Reactive Cobalt–Oxo Complexes of Tetrapyrrolic Macrocycles and N-based Ligand in Oxidative Transformation Reactions
Abstract
:1. Introduction
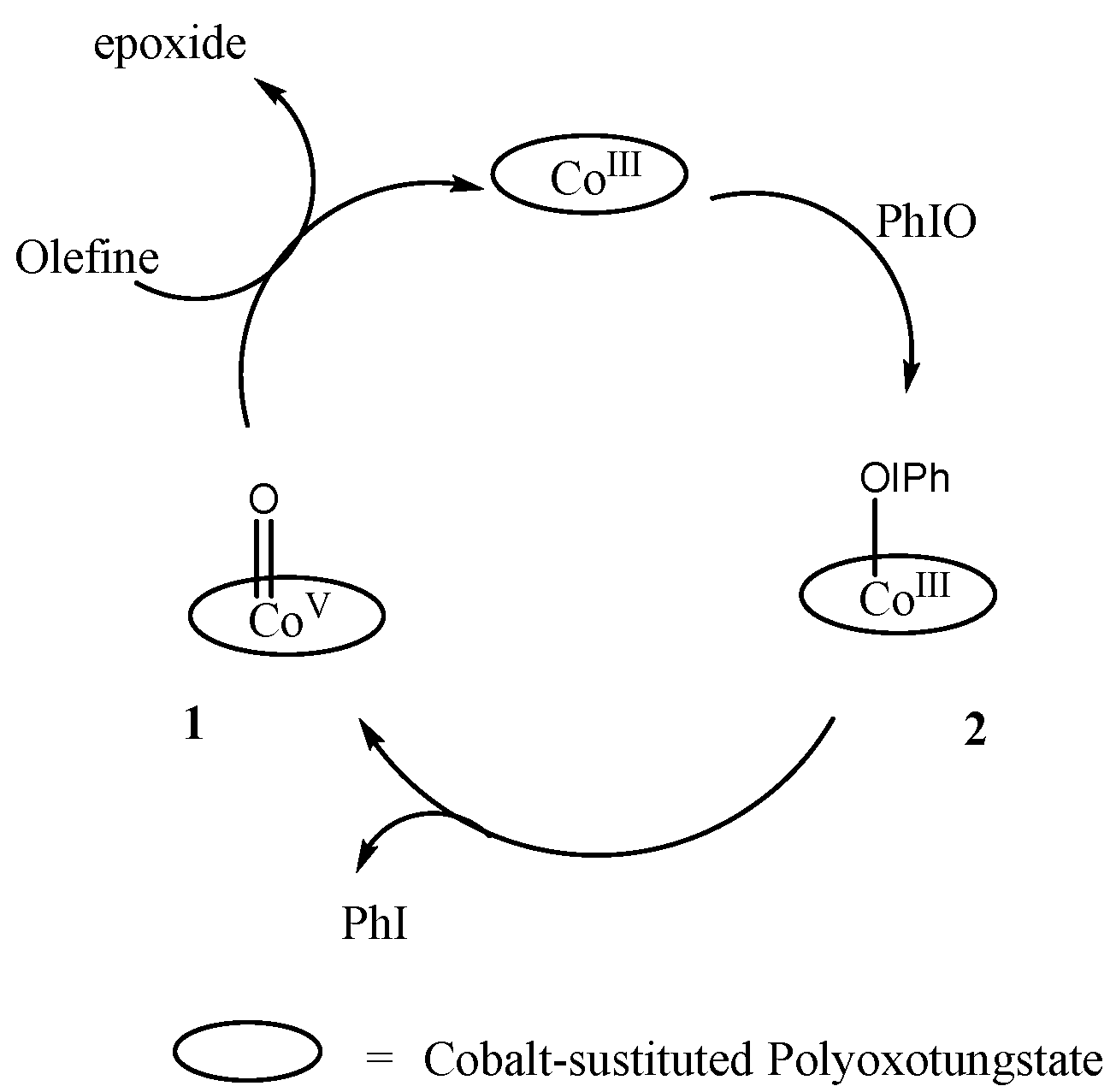
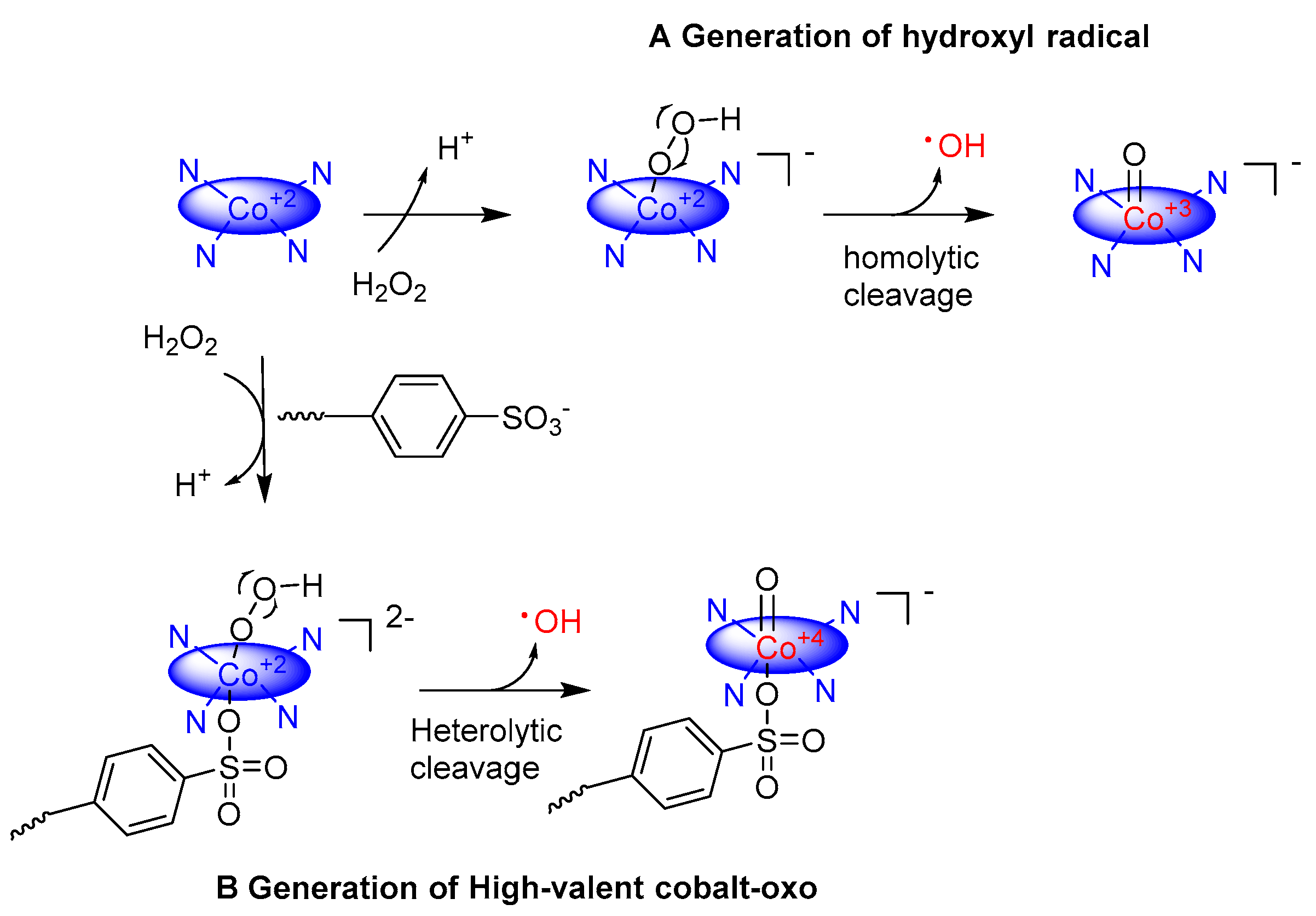
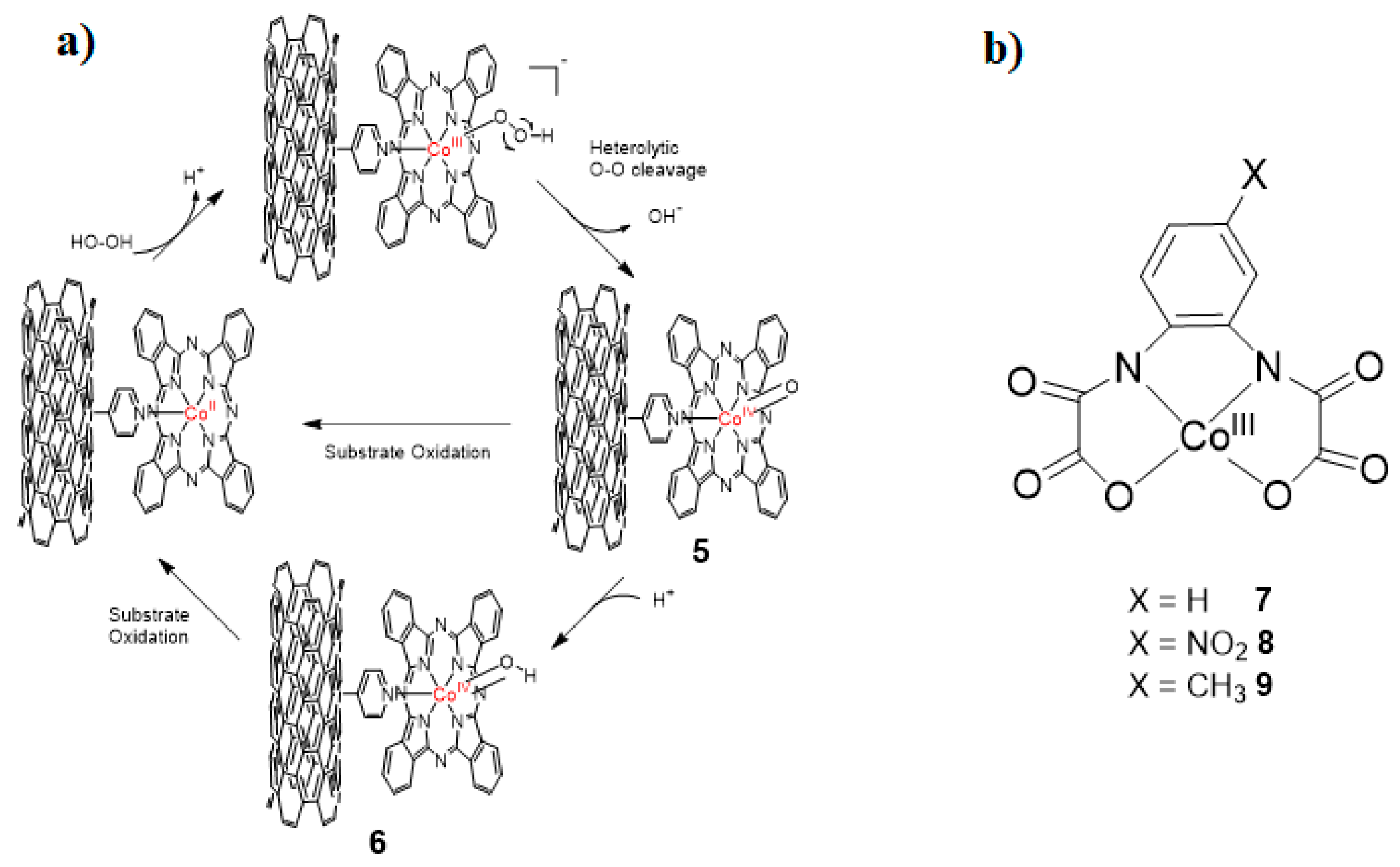
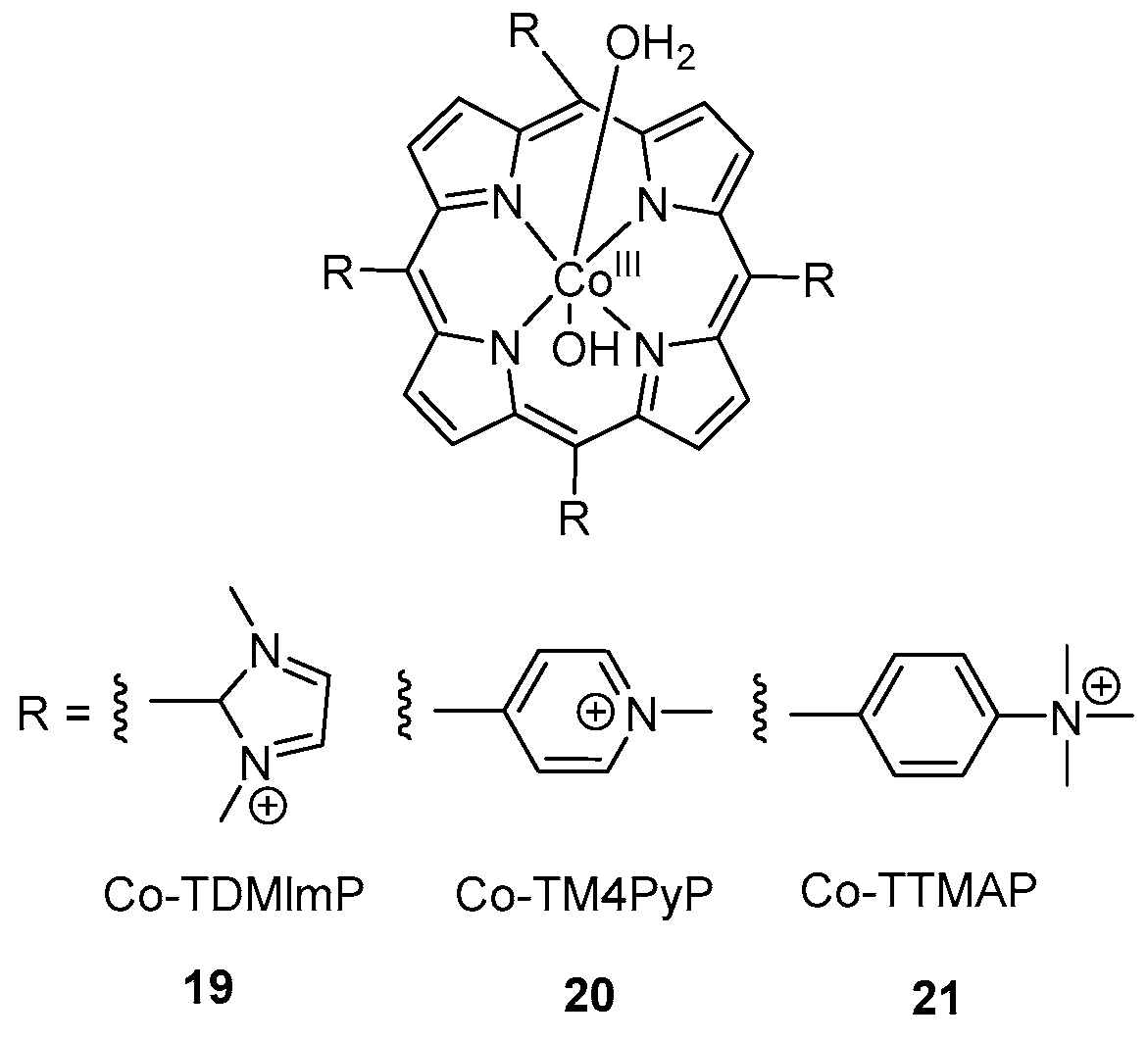
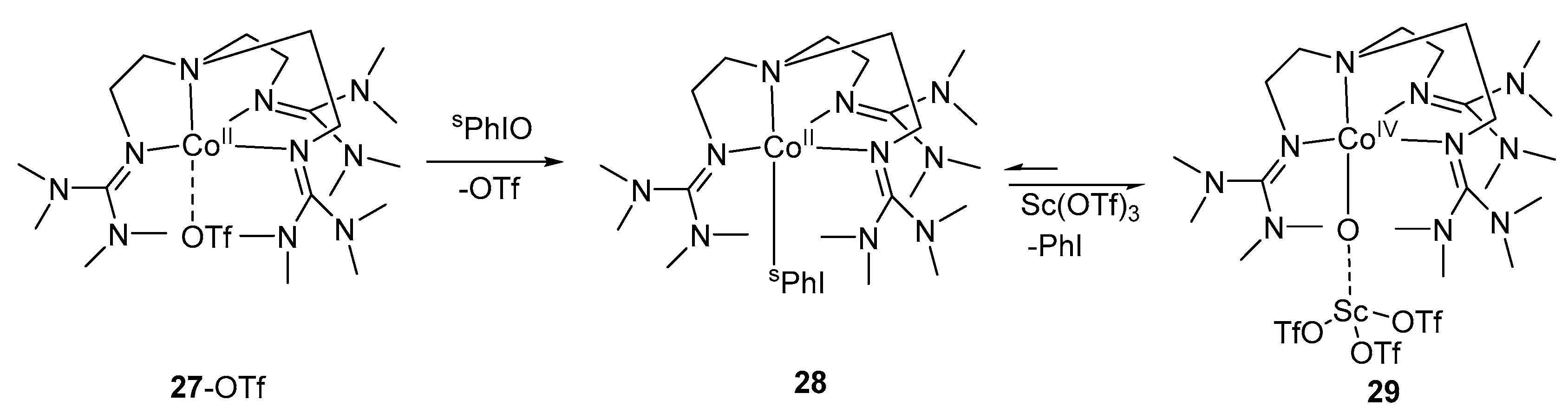
2. Cobalt–Oxo Species Involved in Oxidation of Organic Substrates
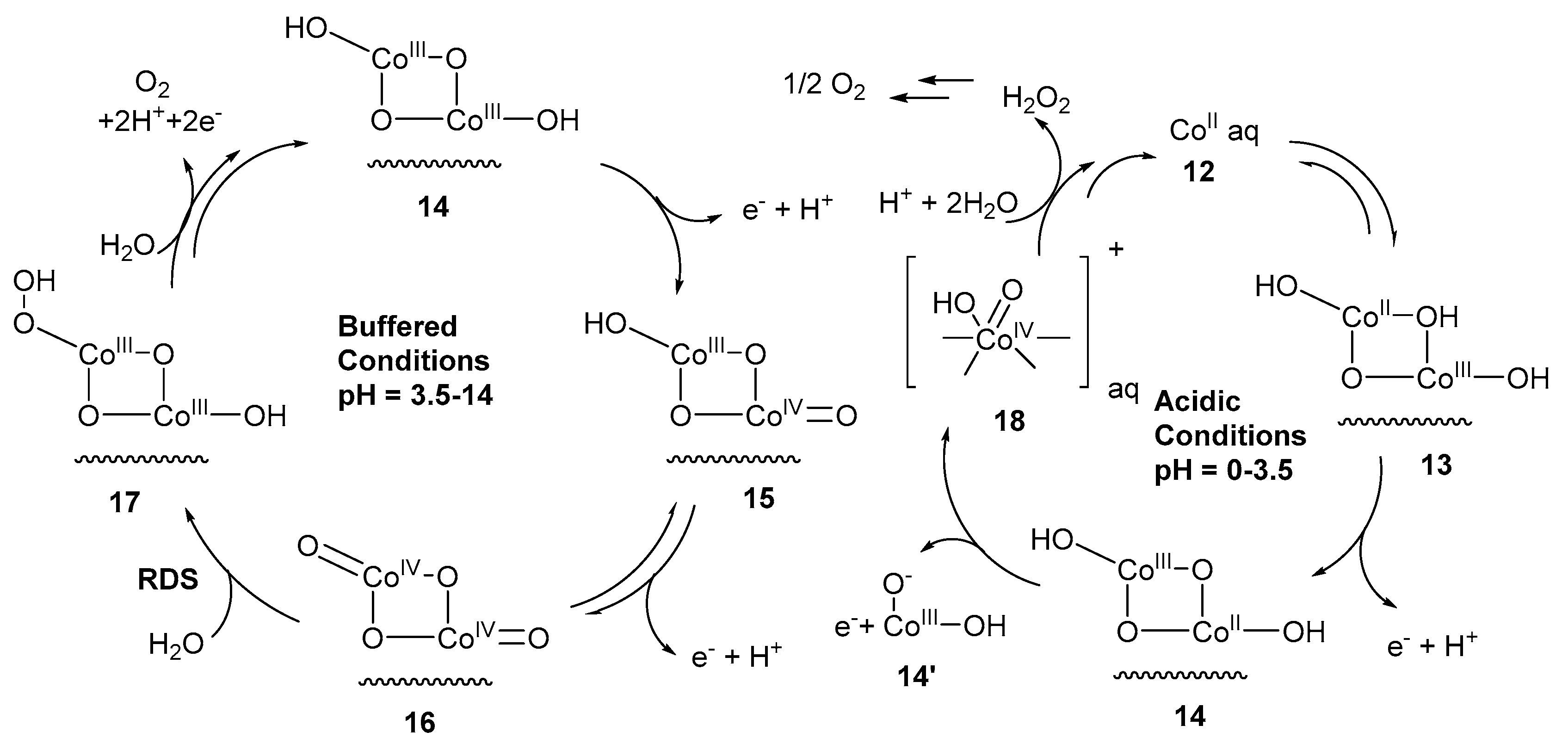
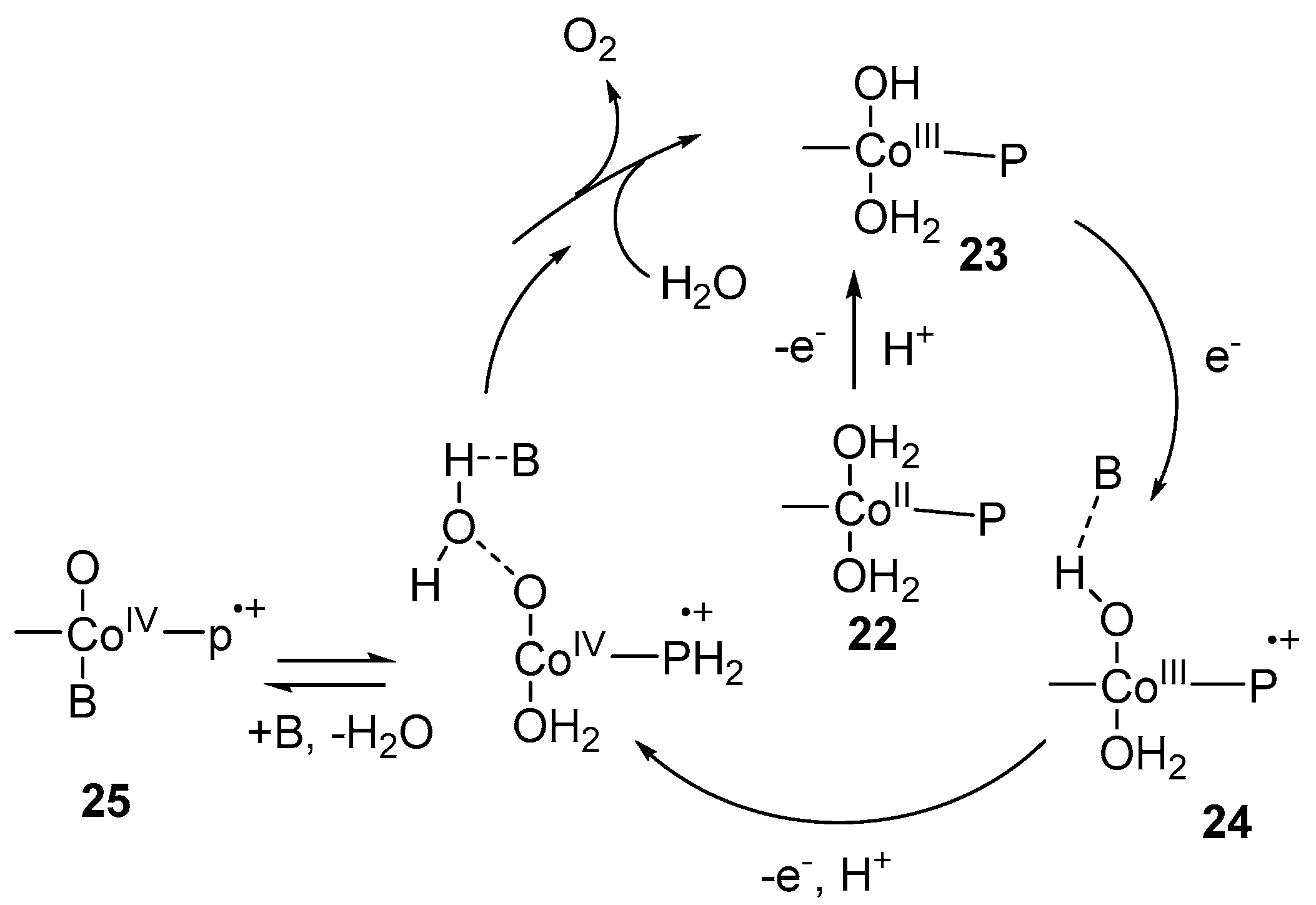
3. Cobalt–Oxo Species Involved in Water Oxidation Reaction
4. Preparation of Cobalt–Oxo Complexes
5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TPFPP | Meso-tetrakis(pentafluorophenyl)porphinato dianion |
| CoTAPc | cobalt tetraaminophthalocyanine |
| EPTAC | 2,3-epoxypropyl triethylammonium chloride |
| Pc | Phthalocyanine |
| TAPc | Tetraaminophthalocyanine |
| MWNCTs | Multiwall carbon nanotubes |
| DMPO | 5,5-Dimethyl-1-pyrroline N-oxide |
| F0C-Co | Co(III) complex of 5,10,15-triphenylcorrole |
| F5C-Co | Co(III) complex of 5,15-bis(phenyl)-10-(pentafluorophenyl)corrole |
| F10C-Co | Co(III) complex of 5,15-bis(pentafluorophenyl)-10-phenylcorrole |
| F15C-Co | Co(III) complex of 5,10,15-tris(pentafluorophenyl)corrole |
| TMG3tren | (tris[2-(N-tetramethylguanidyl)ethyl]amine) |
| sPhIO | 2-(tert-butylsulfonyl)iodosylbenzene |
| TMAL | Tetraamido macrocyclic ligand |
| 13-TMC | 1,4,7,10-tetramethyl-1,4,7,10-tetraazacyclotridecane |
| Co-TDMImP | Co(III) complex of 5,10,15,20-tetrakis(1,3-dimethylimidazolium-2-yl)porphyrin |
| Co-TM4PyP | Co(III) complex of 5,10,15,20-tetrakis(N-methylpyridinium-4-yl)porphyrin |
| Co-TTMAP | Co(III) complex of 5,10,15,20-tetrakis(N,N,N-trimethylanilinium-4-yl)porphyrin |
References
- Sono, M.; Roach, M.P.; Coulter, E.D.; Dawson, J.H. Heme-containing oxygenases. Chem. Rev. 1996, 96, 2841–2888. [Google Scholar] [CrossRef] [PubMed]
- Meunier, B.; de Visser, S.P.; Shaik, S. Mechanism of oxidation reactions catalyzed by cytochrome p450 enzymes. Chem. Rev. 2004, 104, 3947–3980. [Google Scholar] [CrossRef] [PubMed]
- Van Eldik, R. Fascinating inorganic/bioinorganic reaction mechanisms. Coord. Chem. Rev. 2007, 251, 1649–1662. [Google Scholar] [CrossRef]
- Costas, M.; Mehn, M.P.; Jensen, M.P.; Que, L. Dioxygen activation at mononuclear nonheme iron active sites: Enzymes, models, and intermediates. Chem. Rev. 2004, 104, 939–986. [Google Scholar] [CrossRef] [PubMed]
- Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Mullins, C.S.; Pecoraro, V.L. Reflections on small molecule manganese models that seek to mimic photosynthetic water oxidation chemistry. Coord. Chem. Rev. 2008, 252, 416–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEvoy, J.P.; Brudvig, G.W. Water-splitting chemistry of photosystem II. Chem. Rev. 2006, 106, 4455–4483. [Google Scholar] [CrossRef]
- Rebelo, L.S.; Silva, M.A.; Medforth, J.C.; Freire, C. Iron(III) fluorinated porphyrins: Greener chemistry from synthesis to oxidative catalysis reactions. Molecules 2016, 21. [Google Scholar] [CrossRef]
- Pereira, F.C.; Simões, M.M.; Tomé, P.J.; Almeida Paz, A.F. Porphyrin-based metal-organic frameworks as heterogeneous catalysts in oxidation reactions. Molecules 2016, 21. [Google Scholar] [CrossRef]
- de Visser, S.P.; Rohde, J.-U.; Lee, Y.-M.; Cho, J.; Nam, W. Intrinsic properties and reactivities of mononuclear nonheme iron–oxygen complexes bearing the tetramethylcyclam ligand. Coord. Chem. Rev. 2013, 25, 381–393. [Google Scholar] [CrossRef]
- Krebs, C.; Galonić Fujimori, D.; Walsh, C.T.; Bollinger, J.M. Non-heme Fe(IV)-oxo intermediates. Acc. Chem. Res. 2007, 40, 484–492. [Google Scholar] [CrossRef]
- Abu-Omar, M.M.; Loaiza, A.; Hontzeas, N. Reaction mechanisms of mononuclear non-heme iron oxygenases. Chem. Rev. 2005, 105, 2227–2252. [Google Scholar] [CrossRef] [PubMed]
- Baglia, R.A.; Zaragoza, J.P.T.; Goldberg, D.P. Biomimetic reactivity of oxygen-derived manganese and iron porphyrinoid complexes. Chem. Rev. 2017, 117, 13320–13352. [Google Scholar] [CrossRef] [PubMed]
- Fukuzumi, S.; Mandal, S.; Mase, K.; Ohkubo, K.; Park, H.; Benet-Buchholz, J.; Nam, W.; Llobet, A. Catalytic four-electron reduction of O2 via rate-determining proton-coupled electron transfer to a dinuclear cobalt-μ-1,2-peroxo complex. J. Am. Chem. Soc. 2012, 134, 9906–9909. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, F.F.; Heims, F.; Kundu, S.; Mebs, S.; Ray, K. Spectroscopic capture and reactivity of S = 1/2 nickel(III)-oxygen intermediates in the reaction of a Ni(II)-salt with mCPBA. Chem. Commun. 2012, 48, 3730–3732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; de Respinis, M.; Frei, H. Time-resolved observations of water oxidation intermediates on a cobalt oxide nanoparticle catalyst. Nat. Chem. 2014, 6, 362–367. [Google Scholar] [CrossRef]
- Pierpont, A.W.; Cundari, T.R. Computational study of methane C-H activation by first-row late transition metal L(n)M=E (M: Fe, Co, Ni) complexes. Inorg. Chem. 2010, 49, 2038–2046. [Google Scholar] [CrossRef]
- Limberg, C. Was ist wirklich nötig, um Komplexe später Übergangsmetalle mit terminalen oxo-liganden zu stabilisieren? Angew. Chem. 2009, 121, 2305–2308. [Google Scholar] [CrossRef]
- Cox, N.; Pantazis Dimitrios, A.; Neese, F.; Lubitz, W. Artificial photosynthesis: Understanding water splitting in nature. Interface Focus 2015, 5, 20150009. [Google Scholar] [CrossRef]
- Yamada, T.; Domen, K. Development of sunlight driven water splitting devices towards future artificial photosynthetic industry. ChemEngineering 2018, 2, 36. [Google Scholar] [CrossRef]
- Young, K.J.; Brennan, B.J.; Tagore, R.; Brudvig, G.W. Photosynthetic water oxidation: Insights from manganese model chemistry. Acc. Chem. Res. 2015, 48, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Tan, J.M.; Besson, C.; Geletii, Y.V.; Musaev, D.G.; Kuznetsov, A.E.; Luo, Z.; Hardcastle, K.I.; Hill, C.L. A fast soluble carbon-free molecular water oxidation catalyst based on abundant metals. Science 2010, 328, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Bozoglian, F.; Mandal, S.; Stewart, B.; Privalov, T.; Llobet, A.; Sun, L. A molecular ruthenium catalyst with water-oxidation activity comparable to that of photosystem II. Nat. Chem. 2012, 4, 418. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.D.L.; Prévot, M.S.; Fagan, R.D.; Zhang, Z.; Sedach, P.A.; Siu, M.K.J.; Trudel, S.; Berlinguette, C.P. Photochemical route for accessing amorphous metal oxide materials for water oxidation catalysis. Science 2013, 340, 60–63. [Google Scholar] [CrossRef]
- Kanan, M.W.; Nocera, D.G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 2008, 321, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Frei, H. Nanostructured cobalt oxide clusters in mesoporous silica as efficient oxygen-evolving catalysts. Angew. Chem. Int. Ed. 2009, 48, 1841–1844. [Google Scholar] [CrossRef]
- Esswein, A.J.; McMurdo, M.J.; Ross, P.N.; Bell, A.T.; Tilley, T.D. Size-dependent activity of Co3O4 nanoparticle anodes for alkaline water electrolysis. J. Phys. Chem. C 2009, 113, 15068–15072. [Google Scholar] [CrossRef]
- Hans Wedepohl, K. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Das, D.; Pattanayak, S.; Singh, K.K.; Garai, B.; Sen Gupta, S. Electrocatalytic water oxidation by a molecular cobalt complex through a high valent cobalt oxo intermediate. Chem. Commun. 2016, 52, 11787–11790. [Google Scholar] [CrossRef] [PubMed]
- Wasylenko, D.J.; Palmer, R.D.; Schott, E.; Berlinguette, C.P. Interrogation of electrocatalytic water oxidation mediated by a cobalt complex. Chem. Commun. 2012, 48, 2107–2109. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, Y.; Li, N.; Lu, W.; Chen, W. Efficient oxidative removal of organic pollutants by ordered mesoporous carbon-supported cobalt phthalocyanine. J. Nanomater. 2016, 2016, 27. [Google Scholar] [CrossRef]
- Li, N.; Lu, W.; Pei, K.; Yao, Y.; Chen, W. Ordered-mesoporous-carbon-bonded cobalt phthalocyanine: A bioinspired catalytic system for controllable hydrogen peroxide activation. ACS Appl. Mater. Interfaces 2014, 6, 5869–5876. [Google Scholar] [CrossRef] [PubMed]
- McAlpin, J.G.; Surendranath, Y.; Dincǎ, M.; Stich, T.A.; Stoian, S.A.; Casey, W.H.; Nocera, D.G.; Britt, R.D. EPR evidence for Co(IV) species produced during water oxidation at neutral pH. J. Am. Chem. Soc. 2010, 132, 6882–6883. [Google Scholar] [CrossRef] [PubMed]
- Hadt, R.G.; Hayes, D.; Brodsky, C.N.; Ullman, A.M.; Casa, D.M.; Upton, M.H.; Nocera, D.G.; Chen, L.X. X-ray spectroscopic characterization of Co(IV) and metal–metal interactions in Co4O4: Electronic structure contributions to the formation of high-valent states relevant to the oxygen evolution reaction. J. Am. Chem. Soc. 2016, 138, 11017–11030. [Google Scholar] [CrossRef] [PubMed]
- Retegan, M.; Krewald, V.; Mamedov, F.; Neese, F.; Lubitz, W.; Cox, N.; Pantazis, D.A. A five-coordinate Mn(IV) intermediate in biological water oxidation: Spectroscopic signature and a pivot mechanism for water binding. Chem. Sci. 2016, 7, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Singh, K.K.; Panda, C.; Weitz, A.; Hendrich, M.P.; Collins, T.J.; Dhar, B.B.; Sen Gupta, S. Formation of a room temperature stable FeV(O) complex: Reactivity toward unactivated C–H bonds. J. Am. Chem. Soc. 2014, 136, 9524–9527. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.J. TAML oxidant activators: A new approach to the activation of hydrogen peroxide for environmentally significant problems. Acc. Chem. Res. 2002, 35, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Que Jr, L.; Tolman, W.B. Biologically inspired oxidation catalysis. Nature 2008, 455, 333–340. [Google Scholar]
- Nam, W.; Yang, S.J.; Kim, H. Catalytic oxygenation of alkenes and alkanes by oxygen donors catalyzed by cobalt-substituted polyoxotungstate. Bull. Korean Chem. Soc. 1996, 17, 625–630. [Google Scholar] [CrossRef]
- Tang, H.; Shen, C.; Lin, M.; Sen, A. Cobalt porphyrin-catalyzed alkane oxidations using dioxygen as oxidant. Inorg. Chim. Acta 2000, 300–302, 1109–1111. [Google Scholar] [CrossRef]
- Sun, C.; Hu, B.; Liu, Z. Rapid aerobic oxidation of alcohols to carbonyl compounds with dioxygen using metallodeuteroporphyrin dimethyl esters as catalysts in the presence of isobutylaldehyde. Heteroat. Chem 2012, 23, 295–303. [Google Scholar] [CrossRef]
- Nguyen, A.I.; Hadt, R.G.; Solomon, E.I.; Tilley, T.D. Efficient C–H bond activations via O2 cleavage by a dianionic cobalt(II) complex. Chem. Sci. 2014, 5, 2874–2878. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.-Y.; Tian, P.; Sun, F.A.; He, M.-Y.; Chen, Q. Highly efficient transformation of alcohol to carbonyl compounds under a hybrid bifunctional catalyst originated from metalloporphyrins and hydrotalcite. J. Catal. 2016, 335, 105–116. [Google Scholar] [CrossRef]
- Zhou, X.; Ji, H. Cobalt porphyrin immobilized on montmorillonite: A highly efficient and reusable catalyst for aerobic oxidation of alcohols to carbonyl compounds. Chin. J. Catal. 2012, 33, 1906–1912. [Google Scholar] [CrossRef]
- Nam, W.; Kim, I.; Kim, Y.; Kim, C. Biomimetic alkane hydroxylation by cobalt(III) porphyrin complex and m-chloroperbenzoic acid. Chem. Commun. 2001, 0, 1262–1263. [Google Scholar] [CrossRef]
- Song, Y.J.; Hyun, M.Y.; Lee, J.H.; Lee, H.G.; Kim, J.H.; Jang, S.P.; Noh, J.Y.; Kim, Y.; Kim, S.-J.; Lee, S.J.; et al. Amide-based nonheme cobalt(III) olefin epoxidation catalyst: Partition of multiple active oxidants CoV=O, CoIV=O, and CoIII‒OO(O)CR. Chem. A Eur. J. 2012, 18, 6094–6101. [Google Scholar] [CrossRef] [PubMed]
- Nesterova, O.V.; Kopylovich, M.N.; Nesterov, D.S. Stereoselective oxidation of alkanes with m-CPBA as an oxidant and cobalt complex with isoindole-based ligands as catalysts. RSC Adv. 2016, 6, 93756–93767. [Google Scholar] [CrossRef]
- Nurdin, L.; Spasyuk, D.M.; Fairburn, L.; Piers, W.E.; Maron, L. Oxygen–oxygen bond cleavage and formation in Co(II)-mediated stoichiometric O2 reduction via the potential intermediacy of a Co(IV) oxyl radical. J. Am. Chem. Soc. 2018, 140, 16094–16105. [Google Scholar] [CrossRef]
- Ghosh, A.; Mitchell, D.A.; Chanda, A.; Ryabov, A.D.; Popescu, D.L.; Upham, E.C.; Collins, G.J.; Collins, T.J. Catalase–peroxidase activity of iron(III)−TAML activators of hydrogen peroxide. J. Am. Chem. Soc. 2008, 130, 15116–15126. [Google Scholar] [CrossRef]
- Zhang, R.; Horner, J.H.; Newcomb, M. Laser flash photolysis generation and kinetic studies of porphyrin-manganese-oxo intermediates. Rate constants for oxidations effected by porphyrin-MnV-Oxo species and apparent disproportionation equilibrium constants for porphyrin-MnIV-oxo species. J. Am. Chem. Soc. 2005, 127, 6573–6582. [Google Scholar] [CrossRef]
- Zhang, Z.; Hao, J.; Yang, W.; Lu, B.; Ke, X.; Zhang, B.; Tang, J. Porous Co3O4 nanorods-reduced graphene oxide with intrinsic peroxidase-like activity and catalysis in the degradation of methylene blue. ACS Appl. Mater. Interfaces 2013, 5, 3809–3815. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, J.; Stern, C.L.; Mirkin, C.A. A coordination chemistry approach to a multieffector enzyme mimic. J. Am. Chem. Soc. 2007, 129, 10074–10075. [Google Scholar] [CrossRef] [PubMed]
- Wulff, G. Enzyme-like catalysis by molecularly imprinted polymers. Chem. Rev. 2002, 102, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lu, W.; Pei, K.; Yao, Y.; Chen, W. Formation of high-valent cobalt-oxo phthalocyanine species in a cellulose matrix for eliminating organic pollutants. Appl. Catal. B 2015, 163, 105–112. [Google Scholar] [CrossRef]
- Afanasiev, P.; Kudrik, E.V.; Albrieux, F.; Briois, V.; Koifman, O.I.; Sorokin, A.B. Generation and characterization of high-valent iron oxo phthalocyanines. Chem. Commun. 2012, 48, 6088–6090. [Google Scholar] [CrossRef] [PubMed]
- Ramdhanie, B.; Telser, J.; Caneschi, A.; Zakharov, L.N.; Rheingold, A.L.; Goldberg, D.P. An example of O2 binding in a Cobalt(II) corrole system and high-valent cobalt–cyano and cobalt–alkynyl complexes. J. Am. Chem. Soc. 2004, 126, 2515–2525. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yao, Y.; Lu, J.; Chen, C.; Lu, W.; Huang, S.; Chen, W. The consortium of heterogeneous cobalt phthalocyanine catalyst and bicarbonate ion as a novel platform for contaminants elimination based on peroxymonosulfate activation. J. Hazard. Mater. 2016, 301, 214–221. [Google Scholar] [CrossRef]
- Li, N.; Lu, W.; Pei, K.; Chen, W. Interfacial peroxidase-like catalytic activity of surface-immobilized cobalt phthalocyanine on multiwall carbon nanotubes. RSC Adv. 2015, 5, 9374–9380. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Wu, C.; Lu, W.; Pei, K.; Chen, W. Bioinspired catalytic generation of high-valent cobalt–oxo species by the axially coordinated CoPc on pyridine-functionalized MWCNTs for the elimination of organic contaminants. Appl. Surf. Sci. 2018, 434, 1112–1121. [Google Scholar] [CrossRef]
- Fernández, I.; Pedro, J.R.; Roselló, A.L.; Ruiz, R.; Castro, I.; Ottenwaelder, X.; Journaux, Y. Alcohol oxidation by dioxygen and aldehydes catalysed by square-planar cobalt(III) complexes of disubstituted oxamides and related ligands. Eur. J. Org. Chem. 2001, 2001, 1235–1247. [Google Scholar]
- Li, N.; Zheng, Y.; Jiang, X.; Zhang, R.; Pei, K.; Chen, W. Carbon-based oxamate cobalt(III) complexes as bioenzyme mimics for contaminant elimination in high backgrounds of complicated constituents. Materials 2017, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zheng, Y.; Jiang, X.; Zhang, R.; Chen, W. Generation of reactive cobalt oxo oxamate radical species for biomimetic oxidation of contaminants. RSC Adv. 2017, 7, 42875–42883. [Google Scholar] [CrossRef] [Green Version]
- Winkler, J.R.; Gray, H.B. Electronic Structures of Oxo-Metal Ions. In Molecular Electronic Structures of Transition Metal Complexes I; Mingos, D.M.P., Day, P., Dahl, J.P., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg,Germany, 2012; pp. 17–28. [Google Scholar]
- Huang, L.-T.; Ali, A.; Wang, H.-H.; Cheng, F.; Liu, H.-Y. Catalytic oxidation of alkene by cobalt corroles. J. Mol. Catal. A: Chem. 2017, 426, 213–222. [Google Scholar] [CrossRef]
- Betley, T.A.; Wu, Q.; Van Voorhis, T.; Nocera, D.G. Electronic design criteria for O–O bond formation via metal–oxo complexes. Inorg. Chem. 2008, 47, 1849–1861. [Google Scholar] [CrossRef] [PubMed]
- Sala, X.; Romero, I.; Rodríguez, M.; Escriche, L.; Llobet, A. Molecular catalysts that oxidize water to dioxygen. Angew. Chem. Int. Ed. 2009, 48, 2842–2852. [Google Scholar] [CrossRef]
- Romain, S.; Vigara, L.; Llobet, A. Oxygen–oxygen bond formation pathways promoted by ruthenium complexes. Acc. Chem. Res. 2009, 42, 1944–1953. [Google Scholar] [CrossRef] [PubMed]
- Cape, J.L.; Hurst, J.K. Detection and mechanistic relevance of transient ligand radicals formed during [Ru(bpy)2(OH2)]2O4+-catalyzed water oxidation. J. Am. Chem. Soc. 2008, 130, 827–829. [Google Scholar] [CrossRef]
- Niishiro, R.; Takano, Y.; Jia, Q.; Yamaguchi, M.; Iwase, A.; Kuang, Y.; Minegishi, T.; Yamada, T.; Domen, K.; Kudo, A. A CoOx-modified SnNb2O6 photoelectrode for highly efficient oxygen evolution from water. Chem. Commun. 2017, 53, 629–632. [Google Scholar] [CrossRef]
- Zhong, M.; Hisatomi, T.; Kuang, Y.; Zhao, J.; Liu, M.; Iwase, A.; Jia, Q.; Nishiyama, H.; Minegishi, T.; Nakabayashi, M.; et al. Surface modification of CoOx loaded BiVO4 photoanodes with ultrathin p-type NiO layers for improved solar water oxidation. J. Am. Chem. Soc. 2015, 137, 5053–5060. [Google Scholar] [CrossRef]
- Hisatomi, T.; Katayama, C.; Moriya, Y.; Minegishi, T.; Katayama, M.; Nishiyama, H.; Yamada, T.; Domen, K. Photocatalytic oxygen evolution using BaNbO2N modified with cobalt oxide under photoexcitation up to 740 nm. Energy Environ. Sci. 2013, 6, 3595–3599. [Google Scholar] [CrossRef]
- Zecchina, A.; Spoto, G.; Coluccia, S. Surface dioxygen adducts on MgO-CoO solid solutions: Analogy with cobalt-based homogeneous oxygen carriers. J. Mol. Catal. 1982, 14, 351–355. [Google Scholar] [CrossRef]
- Barraclough, C.G.; Lawrance, G.A.; Lay, P.A. Characterization of binuclear. mu.-peroxo and. mu.-superoxo cobalt(III) amine complexes from Raman spectroscopy. Inorg. Chem. 1978, 17, 3317–3322. [Google Scholar] [CrossRef]
- Gerken, J.B.; McAlpin, J.G.; Chen, J.Y.C.; Rigsby, M.L.; Casey, W.H.; Britt, R.D.; Stahl, S.S. Electrochemical water oxidation with cobalt-based electrocatalysts from pH 0–14: The thermodynamic basis for catalyst structure, stability, and activity. J. Am. Chem. Soc. 2011, 133, 14431–14442. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Fukuda, K.; Osada, M.; Nakai, I.; Izumi, F.; Dilanian, R.A.; Kato, K.; Takata, M.; Sakurai, H.; Takayama-Muromachi, E.; et al. Chemical composition and crystal structure of superconducting sodium cobalt oxide bilayer-hydrate. J. Mater. Chem. 2004, 14, 1448–1453. [Google Scholar] [CrossRef]
- Amatucci, G.G.; Tarascon, J.M.; Klein, L.C. CoO2, the end member of the Li x CoO2 solid solution. J. Electrochem. Soc. 1996, 143, 1114–1123. [Google Scholar] [CrossRef]
- Motohashi, T.; Katsumata, Y.; Ono, T.; Kanno, R.; Karppinen, M.; Yamauchi, H. Synthesis and properties of CoO2, the x = 0 end member of the LixCoO2 and NaxCoO2 systems. Chem. Mater. 2007, 19, 5063–5066. [Google Scholar] [CrossRef]
- Brunschwig, B.S.; Chou, M.H.; Creutz, C.; Ghosh, P.; Sutin, N. Mechanisms of water oxidation to oxygen: Cobalt(IV) as an intermediate in the aquocobalt(II)-catalyzed reaction. J. Am. Chem. Soc. 1983, 105, 4832–4833. [Google Scholar] [CrossRef]
- Risch, M.; Ringleb, F.; Kohlhoff, M.; Bogdanoff, P.; Chernev, P.; Zaharieva, I.; Dau, H. Water oxidation by amorphous cobalt-based oxides: In situ tracking of redox transitions and mode of catalysis. Energy Environ. Sci. 2015, 8, 661–674. [Google Scholar] [CrossRef]
- Koroidov, S.; Anderlund, M.F.; Styring, S.; Thapper, A.; Messinger, J. First turnover analysis of water-oxidation catalyzed by Co-oxide nanoparticles. Energy Environ. Sci. 2015, 8, 2492–2503. [Google Scholar] [CrossRef] [Green Version]
- Wasylenko, D.J.; Palmer, R.D.; Berlinguette, C.P. Homogeneous water oxidation catalysts containing a single metal site. Chem. Commun. 2013, 49, 218–227. [Google Scholar] [CrossRef]
- Fillol, J.L.; Codolà, Z.; Garcia-Bosch, I.; Gómez, L.; Pla, J.J.; Costas, M. Efficient water oxidation catalysts based on readily available iron coordination complexes. Nat. Chem. 2011, 3, 807. [Google Scholar] [CrossRef] [PubMed]
- Ellis, W.C.; McDaniel, N.D.; Bernhard, S.; Collins, T.J. Fast water oxidation using iron. J. Am. Chem. Soc. 2010, 132, 10990–10991. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, J.J.; Jurss, J.W.; Brennaman, M.K.; Hoertz, P.G.; Patrocinio, A.O.T.; Murakami Iha, N.Y.; Templeton, J.L.; Meyer, T.J. Making oxygen with ruthenium complexes. Acc. Chem. Res. 2009, 42, 1954–1965. [Google Scholar] [CrossRef] [PubMed]
- Hetterscheid, D.G.H.; Reek, J.N.H. Mononuclear water oxidation catalysts. Angew. Chem. Int. Ed. 2012, 51, 9740–9747. [Google Scholar] [CrossRef] [PubMed]
- Sartorel, A.; Bonchio, M.; Campagna, S.; Scandola, F. Tetrametallic molecular catalysts for photochemical water oxidation. Chem. Soc. Rev. 2013, 42, 2262–2280. [Google Scholar] [CrossRef] [PubMed]
- Codolà, Z.; Garcia-Bosch, I.; Acuña-Parés, F.; Prat, I.; Luis, J.M.; Costas, M.; Lloret-Fillol, J. Electronic effects on single-site iron catalysts for water oxidation. Chem. A Eur. J. 2013, 19, 8042–8047. [Google Scholar] [CrossRef] [PubMed]
- Wasylenko, D.J.; Ganesamoorthy, C.; Koivisto, B.D.; Henderson, M.A.; Berlinguette, C.P. Insight into water oxidation by mononuclear polypyridyl Ru catalysts. Inorg. Chem. 2010, 49, 2202–2209. [Google Scholar] [CrossRef] [PubMed]
- Wasylenko, D.J.; Ganesamoorthy, C.; Borau-Garcia, J.; Berlinguette, C.P. Electrochemical evidence for catalyticwater oxidation mediated by a high-valent cobalt complex. Chem. Commun. 2011, 47, 4249–4251. [Google Scholar] [CrossRef]
- Crandell, D.W.; Ghosh, S.; Berlinguette, C.P.; Baik, M.H. How a [Co(IV) a bond and a half O](2+) fragment oxidizes water: Involvement of a biradicaloid [Co(II)-(⋅O⋅)](2+) species in forming the O–O bond. ChemSusChem 2015, 8, 844–852. [Google Scholar] [CrossRef]
- Nakazono, T.; Parent, A.R.; Sakai, K. Cobalt porphyrins as homogeneous catalysts for water oxidation. Chem. Commun. 2013, 49, 6325–6327. [Google Scholar] [CrossRef]
- Wang, D.; Groves, J.T. Efficient water oxidation catalyzed by homogeneous cationic cobalt porphyrins with critical roles for the buffer base. Proc. Natl. Acad. Sci. USA 2013, 110, 15579–15584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzolato, E.; Natali, M.; Posocco, B.; Montellano López, A.; Bazzan, I.; Di Valentin, M.; Galloni, P.; Conte, V.; Bonchio, M.; Scandola, F.; Sartorel, A. Light driven water oxidation by a single site cobalt salophen catalyst. Chem. Commun. 2013, 49, 9941–9943. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, C.N.; Hadt, R.G.; Hayes, D.; Reinhart, B.J.; Li, N.; Chen, L.X.; Nocera, D.G. In situ characterization of cofacial Co(IV) centers in Co4O4 cubane: Modeling the high-valent active site in oxygen-evolving catalysts. Proc. Natl. Acad. Sci. USA 2017, 114, 3855–3860. [Google Scholar] [CrossRef] [PubMed]
- Symes, M.D.; Surendranath, Y.; Lutterman, D.A.; Nocera, D.G. Bidirectional and unidirectional PCET in a molecular model of a cobalt-based oxygen-evolving catalyst. J. Am. Chem. Soc. 2011, 133, 5174–5177. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.I.; Ziegler, M.S.; Oña-Burgos, P.; Sturzbecher-Hohne, M.; Kim, W.; Bellone, D.E.; Tilley, T.D. Mechanistic investigations of water oxidation by a molecular cobalt oxide analogue: Evidence for a highly oxidized intermediate and exclusive terminal oxo participation. J. Am. Chem. Soc. 2015, 137, 12865–12872. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.I.; Wang, J.; Levine, D.S.; Ziegler, M.S.; Tilley, T.D. Synthetic control and empirical prediction of redox potentials for Co4O4 cubanes over a 1.4 V range: Implications for catalyst design and evaluation of high-valent intermediates in water oxidation. Chem. Sci. 2017, 8, 4274–4284. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-P.; Van Voorhis, T. Direct-coupling O2 bond forming a pathway in cobalt oxide water oxidation catalysts. J. Phys. Chem. Lett. 2011, 2, 2200–2204. [Google Scholar] [CrossRef]
- Fernando, A.; Aikens, C.M. Reaction pathways for water oxidation to molecular oxygen mediated by model cobalt oxide dimer and cubane catalysts. J. Phys. Chem. C 2015, 119, 11072–11085. [Google Scholar] [CrossRef]
- Li, X.; Siegbahn, P.E.M. Water oxidation mechanism for synthetic Co–Oxides with small nuclearity. J. Am. Chem. Soc. 2013, 135, 13804–13813. [Google Scholar] [CrossRef]
- Mattioli, G.; Giannozzi, P.; Amore Bonapasta, A.; Guidoni, L. Reaction pathways for oxygen evolution promoted by cobalt catalyst. J. Am. Chem. Soc. 2013, 135, 15353–15363. [Google Scholar] [CrossRef]
- Xu, L.; Lei, H.; Zhang, Z.; Yao, Z.; Li, J.; Yu, Z.; Cao, R. The effect of the trans axial ligand of cobalt corroles on water oxidation activity in neutral aqueous solutions. Phys. Chem. Chem. Phys. 2017, 19, 9755–9761. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, F.F.; Kundu, S.; Risch, M.; Pandian, S.; Heims, F.; Pryjomska-Ray, I.; Haack, P.; Metzinger, R.; Bill, E.; Dau, H.; et al. An oxocobalt(IV) complex stabilized by lewis acid interactions with scandium(III) ions. Angew. Chem. Int. Ed. 2010, 50, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Pfaff, F.F.; Kwon, E.; Wang, Y.; Seo, M.-S.; Bill, E.; Ray, K.; Nam, W. Spectroscopic capture and reactivity of a low-spin cobalt(IV)–oxo complex stabilized by binding redox-inactive metal ions. Angew. Chem. 2014, 126, 10571–10575. [Google Scholar] [CrossRef]
- Wang, B.; Lee, Y.-M.; Tcho, W.-Y.; Tussupbayev, S.; Kim, S.-T.; Kim, Y.; Seo, M.S.; Cho, K.-B.; Dede, Y.; Keegan, B.C.; et al. Synthesis and reactivity of a mononuclear non-haem cobalt(IV)–oxo complex. Nat. Commun. 2017, 8, 14839. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Y.; Yam, F.; Xie, Y.-T.; Li, X.-Y.; Chang, C.K. A bulky bis-pocket manganese(V)−oxo corrole complex: Observation of oxygen atom transfer between triply bonded MnV≡O and alkene. J. Am. Chem. Soc. 2009, 131, 12890–12891. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; So, H.; Yoon, H.; Cho, K.-B.; Lee, Y.-M.; Fukuzumi, S.; Nam, W. Reactivity comparison of high-valent iron(IV)–oxo complexes bearing N-tetramethylated cyclam ligands with different ring size. Dalton Trans. 2013, 42, 7842–7845. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.K.; Hill, E.A.; Filatov, A.S.; Anderson, J.S. Isolation of a terminal Co(III)–oxo complex. J. Am. Chem. Soc. 2018, 140, 13176–13180. [Google Scholar] [CrossRef] [PubMed]



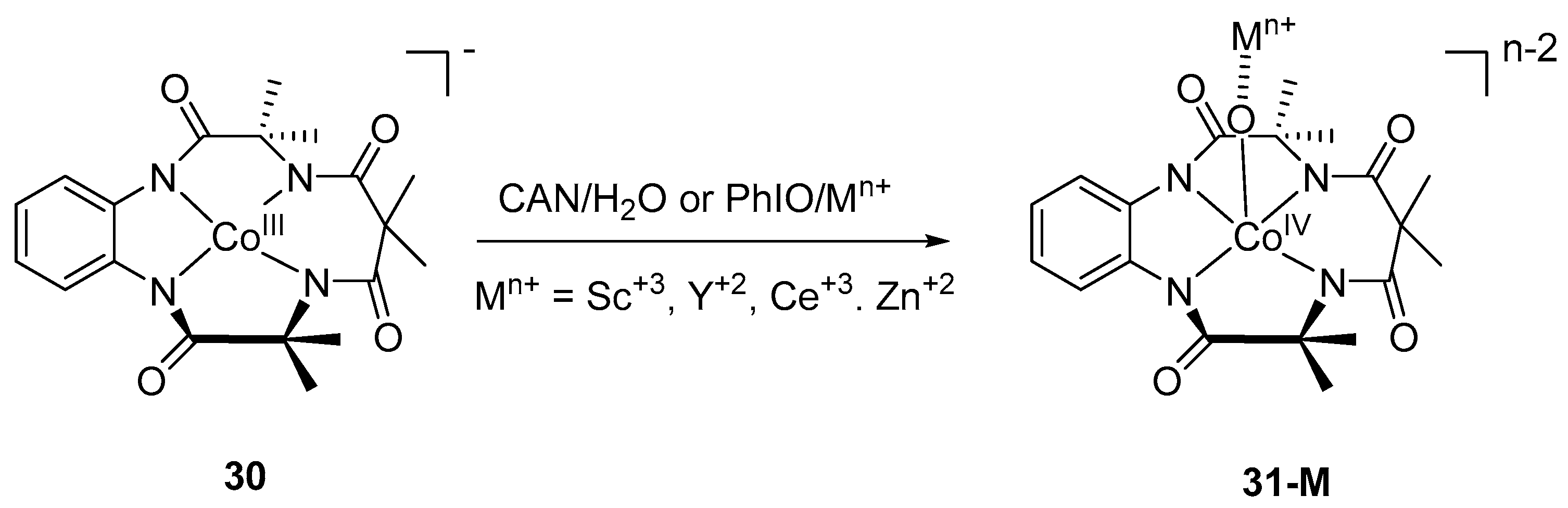
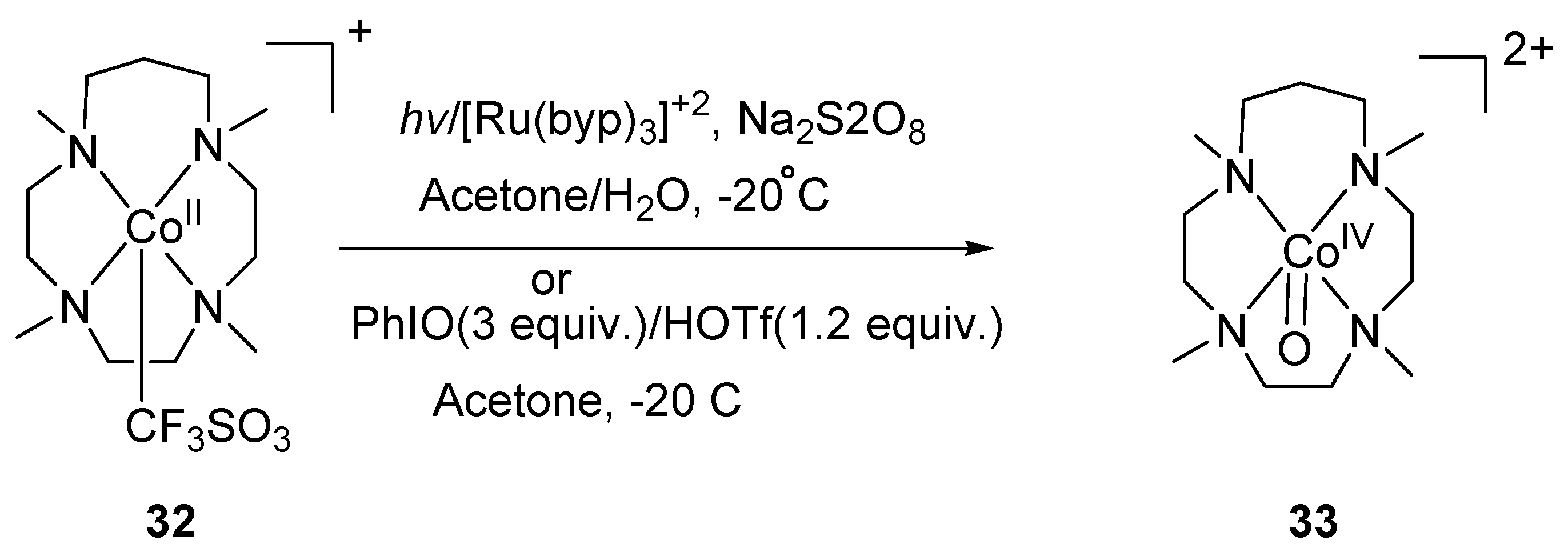
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Akram, W.; Liu, H.-Y. Reactive Cobalt–Oxo Complexes of Tetrapyrrolic Macrocycles and N-based Ligand in Oxidative Transformation Reactions. Molecules 2019, 24, 78. https://doi.org/10.3390/molecules24010078
Ali A, Akram W, Liu H-Y. Reactive Cobalt–Oxo Complexes of Tetrapyrrolic Macrocycles and N-based Ligand in Oxidative Transformation Reactions. Molecules. 2019; 24(1):78. https://doi.org/10.3390/molecules24010078
Chicago/Turabian StyleAli, Atif, Waseem Akram, and Hai-Yang Liu. 2019. "Reactive Cobalt–Oxo Complexes of Tetrapyrrolic Macrocycles and N-based Ligand in Oxidative Transformation Reactions" Molecules 24, no. 1: 78. https://doi.org/10.3390/molecules24010078
APA StyleAli, A., Akram, W., & Liu, H.-Y. (2019). Reactive Cobalt–Oxo Complexes of Tetrapyrrolic Macrocycles and N-based Ligand in Oxidative Transformation Reactions. Molecules, 24(1), 78. https://doi.org/10.3390/molecules24010078





