Synthesis of Cyanate Esters Based on Mono-O-Methylated Bisphenols with Sulfur-Containing Bridges
Abstract
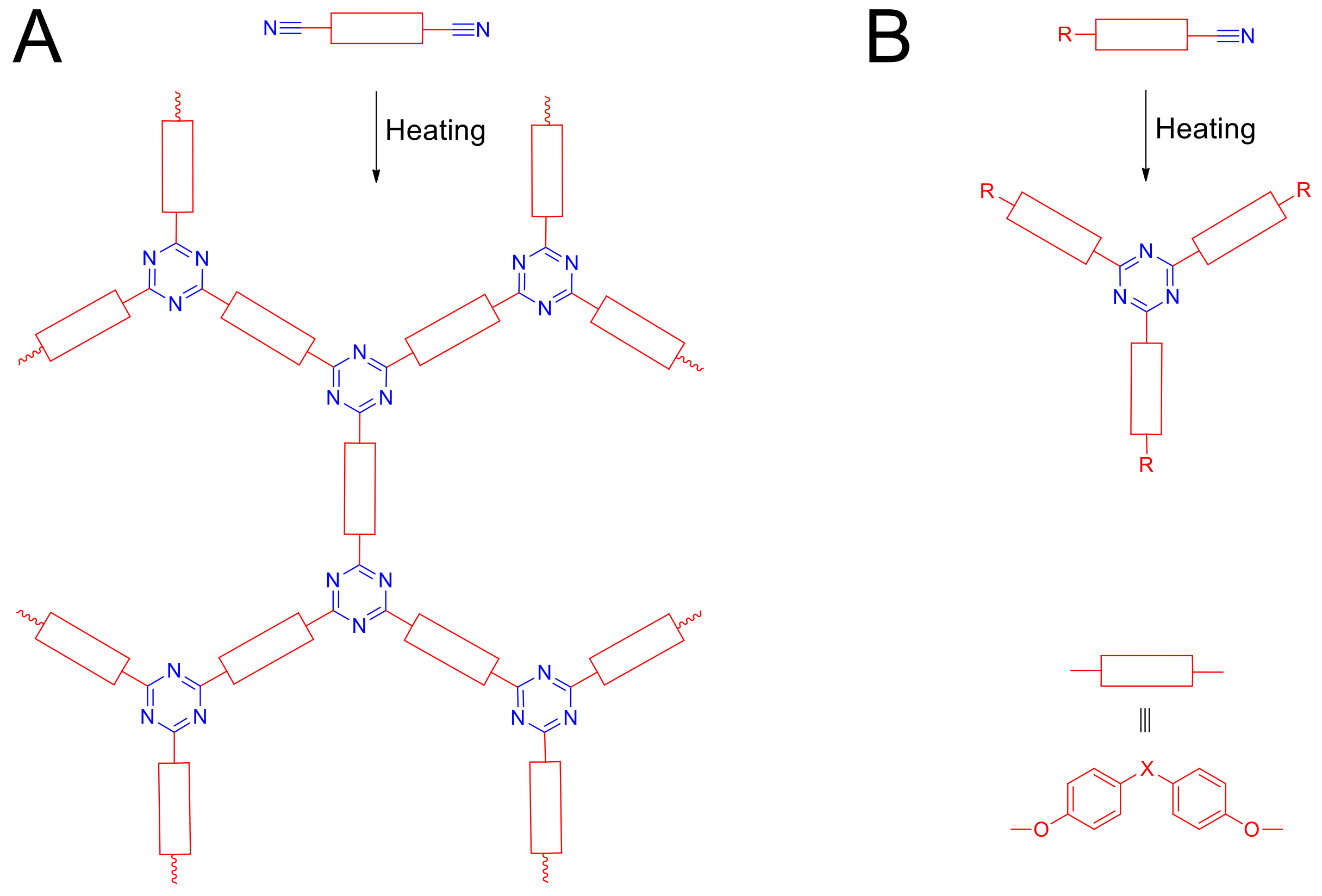
1. Introduction
2. Results and Discussion
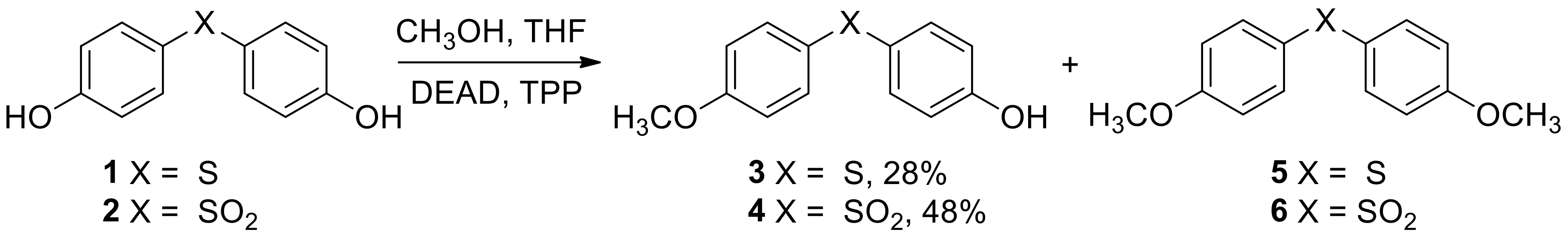
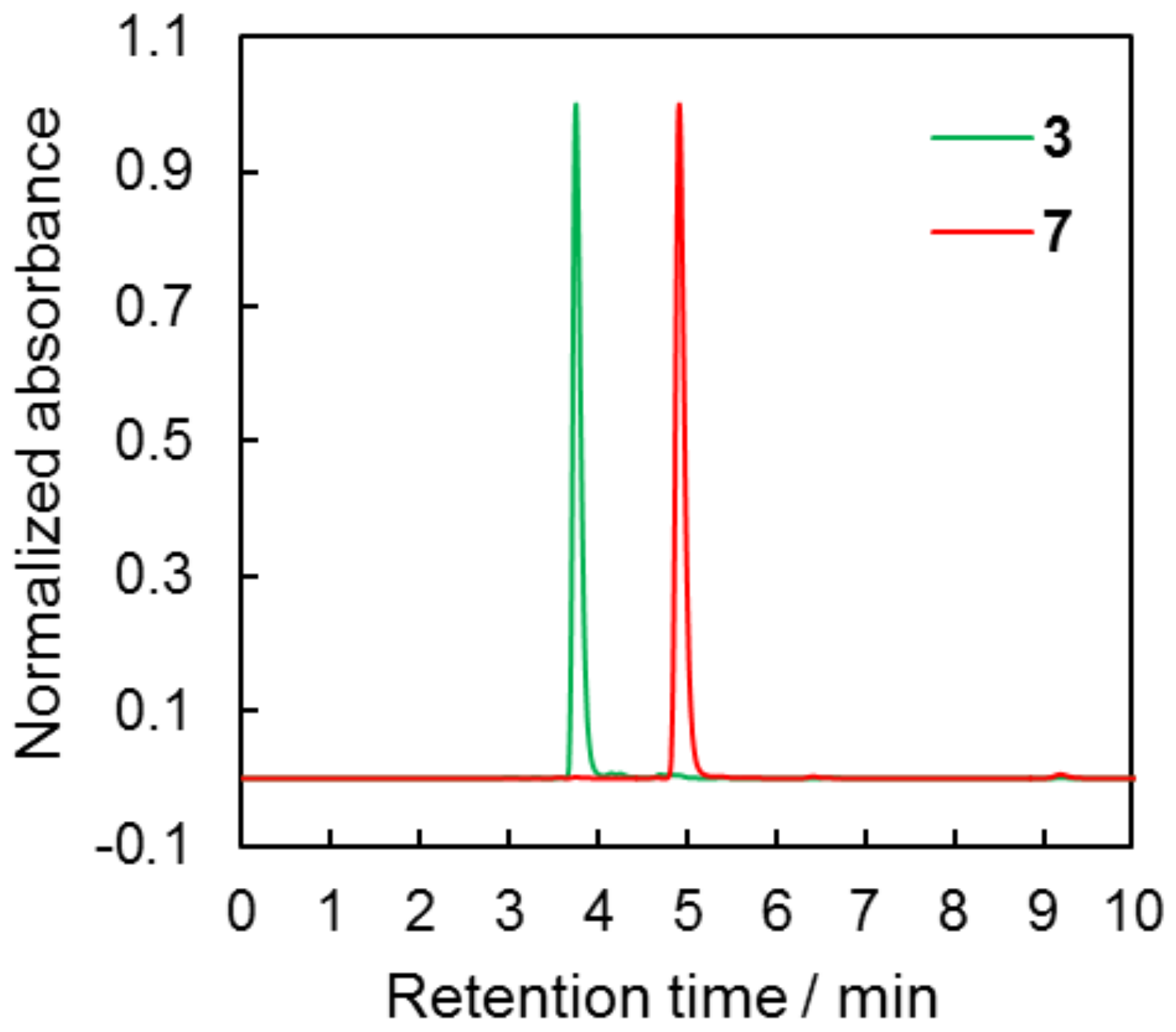
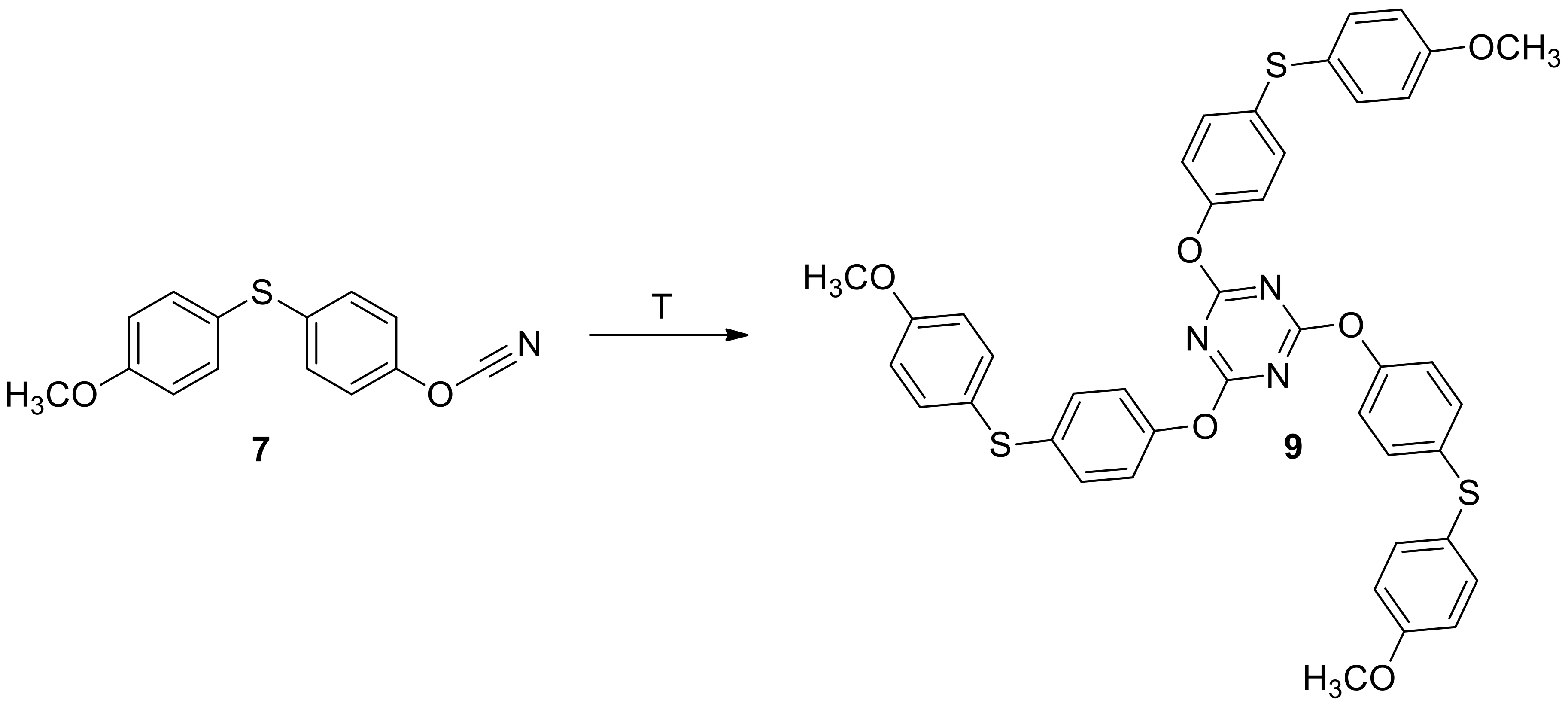
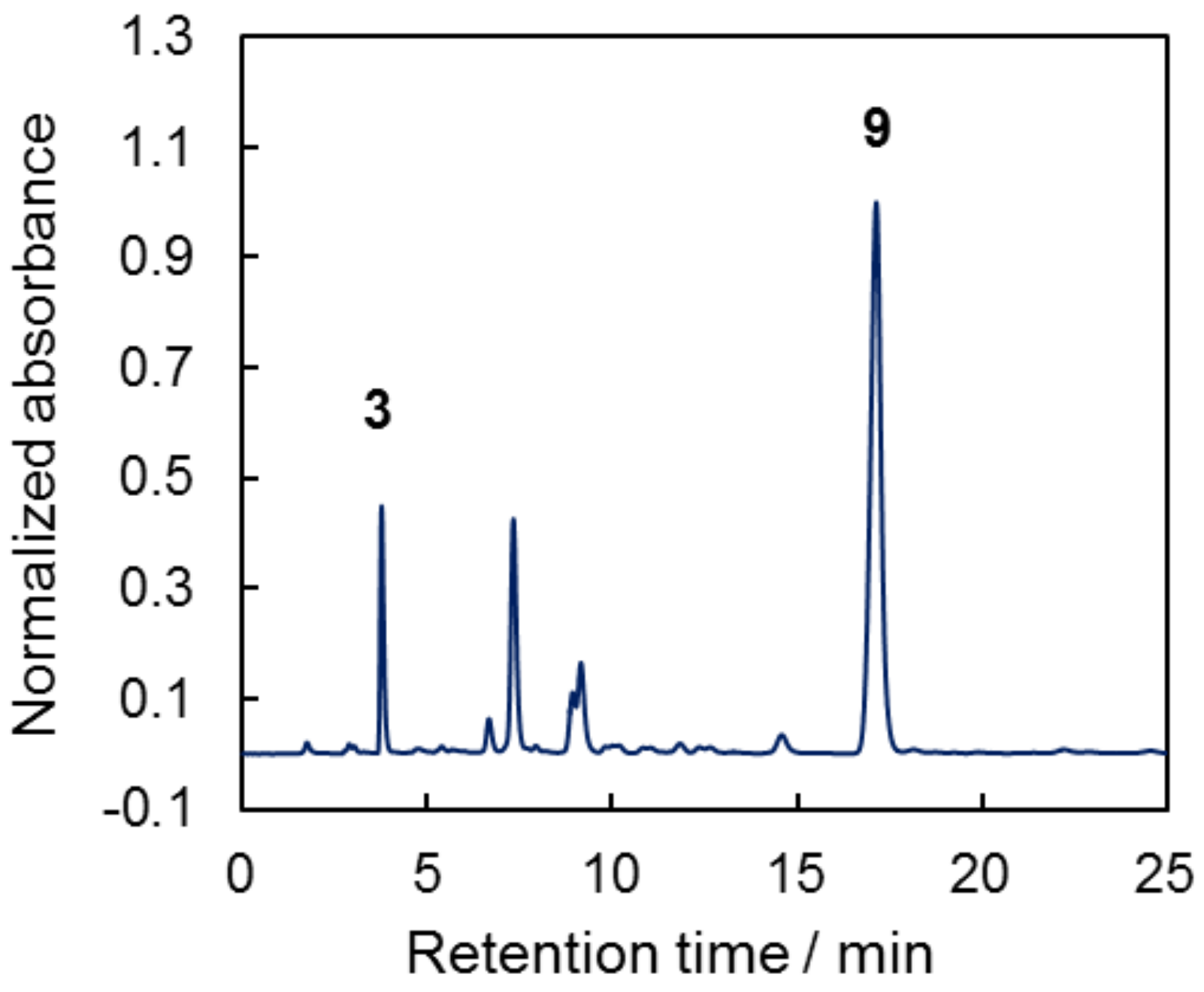
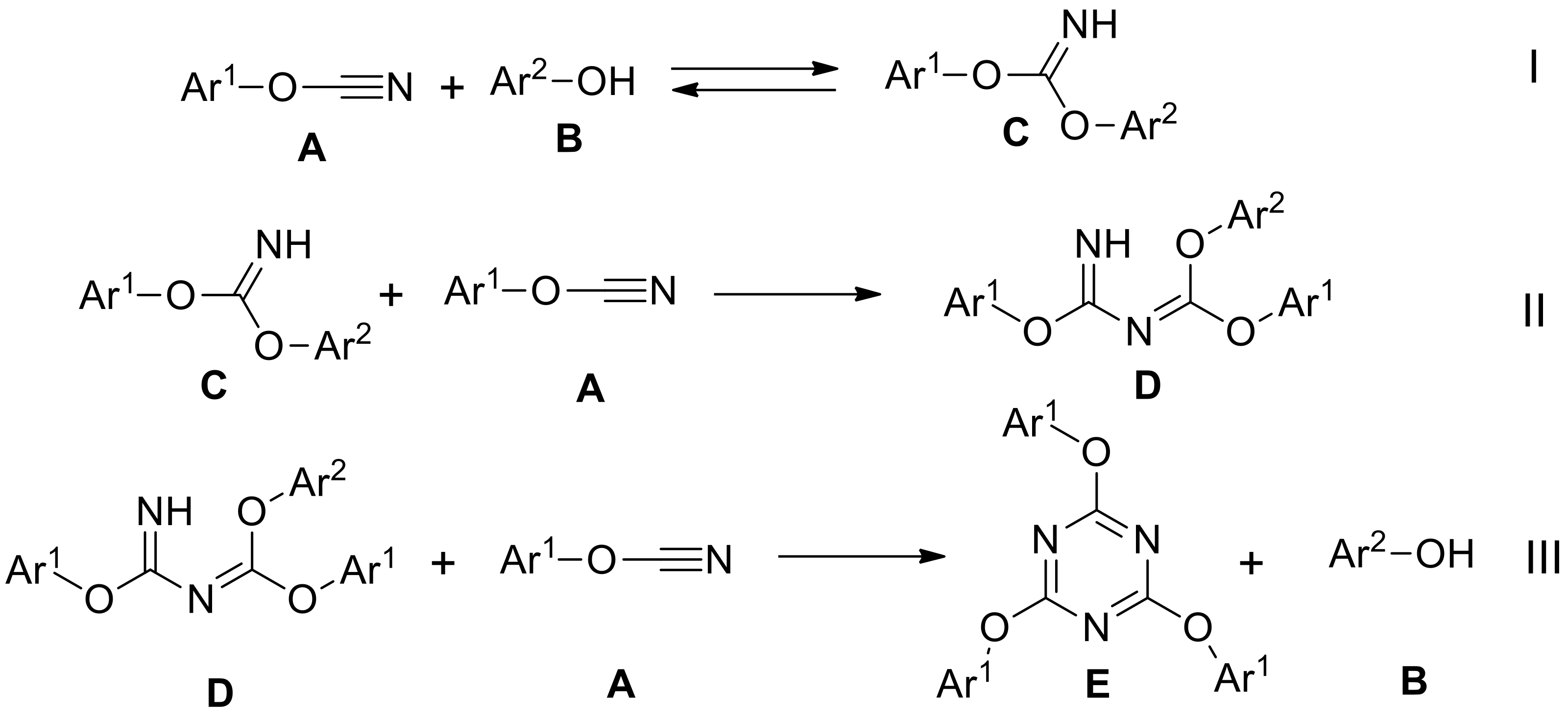
2.1. Synthesis and Characterization of Target Compounds
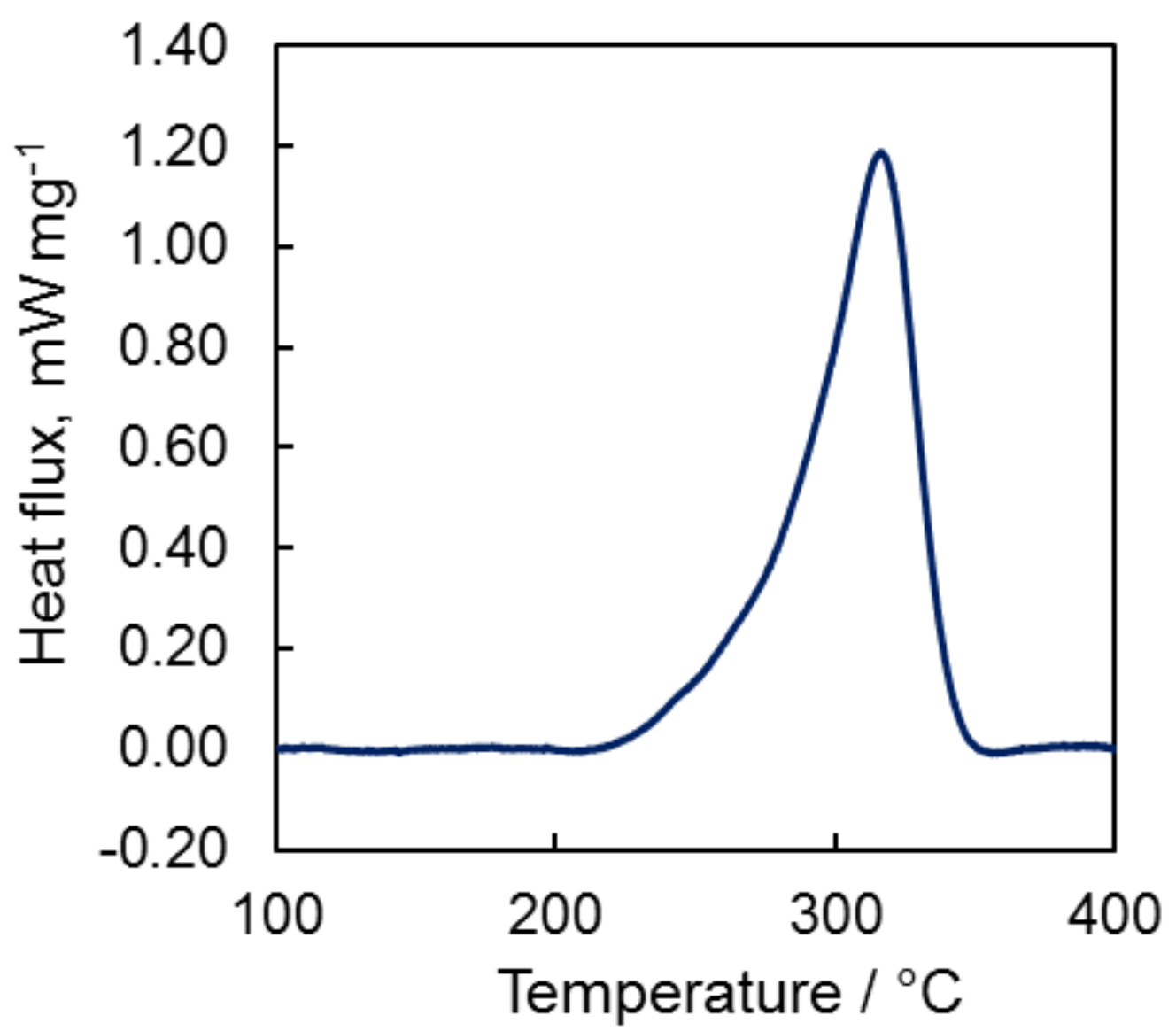
2.2. Differential Scanning Calorimetry Study
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Synthesis Details and Characterization
3.3.1. Synthesis and Characterization of 4-((4′-methoxyphenyl)thio)phenol 3
3.3.2. Synthesis and Characterization of 4-((4′-methoxyphenyl)sulfonyl)phenol 4
3.3.3. Synthesis and Characterization of (4-cyanatophenyl)(4′-methoxyphenyl)sulfane 7
3.3.4. Synthesis of 1-cyanato-4-((4-methoxyphenyl)sulfonyl)benzene 8
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hamerton, I. Chemistry and Technology of Cyanate Ester Resins; Springer-Science+Business Media, B.V.: New York, NY, USA, 1994. [Google Scholar]
- Harismendy, I.; Gómez, C.M.; Del Río, M.; Mondragon, I. Cure monitoring of catalysed cyanate ester resins. Polym. Int. 2000, 49, 735–742. [Google Scholar] [CrossRef]
- Mathew, D.; Nair, C.P.R.; Krishnan, K.; Ninan, K.N. Catalysis of the cure reaction of Bisphenol A dicyanate. A DSC study. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 1103–1114. [Google Scholar] [CrossRef]
- Siddiqui, A.O.; Sudher, P.; Murthy, B.V.S.R. Cure kinetics modeling of cyanate-ester resin system. Thermochim. Acta 2013, 554, 8–14. [Google Scholar] [CrossRef]
- Guenthner, A.J.; Sahagun, C.M.; Lamison, K.R.; Reams, J.T.; Haddad, T.S.; Mabry, J.M. Effect of Nanoparticle Functionalization on the Performance of Polycyanurate/Silica Nanocomposites. Ind. Eng. Chem. Res. 2016, 55, 7096. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Effect of viscosity on the kinetics of initial cure stages. Macromol. Chem. Phys. 2000, 201, 199–203. [Google Scholar] [CrossRef]
- Grenier-Loustalot, M.F.; Lartigau, C.; Metras, F.; Grenier, P. Mechanism of thermal polymerization of cyanate ester systems: Chromatographic and spectroscopic studies. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 2955–2966. [Google Scholar] [CrossRef]
- Mitsunobu, O.; Yamada, M. Preparation of Esters of Carboxylic and Phosphoric Acid via Quaternary Phosphonium Salts. Bull. Chem. Soc. Jpn. 1967, 40, 2380–2382. [Google Scholar] [CrossRef]
- Grigat, E.; Putter, R. Synthesis and Reactions of Cyanic Esters. Angew. Chem. Int. Ed. Engl. 1967, 6, 206–218. [Google Scholar] [CrossRef]
- Martin, D. Cyansaurephenylester. Angew. Chemie 1964, 76, 303. [Google Scholar] [CrossRef]
- Jensen, K.A.; Holm, A. Alkyl cyanates. II a new route to alkyl cyanates. Acta Chem. Scand. 1964, 18, 2417–2418. [Google Scholar] [CrossRef]
- Gómez, C.M.; Recalde, I.B.; Mondragon, I. Kinetic parameters of a cyanate ester resin catalyzed with different proportions of nonylphenol and cobalt acetylacetonate catalyst. Eur. Polym. J. 2005, 41, 2734–2741. [Google Scholar] [CrossRef]
- Osei-Owusu, A.; Martin, G. Analysis of the Curing Behavior of Cyanate Ester Resin Systems. Polym. Eng. Sci. 1991, 31, 1604–1609. [Google Scholar] [CrossRef]
- Osei-Owusu, A.; Martin, G.C.; Gotro, J.T. Catalysis and kinetics of cyclotrimerization of cyanate ester resin systems. Polym. Eng. Sci. 1992, 32, 535–541. [Google Scholar] [CrossRef]
- Simon, S.L.; Gillham, J.K. Cure kinetics of a thermosetting liquid dicyanate ester monomer/high-Tg polycyanurate material. J. Appl. Polym. Sci. 1993, 47, 461–485. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 3, 4, 7 are available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galukhin, A.; Nosov, R. Synthesis of Cyanate Esters Based on Mono-O-Methylated Bisphenols with Sulfur-Containing Bridges. Molecules 2019, 24, 177. https://doi.org/10.3390/molecules24010177
Galukhin A, Nosov R. Synthesis of Cyanate Esters Based on Mono-O-Methylated Bisphenols with Sulfur-Containing Bridges. Molecules. 2019; 24(1):177. https://doi.org/10.3390/molecules24010177
Chicago/Turabian StyleGalukhin, Andrey, and Roman Nosov. 2019. "Synthesis of Cyanate Esters Based on Mono-O-Methylated Bisphenols with Sulfur-Containing Bridges" Molecules 24, no. 1: 177. https://doi.org/10.3390/molecules24010177
APA StyleGalukhin, A., & Nosov, R. (2019). Synthesis of Cyanate Esters Based on Mono-O-Methylated Bisphenols with Sulfur-Containing Bridges. Molecules, 24(1), 177. https://doi.org/10.3390/molecules24010177





