Effect of Planarity of Aromatic Rings Appended to o-Carborane on Photophysical Properties: A Series of o-Carboranyl Compounds Based on 2-Phenylpyridine- and 2-(Benzo[b]thiophen-2-yl)pyridine
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Considerations
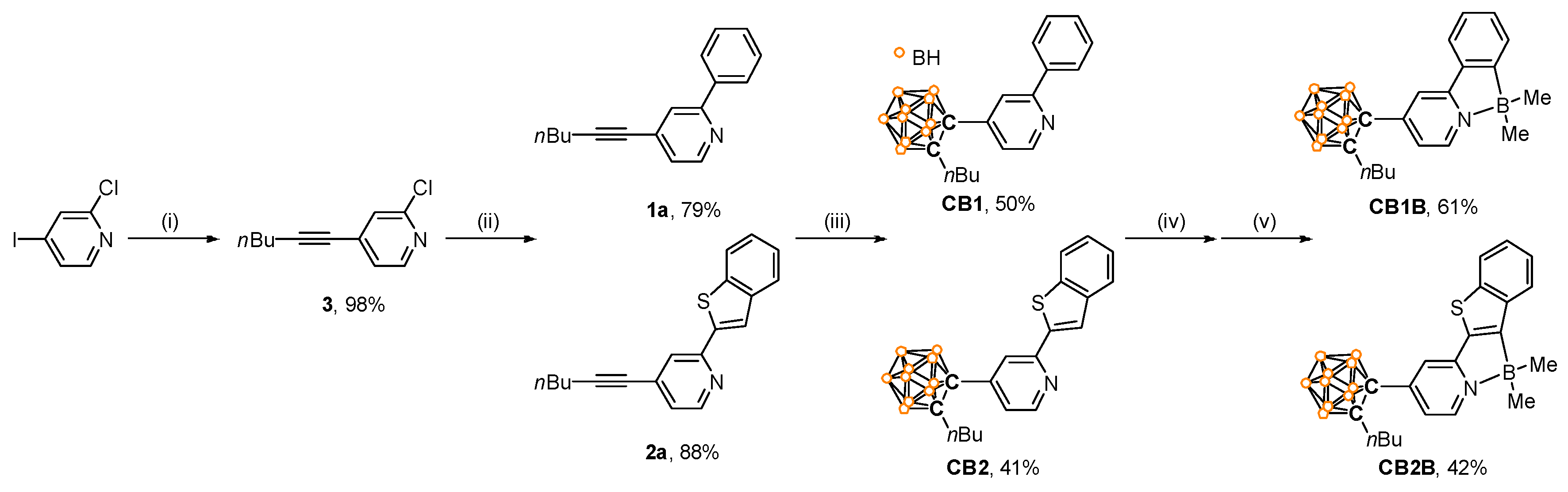
2.2. Synthesis of 2-Chloro-4-(hex-1-yn-1-yl)pyridine (3)
2.3. Synthesis of 1a
2.4. Synthesis of 2a
2.5. Synthesis of CB1
2.6. Synthesis of CB2
2.7. Synthesis of CB1B
2.8. Synthesis of CB2B
2.9. UV-vis Absorption and PL Measurements
2.10. X-ray Crystallography
2.11. Theoretical Calculations
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Photophysical Properties
3.3. Theoretical Calculations and Orbital Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Spokoyny, A.M. New ligand platforms featuring boron-rich clusters as organomimetic substituents. Pure Appl. Chem. 2013, 85, 903–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bregadze, V.I. Dicarba-closo-dodecaboranes C2B10H12 and their derivatives. Chem. Rev. 1992, 92, 209–223. [Google Scholar] [CrossRef]
- González-Campo, A.; Juárez-Pérez, E.J.; Viñas, C.; Boury, B.; Sillanpää, R.; Kivekäs, R.; Núñez, R. Carboranyl Substituted Siloxanes and Octasilsesquioxanes: Synthesis, Characterization, and Reactivity. Macromolecules 2008, 41, 8458–8466. [Google Scholar] [CrossRef]
- Issa, F.; Kassiou, M.; Rendina, L.M. Boron in Drug Discovery: Carboranes as Unique Pharmacophores in Biologically Active Compounds. Chem. Rev. 2011, 111, 5701–5722. [Google Scholar] [CrossRef]
- Wee, K.-R.; Cho, Y.-J.; Jeong, S.; Kwon, S.; Lee, J.-D.; Suh, I.-H.; Kang, S.O. Carborane-Based Optoelectronically Active Organic Molecules: Wide Band Gap Host Materials for Blue Phosphorescence. J. Am. Chem. Soc. 2012, 134, 17982–17990. [Google Scholar] [CrossRef]
- Ferrer-Ugalde, A.; Juárez-Pérez, E.J.; Teixidor, F.; Viñas, C.; Núñez, R. Synthesis, Characterization, and Thermal Behavior of Carboranyl-Styrene Decorated Octasilsesquioxanes: Influence of the Carborane Clusters on Photoluminescence. Chem-Eur. J. 2013, 19, 17021–17030. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Kim, H.; Lee, K.M.; Lee, Y.S.; Lee, M.H. Phosphorescence Color Tuning of Cyclometalated Iridium Complexes by o-Carborane Substitution. Inorg. Chem. 2012, 52, 160–168. [Google Scholar] [CrossRef]
- Bae, H.J.; Chung, J.; Kim, H.; Park, J.; Lee, K.M.; Koh, T.-W.; Lee, M.H. Deep Red Phosphorescence of Cyclometalated Iridium Complexes by o-Carborane Substitution. Inorg. Chem. 2013, 53, 128–138. [Google Scholar] [CrossRef]
- Asay, M.J.; Fisher, S.P.; Lee, S.E.; Tham, F.S.; Borchardt, D.; Lavallo, V. Synthesis of unsymmetrical N-carboranyl NHCs: Directing effect of the carborane anion. Chem. Commun. 2015, 51, 5359–5362. [Google Scholar] [CrossRef]
- Lee, Y.H.; Park, J.; Jo, S.-J.; Kim, M.; Lee, J.; Lee, S.U.; Lee, M.H. Manipulation of Phosphorescence Efficiency of Cyclometalated Iridium Complexes by Substituted o-Carboranes. Chem.-Eur. J. 2014, 21, 2052–2061. [Google Scholar] [CrossRef]
- Núñez, R.; Tarrés, M.; Ferrer-Ugalde, A.; de Biani, F.F.; Teixidor, F. Electrochemistry and Photoluminescence of Icosahedral Carboranes, Boranes, Metallacarboranes, and Their Derivatives. Chem. Rev. 2016, 116, 14307–14378. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Thilagar, P. Boron clusters in luminescent materials. Chem. Commun. 2016, 52, 1070–1093. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, R.M.; Saleh, L.M.A.; Axtell, J.C.; Martin, J.L.; Stevens, S.L.; Royappa, A.T.; Spokoyny, A.M. B–N, B–O, and B–CN Bond Formation via Palladium-Catalyzed Cross-Coupling of B-Bromo-Carboranes. J. Am. Chem. Soc. 2016, 138, 9081–9084. [Google Scholar] [CrossRef] [PubMed]
- Kirlikovali, K.O.; Axtell, J.C.; Gonzalez, A.; Phung, A.C.; Khan, S.I.; Spokoyny, A.M. Luminescent metal complexes featuring photophysically innocent boron cluster ligands. Chem. Sci. 2016, 7, 5132–5138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, L.M.A.; Dziedzic, R.M.; Khan, S.I.; Spokoyny, A.M. Forging Unsupported Metal-Boryl Bonds with Icosahedral Carboranes. Chem.-Eur. J. 2016, 22, 8466–8470. [Google Scholar] [CrossRef] [PubMed]
- Eleazer, B.J.; Smith, M.D.; Popov, A.A.; Peryshkov, D.V. (BB)-Carboryne Complex of Ruthenium: Synthesis by Double B–H Activation at a Single Metal Center. J. Am. Chem. Soc. 2016, 138, 10531–10538. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.O.; Smith, M.D.; Peryshkov, D.V. Synthesis of the First Example of the 12-Vertex-closo/12-Vertex-nido Biscarborane Cluster by a Metal-Free B−H Activation at a Phosphorus(III) Center. Chem.-Eur. J. 2016, 22, 6764–6767. [Google Scholar] [CrossRef]
- Chan, A.L.; Estrada, J.; Kefalidis, C.E.; Lavallo, V. Changing the Charge: Electrostatic Effects in Pd-Catalyzed Cross-Coupling. Organometallics 2016, 35, 3257–3260. [Google Scholar] [CrossRef]
- Fisher, S.P.; El-Hellani, A.; Tham, F.S.; Lavallo, V. Anionic and zwitterionic carboranyl N-heterocyclic carbene Au(i) complexes. Dalton Trans. 2016, 45, 9762–9765. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S.; Lee, Y.H.; Jung, J.; Yoo, S.; Lee, M.H. Homoleptic Tris-Cyclometalated Iridium Complexes with Substituted o-Carboranes: Green Phosphorescent Emitters for Highly Efficient Solution-Processed Organic Light-Emitting Diodes. Inorg. Chem. 2016, 55, 909–917. [Google Scholar] [CrossRef]
- Tu, D.; Leong, P.; Guo, S.; Yan, H.; Lu, C.; Zhao, Q. Highly Emissive Organic Single-Molecule White Emitters by Engineering o-Carborane-Based Luminophores. Angew. Chem. Int. Ed. 2017, 56, 11370–11374. [Google Scholar] [CrossRef] [PubMed]
- Kirlikovali, K.O.; Axtell, J.C.; Anderson, K.; Djurovich, P.I.; Rheingold, A.L.; Spokoyny, A.M. Fine-Tuning Electronic Properties of Luminescent Pt(II) Complexes via Vertex-Differentiated Coordination of Sterically Invariant Carborane-Based Ligands. Organometallics 2018, 37, 3122–3131. [Google Scholar] [CrossRef]
- Nar, I.; Atsay, A.; Altındal, A.; Hamuryudan, E. o-Carborane, Ferrocene, and Phthalocyanine Triad for High-Mobility Organic Field-Effect Transistors. Inorg. Chem. 2018, 57, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Grimes, R.N. Carboranes, 2nd ed.; Academic Press: London, UK, 2011. [Google Scholar]
- Poater, J.; Solà, M.; Viñas, C.; Teixidor, F. π Aromaticity and Three-Dimensional Aromaticity: Two sides of the Same Coin? Angew. Chem. Int. Ed. 2014, 53, 12191–12195. [Google Scholar] [CrossRef] [PubMed]
- Poater, J.; Solà, M.; Viñas, C.; Teixidor, F. Hückel’s Rule of Aromaticity Categorizes Aromatic closo Boron Hydride Clusters. Chem.-Eur. J. 2016, 22, 7437–7443. [Google Scholar] [CrossRef] [PubMed]
- Núñez, R.; Romero, I.; Teixidor, F.; Viñas, C. Icosahedral boron clusters: A perfect tool for the enhancement of polymer features. Chem. Soc. Rev. 2016, 45, 5147–5173. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-González, J.; Sánchez-Arderiu, V.; Viñas, C.; Parella, T.; Teixidor, F.; Núñez, R. Redox-Active Metallacarborane-Decorated Octasilsesquioxanes. Inorg. Chem. 2016, 55, 11630–11634. [Google Scholar] [CrossRef]
- Kokado, K.; Chujo, Y. Multicolor Tuning of Aggregation-Induced Emission through Substituent Variation of Diphenyl-o-carborane. J. Org. Chem. 2011, 76, 316–319. [Google Scholar] [CrossRef]
- Dash, B.P.; Satapathy, R.; Gaillard, E.R.; Norton, K.M.; Maguire, J.A.; Chug, N.; Hosmane, N.S. Enhanced π-Conjugation and Emission via Icosahedral Carboranes: Synthetic and Spectroscopic Investigation. Inorg. Chem. 2011, 50, 5485–5493. [Google Scholar] [CrossRef]
- Wee, K.-R.; Han, W.-S.; Cho, D.W.; Kwon, S.; Pac, C.; Kang, S.O. Carborane Photochemistry Triggered by Aryl Substitution: Carborane-Based Dyads with Phenyl Carbazoles. Angew. Chem. Int. Ed. 2012, 51, 2677–2680. [Google Scholar] [CrossRef]
- Weber, L.; Kahlert, J.; Brockhinke, R.; Böhling, L.; Brockhinke, A.; Stammler, H.-G.; Fox, M.A. Luminescence Properties of C-Diazaborolyl-ortho-Carboranes as Donor-Acceptor Systems. Chem.-Eur. J. 2012, 18, 8347–8357. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.J.; Kim, H.; Lee, K.M.; Kim, T.; Eo, M.; Lee, Y.S.; Lee, M.H. Heteroleptic tris-cyclometalated iridium(iii) complexes supported by an o-carboranyl-pyridine ligand. Dalton Trans. 2013, 42, 8549–8552. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.; Kahlert, J.; Brockhinke, R.; Böhling, L.; Halama, J.; Brockhinke, A.; Stammler, H.-G.; Neumann, B.; Nervi, C.; Harder, R.A.; et al. C,C′-Bis(benzodiazaborolyl)dicarba-closo-dodecaboranes: Synthesis, structures, photophysics and electrochemistry. Dalton Trans. 2013, 42, 10982–10996. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.; Kahlert, J.; Böhling, L.; Brockhinke, A.; Stammler, H.-G.; Neumann, B.; Harder, R.A.; Low, P.J.; Fox, M.A. Electrochemical and spectroelectrochemical studies of C-benzodiazaborolyl-ortho-carboranes. Dalton Trans. 2013, 42, 2266–2281. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Wee, K.-R.; Cho, Y.-J.; Kang, S.O. Carborane Dyads for Photoinduced Electron Transfer: Photophysical Studies on Carbazole and Phenyl-o-carborane Molecular Assemblies. Chem.-Eur. J. 2014, 20, 5953–5960. [Google Scholar] [CrossRef]
- Ferrer-Ugalde, A.; González-Campo, A.; Viñas, C.; Rodríguez-Romero, J.; Santillan, R.; Farfán, N.; Sillanpää, R.; Sousa-Pedrares, A.; Núñez, R.; Teixidor, F. Fluorescence of New o-Carborane Compounds with Different Fluorophores: Can it be Tuned. Chem.-Eur. J. 2014, 20, 9940–9951. [Google Scholar] [CrossRef]
- Bae, H.J.; Kim, H.; Lee, K.M.; Kim, T.; Lee, Y.S.; Do, Y.; Lee, M.H. Through-space charge transfer and emission color tuning of di-o-carborane substituted benzene. Dalton Trans. 2014, 43, 4978–4985. [Google Scholar] [CrossRef]
- Lee, Y.H.; Park, J.; Lee, J.; Lee, S.U.; Lee, M.H. Iridium Cyclometalates with Tethered o-Carboranes: Impact of Restricted Rotation of o-Carborane on Phosphorescence Efficiency. J. Am. Chem. Soc. 2015, 137, 8018–8021. [Google Scholar] [CrossRef]
- Naito, H.; Morisaki, Y.; Chujo, Y. o-Carborane-Based Anthracene: A Variety of Emission Behaviors. Angew. Chem. Int. Ed. 2015, 54, 5084–5087. [Google Scholar] [CrossRef]
- Kim, T.; Lee, J.; Lee, S.U.; Lee, M.H. o-Carboranyl–Phosphine as a New Class of Strong-Field Ancillary Ligand in Cyclometalated Iridium(III) Complexes: Toward Blue Phosphorescence. Organometallics 2015, 34, 3455–3458. [Google Scholar] [CrossRef]
- Choi, B.H.; Lee, J.H.; Hwang, H.; Lee, K.M.; Park, M.H. Novel Dimeric o-Carboranyl Triarylborane: Intriguing Ratiometric Color-Tunable Sensor via Aggregation-Induced Emission by Fluoride Anions. Organometallics 2016, 35, 1771–1777. [Google Scholar] [CrossRef]
- Wee, K.-R.; Cho, Y.-J.; Song, J.K.; Kang, S.O. Two-Dimensional Hybrid Nanosheets of Tungsten Disulfide and Reduced Graphene Oxide as Catalysts for Enhanced Hydrogen Evolution. Angew. Chem. Int. Ed. 2013, 52, 1–5. [Google Scholar]
- Naito, H.; Nishino, K.; Morisaki, Y.; Tanaka, K.; Chujo, Y. Solid-State Emission of the Anthracene-o-Carborane Dyad from the Twisted-Intramolecular Charge Transfer in the Crystalline State. Angew. Chem. Int. Ed. 2017, 56, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Guo, J.; Cao, Y.; Zhao, J.; Jia, W.; Chen, Y.; Jia, D. Mechanically triggered reversible stepwise tricolor switching and thermochromism of anthracene-o-carborane dyad. Chem. Sci. 2018, 9, 5270–5277. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, C.; Peng, X.; Chen, Y.; Qi, Q.; Luo, X.; Lai, W.-Y.; Huang, W. Stimuli-responsive solid-state emission from o-carborane-tetraphenylethene dyads induced by twisted intramolecular charge transfer in the crystalline state. J. Mater. Chem. C 2018, 6, 19–28. [Google Scholar] [CrossRef]
- Nishino, K.; Yamamoto, H.; Tanaka, K.; Chujo, Y. Development of Solid-State Emissive Materials Based on Multifunctional o-Carborane–Pyrene Dyads. Org. Lett. 2016, 18, 4064–4067. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.V.; Cheetham, N.J.; Little, M.; Dyson, M.; White, A.J.P.; Beavis, P.; Warriner, C.N.; Swain, A.C.; Stavrinou, P.N.; Heeney, M. Carborane-Induced Excimer Emission of Severely Twisted Bis-o-Carboranyl Chrysene. Angew. Chem. Int. Ed. 2018, 57. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Cho, Y.-J.; Jin, G.F.; Han, W.-S.; Son, H.-J.; Cho, D.W.; Kang, S.O. Intriguing emission properties of triphenylamine–carborane systems. Phys. Chem. Chem. Phys. 2015, 17, 15679–15682. [Google Scholar] [CrossRef]
- Wan, Y.; Li, J.; Peng, X.; Huang, C.; Qi, Q.; Lai, W.-Y.; Huang, W. Intramolecular charge transfer induced emission from triphenylamine-o-carborane dyads. RSC Adv. 2017, 7, 35543–35548. [Google Scholar] [CrossRef]
- Nishino, K.; Uemura, K.; Gon, M.; Tanaka, K.; Chujo, Y. Enhancement of Aggregation-Induced Emission by Introducing Multiple o-Carborane Substitutions into Triphenylamine. Molecules 2017, 22, 2009. [Google Scholar] [CrossRef]
- Naito, H.; Nishino, K.; Morisaki, Y.; Tanaka, K.; Chujo, Y. Luminescence Color Tuning from Blue to Near Infrared of Stable Luminescent Solid Materials Based on Bis-o-Carborane-Substituted Oligoacenes. Chem. Asian J. 2017, 12, 2134–2138. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Nishino, K.; Morisaki, Y.; Tanaka, K.; Chujo, Y. Highly-efficient solid-state emissions of anthracene-o-carborane dyads with various substituents and their thermochromic luminescence properties. J. Mater. Chem. C 2017, 5. [Google Scholar] [CrossRef]
- Wu, X.; Guo, J.; Quan, Y.; Jia, W.; Jia, D.; Chen, Y.; Xie, Z. Cage carbon-substitute does matter for aggregation-induced emission features of o-carborane-functionalized anthracene triads. J. Mater. Chem. C 2018, 6, 4140–4149. [Google Scholar] [CrossRef]
- Mori, H.; Nishino, K.; Wada, K.; Morisaki, Y.; Tanaka, K.; Chujo, Y. Modulation of luminescence chromic behaviors and environment-responsive intensity changes by substituents in bis-o-carborane-substituted conjugated molecules. Mater. Chem. Front. 2018, 2, 573–579. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, J.; Wu, X.; Jia, D.; Tong, F. Color-tuning aggregation-induced emission of o-Carborane-bis(1,3,5-triaryl-2-pyrazoline) triads: Preparation and investigation of the photophysics. Dyes Pigm. 2018, 148, 180–188. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Lee, J.-D.; Cho, Y.-J.; Son, M.R.; Son, H.-J.; Cho, D.W.; Kang, S.O. Excitation spectroscopic and synchronous fluorescence spectroscopic analysis of the origin of aggregation-induced emission in N,N-diphenyl-1-naphthylamine-o-carborane derivatives. Phys. Chem. Chem. Phys. 2018, 20, 17458–17463. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.; Yu, S.; Lee, J.H.; Hwang, H.; Lee, K.M. Biphenyl- and Fluorene-Based o-Carboranyl Compounds: Alteration of Photophysical Properties by Distortion of Biphenyl Rings. Organometallics 2017, 36, 1522–1529. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Phys. Chem. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Runge, E.; Gross, E.K.U. Density-Functional Theory for Time-Dependent Systems. Phys. Rev. Lett. 1984, 52, 997–1000. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision D.01; Gaussian. Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. cclib: A library for package-independent computational chemistry algorithms. J. Comp. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Berry, T.E.; Wegner, P.A. The Electronic Properties of the 1,2- and 1,7-Dicarbaclovododecaborane(12) Groups Bonded at Carbon. J. Am. Chem. Soc. 1965, 87, 4746–4750. [Google Scholar] [CrossRef] [PubMed]
- Paxson, T.E.; Callahan, K.P.; Hawthorne, M.F. Improved synthesis of biscarborane and its precursor ethynylcarborane. Inorg. Chem. 1973, 12, 708–709. [Google Scholar] [CrossRef]
- Jiang, W.; Knobler, C.B.; Hawthorne, M.F. Synthesis and Structural Characterization of Bis- and Tris(closo-1,2-C2B10H11-1-yl)-Substituted Biphenyl and Benzene. Inorg. Chem. 1996, 35, 3056–3058. [Google Scholar] [CrossRef]
Sample Availability: Samples of the o-carboranyl compounds (CB1, CB2, CB1B, and CB2B) are available from the authors. |



| Compound | λabs1/nm(ε × 10−3 M−1 cm−1) | λex/nm | λem/nm | Φem3 | ||||
| 298 K 1 | 77 K 1 | Film 2 | 298 K 1 | Film 2 | ||||
| CB1 | 286 (8.8) | 286 | − 4 | 445 | − 4 | <0.01 | <0.01 | |
| CB1B | 283 (12.4), 343 (8.8) | 337 | 451 | 384, 454 | 424 | 0.03 | 0.08 | |
| CB2 | 328 (19.6) | 328 | 552 | 487 | 510 | 0.13 | 0.25 | |
| CB2B | 314 (21.4), 391 (7.0) | 391 | 505 | 456 | 473 | 0.06 | 0.09 | |
| Compound | τ/ns | kr5/× 108s−1 | knr6/× 108s−1 | |||||
| 298 K 1 | Film 2 | 298 K 1 | Film 2 | 298 K 1 | Film 2 | |||
| CB1 | − 4 | − 4 | - | - | - | - | ||
| CB1B | 5.5 | 5.0 | 0.05 | 0.16 | 1.76 | 1.84 | ||
| CB2 | 5.9 | 0.24 | 0.22 | 10.4 | 1.47 | 31.3 | ||
| CB2B | 1.2 | 0.83 | 0.50 | 1.1 | 7.8 | 11.0 | ||

| CB1 | CB1B | CB2 | CB2B | |||||
|---|---|---|---|---|---|---|---|---|
| S0 | S1 | S0 | S1 | S0 | S1 | S0 | S1 | |
| C−C | 1.72 | 2.39 | 1.73 | 2.42 | 1.72 | 2.38 | 1.74 | 2.40 |
| Ψcalc | 22.2 | 0.3 | - | - | 0.8 | 1.2 | - | - |
| Ψexp1 | - | - | 2.9 | - | ||||
| λcalc/nm | fcalc | Assignment | |
|---|---|---|---|
| CB1 | 468.98 | 0.0282 | HOMO → LUMO (99.6%) |
| CB1B | 466.86 360.99 | 0.2415 0.2817 | HOMO → LUMO (99.6%) HOMO → LUMO+1 (74.8%) |
| CB2 | 515.13 | 0.0137 | HOMO → LUMO (99.7%) |
| CB2B | 522.90 433.83 | 0.1849 0.1971 | HOMO → LUMO (99.8%) HOMO → LUMO+1 (70.2%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.; Kim, S.; Bae, H.J.; Lee, J.H.; Hwang, H.; Park, M.H.; Lee, K.M. Effect of Planarity of Aromatic Rings Appended to o-Carborane on Photophysical Properties: A Series of o-Carboranyl Compounds Based on 2-Phenylpyridine- and 2-(Benzo[b]thiophen-2-yl)pyridine. Molecules 2019, 24, 201. https://doi.org/10.3390/molecules24010201
Jin H, Kim S, Bae HJ, Lee JH, Hwang H, Park MH, Lee KM. Effect of Planarity of Aromatic Rings Appended to o-Carborane on Photophysical Properties: A Series of o-Carboranyl Compounds Based on 2-Phenylpyridine- and 2-(Benzo[b]thiophen-2-yl)pyridine. Molecules. 2019; 24(1):201. https://doi.org/10.3390/molecules24010201
Chicago/Turabian StyleJin, Hyomin, Seonah Kim, Hye Jin Bae, Ji Hye Lee, Hyonseok Hwang, Myung Hwan Park, and Kang Mun Lee. 2019. "Effect of Planarity of Aromatic Rings Appended to o-Carborane on Photophysical Properties: A Series of o-Carboranyl Compounds Based on 2-Phenylpyridine- and 2-(Benzo[b]thiophen-2-yl)pyridine" Molecules 24, no. 1: 201. https://doi.org/10.3390/molecules24010201
APA StyleJin, H., Kim, S., Bae, H. J., Lee, J. H., Hwang, H., Park, M. H., & Lee, K. M. (2019). Effect of Planarity of Aromatic Rings Appended to o-Carborane on Photophysical Properties: A Series of o-Carboranyl Compounds Based on 2-Phenylpyridine- and 2-(Benzo[b]thiophen-2-yl)pyridine. Molecules, 24(1), 201. https://doi.org/10.3390/molecules24010201





