRef]
- Gao, X.; Yu, B.; Zhao, Y.; Hao, L.; Liu, Z. Hydrosilane-promoted cyclization of 2-aminothiophenols by CO2to benzothiazoles. RSC Adv. 2014, 4, 56957–56960. [Google Scholar] [CrossRef]
- Gao, X.; Yu, B.; Yang, Z.; Zhao, Y.; Zhang, H.; Hao, L.; Han, B.; Liu, Z. Ionic Liquid-Catalyzed C–S Bond Construction using CO2 as a C1 Building Block under Mild Conditions: A Metal-Free Route to Synthesis of Benzothiazoles. ACS Catal. 2015, 5, 6648–6652. [Google Scholar] [CrossRef]
- Chun, S.; Yang, S.; Chung, Y.K. Synthesis of benzothiazoles from 2-aminobenzenethiols in the presence of a reusable polythiazolium precatalyst under atmospheric pressure of carbon dioxide. Tetrahedron 2017, 73, 3438–3442. [Google Scholar] [CrossRef]
- Bhanage, B.; Nale, D. N-Substituted Formamides as C1-Sources for the Synthesis of Benzimidazole and Benzothiazole Derivatives by Using Zinc Catalysts. Synlett 2015, 26, 2835–2842. [Google Scholar] [CrossRef]
- Sharma, S.; Bhattacherjee, D.; Das, P. Oxalic/malonic acids as carbon building blocks for benzazole, quinazoline and quinazolinone synthesis. Org. Biomol. Chem. 2018, 16, 1337–1342. [Google Scholar] [CrossRef]
- Tian, Q.; Gan, Z.; Wang, X.; Li, D.; Luo, W.; Wang, H.; Dai, Z.; Yuan, J. Imidazolium Chloride: An Efficient Catalyst for Transamidation of Primary Amines. Molecules 2018, 23, 2234. [Google Scholar] [CrossRef]
- Gan, Z.; Tian, Q.; Shang, S.; Luo, W.; Dai, Z.; Wang, H.; Li, D.; Wang, X.; Yuan, J. Imidazolium chloride-catalyzed synthesis of benzimidazoles and 2-substituted benzimidazoles from o-phenylenediamines and DMF derivatives. Tetrahedron 2018, 74, 7450–7456. [Google Scholar] [CrossRef]
- Srinivas, M.; Hudwekar, A.D.; Venkateswarlu, V.; Reddy, G.L.; Kumar, K.A.A.; Vishwakarma, R.A.; Sawant, S.D. A metal-free approach for transamidation of amides with amines in aqueous media. Tetrahedron Lett. 2015, 56, 4775–4779. [Google Scholar] [CrossRef]
- Muzart, J. N,N-Dimethylformamide: Much more than a solvent. Tetrahedron 2009, 65, 8313–8323. [Google Scholar] [CrossRef]
- Le Bras, J.; Muzart, J. Recent Uses of N,N-Dimethylformamide and N,N-Dimethylacetamide as Reagents. Molecules 2018, 23, 1939. [Google Scholar] [CrossRef] [PubMed]
- Suchy, M.; Elmehriki, A.A.H.; Hudson, R.H.E. A Remarkably Simple Protocol for the N-Formylation of Amino Acid Esters and Primary Amines. Org. Lett. 2011, 13, 3952–3955. [Google Scholar] [CrossRef] [PubMed]
- Mayo, M.S.; Yu, X.; Zhou, X.; Feng, X.; Yamamoto, Y.; Bao, M. Convenient synthesis of benzothiazoles and benzimidazoles through Bronsted acid catalyzed cyclization of 2-amino thiophenols/anilines with beta-diketones. Org. Lett. 2014, 16, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Bochatay, V.N.; Boissarie, P.J.; Murphy, J.A.; Suckling, C.J.; Lang, S. Mechanistic exploration of the palladium-catalyzed process for the synthesis of benzoxazoles and benzothiazoles. J. Org. Chem. 2013, 78, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Rancan, E.; Aricò, F.; Quartarone, G.; Ronchin, L.; Vavasori, A. Acid Catalyzed Direct-Amidation–Dehydrocyclization of 2-Hydroxy-acetophenones to Benzoxazoles by a One-Pot Sustainable Synthesis. Catal. Lett. 2015, 145, 939–946. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.; Nie, S.; Xu, D.; Yu, M.; Yao, X. Ligand-promoted, copper nanoparticles catalyzed one-pot synthesis of substituted benzoxazoles from 2-bromoanilines and acyl chlorides. Tetrahedron Lett. 2015, 56, 6827–6832. [Google Scholar] [CrossRef]
- Teo, Y.C.; Riduan, S.N.; Zhang, Y. Iodine-mediated arylation of benzoxazoles with aldehydes. Green Chem. 2013, 15, 2365–2368. [Google Scholar] [CrossRef]
- Azizian, J.; Torabi, P.; Noei, J. Synthesis of benzimidazoles and benzoxazoles using TiCl 3 OTf in ethanol at room temperature. Tetrahedron Lett. 2016, 57, 185–188. [Google Scholar] [CrossRef]
- Aksenov, N.A.; Aksenov, A.V.; Nadein, O.N.; Aksenov, D.A.; Smirnov, A.N.; Rubin, M. One-pot synthesis of benzoxazoles via the metal-free ortho-C–H functionalization of phenols with nitroalkanes. RSC Adv. 2015, 5, 71620–71626. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, R.; Marrot, J.; Coeffard, V.; Xiong, Y. Hypervalent iodine-mediated synthesis of benzoxazoles and benzimidazoles via an oxidative rearrangement. Tetrahedron 2015, 71, 700–708. [Google Scholar] [CrossRef]
- Wang, L.; Ma, Z.-G.; Wei, X.-J.; Meng, Q.-Y.; Yang, D.-T.; Du, S.-F.; Chen, Z.-F.; Wu, L.-Z.; Liu, Q. Synthesis of 2-substituted pyrimidines and benzoxazoles via a visible-light-driven organocatalytic aerobic oxidation: Enhancement of the reaction rate and selectivity by a base. Green Chem. 2014, 16, 3752–3757. [Google Scholar] [CrossRef]
- Mortimer, C.G.; Wells, G.; Crochard, J.P.; Stone, E.L.; Bradshaw, T.D.; Stevens, M.F.; Westwell, A.D. Antitumor benzothiazoles. 26.(1) 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (GW 610, NSC 721648), a simple fluorinated 2-arylbenzothiazole, shows potent and selective inhibitory activity against lung, colon, and breast cancer cell lines. J. Med. Chem. 2006, 49, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tang, J. Solid phase synthesis of benzothiazole and thiophene derivatives based on resin-bound cyclic malonic acid ester. Tetrahedron 2003, 59, 4851–4856. [Google Scholar] [CrossRef]
- Shimada, T.; Yamamoto, Y. Carbon—Carbon Bond Cleavage of Diynes Through the Hydroamination with Transition Metal Catalysts. J. Am. Chem. Soc. 2003, 125, 6646–6647. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, S.; Liu, X. Direct arylation of benzothiazoles and benzoxazoles with aryl boronic acids. Chemistry 2011, 17, 1105–1108. [Google Scholar] [CrossRef]
- Kumar, P.; Meenakshi; Kumar, S.; Kumar, A.; Hussain, K.; Kumar, S. Solvent-Free One Pot Synthesis of 2-aryl/Heteroarylbenzothiazoles Using Hypervalent Iodine (III) Reagents. J. Heterocycl. Chem. 2012, 49, 1243–1249. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, D.; Wang, X.; Zhou, P.; Wu, W.; Jiang, H. Controllable assembly of the benzothiazole framework using a C[triple bond, length as m-dash]C triple bond as a one-carbon synthon. Chem. Commun. 2018, 54, 1742–1745. [Google Scholar] [CrossRef]
- Lu, S.C.; Li, H.S.; Xu, S.; Duan, G.Y. Silver-catalyzed C2-selective direct alkylation of heteroarenes with tertiary cycloalkanols. Org. Biomol. Chem. 2017, 15, 324–327. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |





| Entry | Substance | Product 1 | Yield b (%) | |
|---|---|---|---|---|
| Substance 1 | Substance 2 | |||
| 1 |  | DMAc |  | 18 |
| 2 |  |  |  | 8 |
| 3 |  | DMA |  | 15 |
| 4 |  |  |  | 10 |

| Entry | Cat (equiv.) | Solvent | Temp | Time | Yield b (%) |
|---|---|---|---|---|---|
| 1 | - | DMA | 140 | 8 | - |
| 2 | HCl (0.5) | DMA | 140 | 8 | trace |
| 3 | Imidazolium chloride (0.3) | DMA | 140 | 8 | 18 |
| 4 | Imidazolium chloride (0.3) | DMA | 150 | 8 | 43 |
| 5 | Imidazolium chloride (0.3) | DMA | 160 | 8 | 60 |
| 6 | Imidazolium chloride (0.3) | DMA | 170 | 8 | 62 |
| 7 | Imidazolium chloride (0.3) | DMA | 180 | 8 | 65 |
| 8 | Imidazolium chloride (0.5) | DMA | 160 | 8 | 87 |
| 9 | Imidazolium chloride (0.8) | DMA | 160 | 8 | 85 |
| 10 | Imidazolium chloride (1.0) | DMA | 160 | 8 | 88 |
| 11 c | Imidazolium chloride (0.5) | Xylenes | 140 | 10 | 15 |
| 12 c | Imidazolium chloride (0.5) | H2O | 100 | 10 | Trace |
| 13 c | Imidazolium chloride (0.5) | Benzene | 90 | 10 | Trace |

| Entry | Substance | Time (h) | Product | Yield b (%) | |
|---|---|---|---|---|---|
| Substance 1 | Substance 2 | ||||
| 1 |  | DMA | 8 |  | 80 |
| 2 C |  |  | 10 |  | 86 |
| 3 C |  |  | 10 |  | 88 |
| 4 C |  |  | 10 |  | 52 |
| 5 |  | DMA | 8 |  | 86 |
| 6 |  | DMA | 8 |  | 84 |
| 7 C |  |  | 10 |  | 83 |
| 8 |  | DMA | 8 |  | 87 |
| 9 C |  |  | 10 |  | 80 |

| Entry | Substance | Time (h) | Product | Yield b (%) | |
|---|---|---|---|---|---|
| Substance 1 | Substance 2 | ||||
| 1 |  |  | 10 |  | 82 |
| 2 |  |  | 10 |  | 85 |
| 3 |  |  | 10 |  | 80 |
| 4 |  |  | 10 |  | 75 |
| 5 |  |  | 10 |  | 87 |
| 6 |  |  | 10 |  | 79 |
| 7 |  |  | 10 |  | 60 |
| 8 |  |  | 10 |  | 82 |
| 9 |  |  | 10 |  | 79 |
| 10 |  |  | 10 |  | 75 |
| 11 |  |  | 10 |  | 82 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Q.; Luo, W.; Gan, Z.; Li, D.; Dai, Z.; Wang, H.; Wang, X.; Yuan, J. Eco-Friendly Syntheses of 2-Substituted Benzoxazoles and 2-Substituted Benzothiazoles from 2-Aminophenols, 2-Aminothiophenols and DMF Derivatives in the Presence of Imidazolium Chloride. Molecules 2019, 24, 174. https://doi.org/10.3390/molecules24010174
Tian Q, Luo W, Gan Z, Li D, Dai Z, Wang H, Wang X, Yuan J. Eco-Friendly Syntheses of 2-Substituted Benzoxazoles and 2-Substituted Benzothiazoles from 2-Aminophenols, 2-Aminothiophenols and DMF Derivatives in the Presence of Imidazolium Chloride. Molecules. 2019; 24(1):174. https://doi.org/10.3390/molecules24010174
Chicago/Turabian StyleTian, Qingqiang, Wen Luo, Zongjie Gan, Dan Li, Zeshu Dai, Huajun Wang, Xuetong Wang, and Jianyong Yuan. 2019. "Eco-Friendly Syntheses of 2-Substituted Benzoxazoles and 2-Substituted Benzothiazoles from 2-Aminophenols, 2-Aminothiophenols and DMF Derivatives in the Presence of Imidazolium Chloride" Molecules 24, no. 1: 174. https://doi.org/10.3390/molecules24010174
APA StyleTian, Q., Luo, W., Gan, Z., Li, D., Dai, Z., Wang, H., Wang, X., & Yuan, J. (2019). Eco-Friendly Syntheses of 2-Substituted Benzoxazoles and 2-Substituted Benzothiazoles from 2-Aminophenols, 2-Aminothiophenols and DMF Derivatives in the Presence of Imidazolium Chloride. Molecules, 24(1), 174. https://doi.org/10.3390/molecules24010174