Cucurbits Plants: A Key Emphasis to Its Pharmacological Potential
Abstract
:1. Introduction
2. Cucurbita Plants: A Brief Overview to Its Ethnopharmacological Uses
3. Cucurbita Plants Phytochemical Composition
4. Looking at Cucurbita Plants Biological Activity
4.1. Antimicrobial Activity of Cucurbita Plants
4.1.1. In Vitro Studies
4.1.2. In Vivo Studies
4.2. Anticancer Activities of Cucurbita Plants
In Vitro Anticancer/Antitumor Effects
5. Clinical Effectiveness of Cucurbita Plants in Humans
5.1. Control of Blood Glucose Level in Diabetic Patients
5.2. Pharmacotherapeutic Effects in Low Urinary Tract Diseases
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marie-Magdeleine, C.; Hoste, H.; Mahieu, M.; Varo, H.; Archimede, H. In vitro effects of Cucurbita moschata seed extracts on Haemonchus contortus. Vet. Parasitol. 2009, 161, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.M.; Yang, S.T. A preliminary study on the cultivating technique of Cucurbita pepo cv Dayangua. Spec. Econ. Amin. Plant 2000, 3, 28–34. [Google Scholar]
- Adnan, M.; Gul, S.; Batool, S.; Fatima, B.; Rehman, A.; Yaqoob, S.; Shabir, H.; Yousaf, T.; Mussarat, S.; Ali, N.; et al. A review on the ethnobotany, phytochemistry, pharmacology and nutritional composition of Cucurbita pepo L. J. Phytopharm. 2017, 6, 133–139. [Google Scholar]
- Andolfo, G.; Di Donato, A.; Darrudi, R.; Errico, A.; Aiese Cigliano, R.; Ercolano, M.R. Draft of Zucchini (Cucurbita pepo L.) Proteome: A Resource for Genetic and Genomic Studies. Front. Genet. 2017, 8, 181. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.D. Overview on Cucurbita maxima. Int. J. Phytopharm. 2012, 2, 68–71. [Google Scholar] [CrossRef]
- Paris, H.S. Historical records, origins, and development of the edible cultivar groups of Cucurbita pepo (Cucurbitaceae). Econ. Bot. 1989, 43, 423–443. [Google Scholar] [CrossRef]
- Ratnam, N. A review on Cucurbita pepo. Int. J. Pharm. Phytochem. Res. 2017, 9, 1190–1194. [Google Scholar] [CrossRef]
- Salehi, B.; Valussi, M.; Jugran, A.K.; Martorell, M.; Ramírez-Alarcón, K.; Stojanović-Radić, Z.Z.; Antolak, H.; Kręgiel, D.; Mileski, K.S.; Sharifi-Rad, M.; et al. Nepeta species: From farm to food applications and phytotherapy. Trends Food Sci. Technol. 2018, 80, 104–122. [Google Scholar] [CrossRef]
- Mishra, A.P.; Sharifi-Rad, M.; Shariati, M.A.; Mabkhot, Y.N.; Al-Showiman, S.S.; Rauf, A.; Salehi, B.; Župunski, M.; Sharifi-Rad, M.; Gusain, P.; et al. Bioactive compounds and health benefits of edible Rumex species-A review. Cell. Mol. Biol. 2018, 64, 27–34. [Google Scholar] [CrossRef]
- Fapohunda, S.; Adewumi, A.; Jegede, D. Cucurbitaceae - the family that nourishes and heals. MicroMedicine 2018, 6, 85–93. [Google Scholar]
- Phillips, K.M.; Ruggio, D.M.; Ashraf-Khorassani, M. Phytosterol composition of nuts and seeds commonly consumed in the United States. J. Agric. Food Chem. 2005, 53, 9436–9445. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.; Galvin, K.; O’Connor, T.P.; Maguire, A.R.; O’Brien, N.M. Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Foods Hum. Nutr. 2007, 62, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Applequist, W.L.; Avula, B.; Schaneberg, B.T.; Wang, Y.H.; Khan, I.A. Comparative fatty acid content of seeds of four Cucurbita species grown in a common (shared) garden. J. Food Compos. Anal. 2006, 19, 606–611. [Google Scholar] [CrossRef]
- Sabudak, T. Fatty acid composition of seed and leaf oils of pumpkin, walnut, almond, maize, sunflower and melon. Chem. Nat. Compd. 2007, 43, 465–467. [Google Scholar] [CrossRef]
- Stevenson, D.G.; Eller, F.J.; Wang, L.; Jane, J.L.; Wang, T.; Inglett, G.E. Oil and tocopherol content and composition of pumpkin seed oil in 12 cultivars. J. Agric. Food Chem. 2007, 55, 4005–4013. [Google Scholar] [CrossRef] [PubMed]
- Glew, R.H.; Glew, R.S.; Chuang, L.T.; Huang, Y.S.; Millson, M.; Constans, D.; Vanderjagt, D.J. Amino acid, mineral and fatty acid content of pumpkin seeds (Cucurbita spp) and Cyperus esculentus nuts in the Republic of Niger. Plant Foods Hum. Nutr. 2006, 61, 49–54. [Google Scholar] [CrossRef]
- Talukdar, S.N.; Hossain, M.N. Phytochemical, Phytotherapeutical and Pharmacological Study of Momordica dioica. Evid.-Based Complement. Altern. Med. 2014, 2014, 806082. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Eswaran, M.B.; Ojha, S.K.; Rao, C.V.; Rawat, A.K.S. Antiulcer activity of hydroalcohol extract of Momordica dioica roxb. Fruit. Indian J. Pharm. Sci. 2011, 73, 572–577. [Google Scholar] [CrossRef]
- Chandrasekar, B.; Mukherjee, B.; Mukherjee, S.K. Blood sugar lowering potentiality of selected Cucurbitaceae plants of Indian origin. Indian J. Med Res. 1989, 90, 300–305. [Google Scholar] [PubMed]
- Huseini, H.F.; Darvishzadeh, F.; Heshmat, R.; Jafariazar, Z.; Raza, M.; Larijani, B. The clinical investigation of Citrullus colocynthis (L.) schrad fruit in treatment of type II diabetic patients: A randomized, double blind, placebo-controlled clinical trial. Phytother. Res. 2009, 23, 1186–1189. [Google Scholar] [CrossRef]
- Rashidi, A.A.; Mirhashemi, S.M.; Taghizadeh, M.; Sarkhail, P. Iranian medicinal plants for diabetes mellitus: A systematic review. Pak. J. Biol. Sci. 2013, 16, 401–411. [Google Scholar]
- Jain, A.; Singhai, A.K. Effect of Momordica dioica Roxb on gentamicin model of acute renal failure. Nat. Prod. Res. 2010, 24, 1379–1389. [Google Scholar] [CrossRef]
- Menendez-Baceta, G.; Aceituno-Mata, L.; Molina, M.; Reyes-García, V.; Tardío, J.; Pardo-De-Santayana, M. Medicinal plants traditionally used in the northwest of the Basque Country (Biscay and Alava), Iberian Peninsula. J. Ethnopharmacol. 2014, 152, 113–134. [Google Scholar] [CrossRef]
- Peter, E.L.; Rumisha, S.F.; Mashoto, K.O.; Malebo, H.M. Ethno-medicinal knowledge and plants traditionally used to treat anemia in Tanzania: A cross sectional survey. J. Ethnopharmacol. 2014, 154, 767–773. [Google Scholar] [CrossRef]
- Kujawska, M.; Pieroni, A. Plants used as food and medicine by polish migrants in Misiones, Argentina. Ecol. Food Nutr. 2015, 54, 255–279. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Morvin Yabesh, J.E.; Prabhu, S.; Manikandan, R.; Muralidharan, B. Quantitative ethnomedicinal study of plants used in the Nelliyampathy hills of Kerala, India. J. Ethnopharmacol. 2015, 161, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Mootoosamy, A.; Wambugu, S. Traditional therapies used to manage diabetes and related complications in Mauritius: A comparative ethnoreligious study. Evid.-Based Complement. Altern. Med. 2016, 2016, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Motti, R.; Motti, P. An ethnobotanical survey of useful plants in the agro Nocerino Sarnese (Campania, southern Italy). Hum. Ecol. 2017, 45, 865–878. [Google Scholar] [CrossRef]
- Nayak, D.; Ashe, S.; Rauta, P.R.; Nayak, B. Assessment of antioxidant, antimicrobial and anti-osteosarcoma potential of four traditionally used Indian medicinal plants. J. Appl. Biomed. 2017, 15, 119–132. [Google Scholar] [CrossRef]
- Ramzan, S.; Soelberg, J.; Jäger, A.K.; Cantarero-Arévalo, L. Traditional medicine among people of Pakistani descent in the capital region of Copenhagen. J. Ethnopharmacol. 2017, 196, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Agyare, C.; Spiegler, V.; Asase, A.; Scholz, M.; Hempel, G.; Hensel, A. An ethnopharmacological survey of medicinal plants traditionally used for cancer treatment in the Ashanti region, Ghana. J. Ethnopharmacol. 2018, 212, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Balcha, A. Medicinal plants used in traditional medicine by Oromo people, Ghimbi District, Southwest Ethiopia. J. Ethnobiol. Ethnomed. 2014, 10, 1–15. [Google Scholar]
- Alonso-Castro, A.J.; Dominguez, F.; Zapata-Morales, J.R.; Carranza-Alvarez, C. Plants used in the traditional medicine of Mesoamerica (Mexico and Central America) and the Caribbean for the treatment of obesity. J. Ethnopharmacol. 2015, 175, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Rigat, M.; Vallès, J.; Dambrosio, U.; Gras, A.; Iglésias, J.; Garnatje, T. Plants with topical uses in the Ripollès district (Pyrenees, Catalonia, Iberian Peninsula): Ethnobotanical survey and pharmacological validation in the literature. J. Ethnopharmacol. 2015, 164, 162–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asowata-Ayodele, A.M.; Afolayan, A.J.; Otunola, G.A. Ethnobotanical survey of culinary herbs and spices used in the traditional medicinal system of Nkonkobe Municipality, Eastern Cape, South Africa. South Afr. J. Bot. 2016, 104, 69–75. [Google Scholar] [CrossRef]
- Jaradat, N.A.; Shawahna, R.; Eid, A.M.; Al-Ramahi, R.; Asma, M.K.; Zaid, A.N. Herbal remedies use by breast cancer patients in the West Bank of Palestine. J. Ethnopharmacol. 2016, 178, 1–8. [Google Scholar] [CrossRef]
- Meragiaw, M.; Asfaw, Z.; Argaw, M. The Status of Ethnobotanical Knowledge of Medicinal Plants and the Impacts of Resettlement in Delanta, Northwestern Wello, Northern Ethiopia. Evid.-Based Complement. Altern. Med. 2016, 2016, 5060247. [Google Scholar] [CrossRef]
- Odoh, U.E.; Uzor, P.F.; Eze, C.L.; Akunne, T.C.; Onyegbulam, C.M.; Osadebe, P.O. Medicinal plants used by the people of Nsukka Local Government Area, south-eastern Nigeria for the treatment of malaria: An ethnobotanical survey. J. Ethnopharmacol. 2018, 218, 1–15. [Google Scholar] [CrossRef]
- Suroowan, S.; Mahomoodally, M.F. A comparative ethnopharmacological analysis of traditional medicine used against respiratory tract diseases in Mauritius. J. Ethnopharmacol. 2016, 177, 61–80. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Meineri, G.; Gai, F.; Longato, E.; Amarowicz, R. Antioxidative activities and phenolic compounds of pumpkin (Cucurbita pepo) seeds and amaranth (Amaranthus caudatus) grain extracts. Nat. Prod. Res. 2017, 31, 2178–2182. [Google Scholar] [CrossRef] [PubMed]
- Azevedo-Meleiro, C.H.; Rodriguez-Amaya, D.B. Qualitative and quantitative differences in carotenoid composition among Cucurbita moschata, Cucurbita maxima, and Cucurbita pepo. J. Agric. Food Chem. 2007, 55, 4027–4033. [Google Scholar] [CrossRef] [PubMed]
- Maria, L.; Carvalho, J.D.; Barros, P.; Luiz, R.; Godoy, D.O.; Pacheco, S.; Henrique, P.; Luiz, J.; Carvalho, V.D.; Regini, M.; et al. Total carotenoid content, α-carotene and β-carotene, of landrace pumpkins (Cucurbita moschata Duch): A preliminary study. Food Res. Int. 2012, 47, 337–340. [Google Scholar]
- Perez Gutierrez, R.M. Review of Cucurbita pepo (Pumpkin) its Phytochemistry and Pharmacology. Med. Chem. 2016, 6, 12–21. [Google Scholar] [CrossRef]
- Chandrika, U.G.; Basnayake, B.M.L.B.; Athukorala, I.; Colombagama, P.W.N.M.; Goonetilleke, A. Carotenoid Content and In Vitro Bioaccessibility of Lutein in Some Leafy Vegetables Popular in Sri Lanka. J. Nutr. Sci. Vitaminol. 2010, 56, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Mi, Y.K.; Eun, J.K.; Young-Nam, K.; Changsun, C.; Bo-Hieu, L. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutr. Res. Pract. 2012, 6, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.Y.; Lin, S.; Kuo, G. Content and distribution of flavonoids among 91 edible plant species. Asia Pac. J. Clin. Nutr. 2008, 17, 275–279. [Google Scholar]
- Sreeramulu, D.; Raghunath, M. Antioxidant activity and phenolic content of roots, tubers and vegetables commonly consumed in India. Food Res. Int. 2010, 43, 1017–1020. [Google Scholar] [CrossRef]
- Koo, M.H.; Suhaila, M. Flavonoid (Myricetin, Quercetin, Kaempferol, Luteolin and Apigenin) Content of Edible Tropical Plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar]
- Mongkolsilp, S.; Pongbupakit, I.; Sae-Lee, N.; Sitthihaworm, W.; Article, O. Radical Scavenging Activity and Total Phenolic Content of Medicinal Plants Used in Primary Health Care Savitree Mongkolsilp, Isara Pongbupakit, Nittaya Sae-Lee and Worapan Sitthithaworn. Swu J. Pharm. Sci. 2004, 9, 32–35. [Google Scholar]
- Iswaldi, I.; Gómez-Caravaca, A.M.; Lozano-Sánchez, J.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Profiling of phenolic and other polar compounds in zucchini (Cucurbita pepo L.) by reverse-phase high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Res. Int. 2013, 50, 77–84. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Van Camp, J.; Trawka, A.; Verhé, R. Phenolic compounds and some quality parameters of pumpkin seed oil. Eur. J. Lipid Sci. Technol. 2010, 112, 208–217. [Google Scholar] [CrossRef]
- Peričin, D.; Krimer, V.; Trivić, S.; Radulović, L. The distribution of phenolic acids in pumpkin’s hull-less seed, skin, oil cake meal, dehulled kernel and hull. Food Chem. 2009, 113, 450–456. [Google Scholar] [CrossRef]
- El-Kamali, H.H.; Mahjoub, S.A.T. Antibacterial activity of Francoeuria crispa, Pulicaria undulata, Ziziphus spina-christi and Cucurbita pepo against seven standard pathogenic bacteria. Ethnobot. Leafl. 2009, 13, 722–733. [Google Scholar]
- Dubey, A.; Mishra, N.; Singh, N. Antimicrobial activity of some selected vegetables. Int. J. Appl. Biol. Pharm. Technol. 2010, 1, 994–999. [Google Scholar]
- Sood, A.; Kaur, P.; Gupta, R. Phytochemical screening and antimicrobial assay of various seeds extract of Cucurbitaceae family. Int. J. Appl. Biol. Pharm. Technol. 2012, 3, 401–409. [Google Scholar]
- Grzybek, M.; Kukula-Koch, W.; Strachecka, A.; Jaworska, A.; Phiri, A.M.; Paleolog, J.; Tomczuk, K. Evaluation of anthelmintic activity and composition of pumpkin (Cucurbita pepo L.) seed extracts—in vitro and in vivo studies. Int. J. Mol. Sci. 2016, 17, 1456. [Google Scholar] [CrossRef]
- Al-Ghazal, A.T. Evaluation of Antibacterial Effect of Cucurbita pepo (Yakten) Extracts on Multi-antibiotic Resistance Bacterial Strains Isolated From Human Urinary Tract Infections. Rafidain J. Sci. 2012, 23, 1–7. [Google Scholar]
- Chonoko, U.G.; Rufai, A.B. Phytochemical screening and antibacterial activity of Curbita pepo (Pumpkin) against Staphylococcus aureus and Salmonella typhi. J. Pure Appl. Sci. 2011, 4, 145–147. [Google Scholar]
- Jasim, S.; Alwan, A.N.; Altimimi, H.W.; Kareem, K.H. Evaluation of antimicrobial activity of flavonoids extract from Cucurbita pepo leaves. Bas. J. Vet. Res. 2010, 9, 10–17. [Google Scholar]
- Noumedem, J.A.K.; Mihasan, M.; Lacmata, S.T.; Stefan, M.; Kuiate, J.R.; Kuete, V. Antibacterial activities of the methanol extracts of ten Cameroonian vegetables against Gram-negative multidrug-resistant bacteria. BMC Complement. Altern. Med. 2013, 13, 26. [Google Scholar] [CrossRef]
- Dar, A.H.; Sofi, S.A. Pumpkin the functional and therapeutic ingredient: A review. Int. J. Food Sci. Nutr. 2017, 2, 165–170. [Google Scholar]
- Abed El-Aziz, A.; Abed El-Aziz, H. Antimicrobial proteins and oil seeds from pumpkin. Nat. Sci. 2011, 9, 105–119. [Google Scholar]
- Elhadi, I.M.; Koko, S.W.; Dahab, M.M.; El Imam, J.M.; El Mageed, M.A.E. Antigiardial activity of some Cucurbita species and Lagenaria siceraria. J. For. Prod. Ind. 2013, 2, 43–47. [Google Scholar]
- Muruganantham, N.; Solomon, S.; Senthamilselvi, M.M. Anti-cancer activity of Cucumis sativus (cucumber) flowers against human liver cancer. Int. J. Pharm. Clin. Res. 2016, 8, 39–41. [Google Scholar]
- Geetha, S. Antimicrobial activity of selected vegetable peels against water borne pathogens. Int. J. Adv. Pharm. Biol. Chem. 2014, 3, 937–940. [Google Scholar]
- Kabbashi, A.S.; Koko, W.S.; Mohammed, S.E.A.; Musa, N.; Elbadri, E.; Dahab, M.M.; Mohammed, A.K. In vitro a moebicidal, antimicrobial and antioxidant activities of the plants Adansonia digitata and Cucurbit maxima. Adv. Med. Plant Res. 2014, 2, 50–57. [Google Scholar]
- Ravishankar, K.; Kiranmayi, G.V.N.; Appa Reddy, G.V.; Sowjanya, V.V.L.; Baba Sainadh, V.; Lakshmi, V.G.; Prasad, T. Preliminary phytochemical screening and In-vitro antibacterial activity of Cucurbita maxima seed extract. Int. J. Res. Pharm. Chem. 2012, 2, 86–91. [Google Scholar]
- Cassel, C.K. Policy challenges and clinical practices. Hosp. Pract. 1993, 28, 9–10. [Google Scholar] [CrossRef]
- Wang, H.X.; Ng, T.B. Isolation of cucurmoschin, a novel antifungal peptide abundant in arginine, glutamate and glycine residues from black pumpkin seeds. Peptides 2003, 24, 969–972. [Google Scholar] [CrossRef]
- Barbieri, L.; Polito, L.; Bolognesi, A.; Ciani, M.; Pelosi, E.; Farini, V.; Stirpe, F. Ribosome-inactivating proteins in edible plants and purification and characterization of a new ribosome-inactivating protein from Cucurbita moschata. Biochim. Biophys. Acta 2006, 760, 783–792. [Google Scholar] [CrossRef]
- Cheong, N.E.; Choi, Y.O.; Kim, W.Y.; Bae, I.S.; Cho, M.J.; Hwang, I.; Lee, S.Y. Purification and characterization of an antifungal PR-5 protein from pumpkin leaves. Mol. Cells 1997, 7, 214–219. [Google Scholar]
- Park, S.C.; Lee, J.R.; Kim, J.Y.; Hwang, I.; Nah, J.W.; Cheong, H.; Hahm, K.S. Pr-1, a novel antifungal protein from pumpkin rinds. Biotechnol. Lett. 2009, 32, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Karanja, J.; Mugendi, J.; Muchugi, A.; Karanja, J.K.; Mugendi, B.J.; Khamis, F.M.; Muchugi, A.N. Nutritional evaluation of some kenyan pumpkins (Cucurbita spp.). Int. J. Agric. For. 2016, 4, 195–200. [Google Scholar]
- Bardaa, S.; Ben Halima, N.; Aloui, F.; Ben Mansour, R.; Jabeur, H.; Bouaziz, M.; Sahnoun, Z. Oil from pumpkin (Cucurbita pepo L.) seeds: Evaluation of its functional properties on wound healing in rats. Lipids Health Dis. 2016, 15, 783–792. [Google Scholar] [CrossRef]
- Seo, J.S.; Burri, B.J.; Quan, Z.; Neidlinger, T.R. Extraction and chromatography of carotenoids from pumpkin. J. Chromatogr. A 2005, 1073, 371–375. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, R.M.; Rijken, P.J.; Rietveld, A.G.; van Vugt, A.C.; Dijkmans, B.A.C. Antioxidant intervention in rheumatoid arthritis: Results of an open pilot study. Clin. Rheumatol. 2008, 27, 771–775. [Google Scholar] [CrossRef]
- Dixon, W.G. Rheumatoid arthritis: Biological drugs and risk of infection. Lancet 2015, 386, 224–225. [Google Scholar] [CrossRef]
- Fokou, E.A.M. Preliminary nutritional evaluation of five species of egusi seeds in Cameroon. Afr. J. Food Agric. Nutr. Dev. 2004, 4, 1–11. [Google Scholar] [CrossRef]
- Duncan, K.L.K.; Duncan, M.D.; Alley, M.C.; Sausville, E.A. Cucurbitacin E-induced disruption of the actin and vimentin cytoskeleton in prostate carcinoma cells. Biochem. Pharmacol. 1996, 52, 1553–1560. [Google Scholar] [CrossRef]
- Fang, X.; Phoebe, C.H.; Pezzuto, J.M.; Fong, H.H.; Farnsworth, N.R.; Yellin, B.; Hecht, S.M. Plant anticancer agents, XXXIV. Cucurbitacins from Elaeocarpus dolichostylus. J. Nat. Prod. 1984, 47, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasam, B.; Seeram, N.P.; Nair, M.G. Anticancer and antiinflammatory activities of cucurbitacins from Cucurbita andreana. Cancer Lett. 2003, 189, 11–16. [Google Scholar] [CrossRef]
- Chan, K.T.; Meng, F.Y.; Li, Q.; Ho, C.Y.; Lam, T.S.; To, Y.; Toh, M. Cucurbitacin B induces apoptosis and S phase cell cycle arrest in BEL-7402 human hepatocellular carcinoma cells and is effective via oral administration. Cancer Lett. 2010, 294, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, M.; Zhang, H.; Sun, C.; Deng, Y. Inhibitory effects of cucurbitacin B on laryngeal squamous cell carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2008, 265, 1225–1232. [Google Scholar] [CrossRef]
- Wakimoto, N.; Yin, D.; O’Kelly, J.; Haritunians, T.; Karlan, B.; Said, J.; Koeffler, H.P. Cucurbitacin B has a potent antiproliferative effect on breast cancer cells in vitro and in vivo. Cancer Sci. 2008, 99, 1793–1797. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.; Sun, C.; Shan, X.; Yang, X.; Li-Ling, J.; Deng, Y. Targeted constitutive activation of signal transducer and activator of transcription 3 in human hepatocellular carcinoma cells by cucurbitacin B. Cancer Chemother. Pharmacol. 2009, 63, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.T.; Li, K.; Liu, S.L.; Chu, K.H.; Toh, M.; Xie, W.D. Cucurbitacin B inhibits STAT3 and the Raf/MEK/ERK pathway in leukemia cell line K562. Cancer Lett. 2010, 289, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.J.; Smiderle, L.A.S.; Carvalho, J.L.V.; Cardoso, F.S.N.; Koblitz, M.G.B. Assessment of carotenoids in pumpkins after different home cooking conditions. Food Sci. Technol. 2014, 34, 365–370. [Google Scholar] [CrossRef] [Green Version]
- Konoshima, T.; Takasaki, M.; Kozuka, M.; Nagao, T.; Okabe, H.; Irino, N.; Nishino, H. Inhibitory effects of cucurbitane triterpenoids on Epstein-Barr virus activation and two-stage carcinogenesis of skin tumor. Biol. Pharm. Bull. 1994, 18, 284–287. [Google Scholar] [CrossRef]
- Shokrzadeh, M.; Azadbakht, M.; Ahangar, N.; Hashemi, A.; Saravi, S. Cytotoxicity of hydro-alcoholic extracts of Cucurbita pepo and Solanum nigrum on HepG2 and CT26 cancer cell lines. Pharmacogn. Mag. 2010, 6, 176. [Google Scholar] [CrossRef]
- Wang, D.C.; Xiang, H.; Li, D.; Gao, H.; Cai, H.; Wu, L.J.; Deng, X.M. Purine-containing cucurbitane triterpenoids from Cucurbita pepo cv dayangua. Phytochemistry 2008, 69, 1434–1438. [Google Scholar] [CrossRef]
- Ren, S.; Ouyang, D.Y.; Saltis, M.; Xu, L.H.; Zha, Q.B.; Cai, J.Y.; He, X.H. Anti-proliferative effect of 23,24-dihydrocucurbitacin F on human prostate cancer cells through induction of actin aggregation and cofilin-actin rod formation. Cancer Chemother. Pharmacol. 2012, 70, 415–424. [Google Scholar] [CrossRef]
- Dakeng, S.; Duangmano, S.; Jiratchariyakul, W.; U-Pratya, Y.; Bögler, O.; Patmasiriwat, P. Inhibition of Wnt signaling by cucurbitacin B in breast cancer cells: Reduction of Wnt-associated proteins and reduced translocation of galectin-3-mediated β-catenin to the nucleus. J. Cell. Biochem. 2012, 113, 49–60. [Google Scholar] [CrossRef]
- Attard, E.; Cuschieri, A.; Scicluna-Spiteri, A.; Brincat, M.P. The effects of cucurbitacin E on two lymphocyte models. Pharm. Biol. 2004, 42, 170–175. [Google Scholar] [CrossRef]
- Alam, U.; Asghar, O.; Azmi, S.; Malik, R.A. General aspects of diabetes mellitus. Handb. Clin. Neurol. 2014, 126, 211–222. [Google Scholar] [PubMed]
- Sharifi-Rad, M.; Fokou, P.V.T.; Sharopov, F.; Martorell, M.; Ademiluyi, A.O.; Rajkovic, J.; Salehi, B.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Antiulcer agents: From plant extracts to phytochemicals in healing promotion. Molecules 2018, 23, 1751. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.P.; Saklani, S.; Salehi, B.; Parcha, V.; Sharifi-Rad, M.; Milella, L.; Iriti, M.; Sharifi-Rad, J.; Srivastava, M. Satyrium nepalense, a high altitude medicinal orchid of Indian Himalayan region: Chemical profile and biological activities of tuber extracts. Cell. Mol. Biol. 2018, 64, 35–43. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Morais-Braga, M.F.B.; Rocha, J.E.; Coutinho, H.D.M.; Salehi, B.; Tabanelli, G.; Montanari, C.; del Mar Contreras, M.; et al. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol. Res. 2018, 215, 76–88. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Tayeboon, G.S.; Niknam, F.; Sharifi-Rad, M.; Mohajeri, M.; Salehi, B.; Iriti, M.; Sharifi-Rad, M. Veronica persica Poir. extract - antibacterial, antifungal and scolicidal activities, and inhibitory potential on acetylcholinesterase, tyrosinase, lipoxygenase and xanthine oxidase. Cell. Mol. Biol. 2018, 64, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Alves Borges Leal, A.L.; et al. Salvia spp. plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Martorell, M.; Rajkovic, J.; Ademiluyi, A.O.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Phytochemicals in Helicobacter pylori infections: What are we doing now? Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Caili, F.; Huan, S.; Quanhong, L. A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods Hum. Nutr. 2006, 61, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Cetto, A.; Heinrich, M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef]
- Jia, W.; Gao, W.; Tang, L. Antidiabetic herbal drugs officially approved in China. Phytother. Res. 2003, 17, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Maiti, K.; Mukherjee, K.; Houghton, P.J. Leads from Indian medicinal plants with hypoglycemic potentials. J. Ethnopharmacol. 2006, 106, 1–28. [Google Scholar] [CrossRef]
- Acosta-Patiño, J.L.; Jiménez-Balderas, E.; Juárez-Oropeza, M.A.; Díaz-Zagoya, J.C. Hypoglycemic action of Cucurbita ficifolia on Type 2 diabetic patients with moderately high blood glucose levels. J. Ethnopharmacol. 2001, 77, 99–101. [Google Scholar] [CrossRef]
- Mahmoodpoor, A.; Medghalchi, M.; Nazemiyeh, H.; Asgharian, P.; Shadvar, K.; Hamishehkar, H. Effect of Cucurbita maxima on control of blood glucose in diabetic critically ill patients. Adv. Pharm. Bull. 2018, 8, 347–351. [Google Scholar] [CrossRef]
- Adams, G.G.; Imran, S.; Wang, S.; Mohammad, A.; Kok, S.; Gray, D.A.; Harding, S.E. The hypoglycaemic effect of pumpkins as anti-diabetic and functional medicines. Food Res. Int. 2011, 44, 862–867. [Google Scholar] [CrossRef]
- Cai, T.; Li, Q.; Yan, H.; Li, N. Study on the hypoglycemic action of pumpkin seed protein. J. Chin. Inst. Food Sci. Technol. 2003, 3, 7–11. [Google Scholar]
- Xiong, X.; Cao, J. Study of extraction and isolation of effective pumpkin polysaccharide component and its reducing glycemia function. Chin. J. Mod. Appl. Pharm. 2001, 18, 662–664. [Google Scholar]
- Gossell-Williams, M.; Davis, A.; O’Connor, N. Inhibition of testosterone-induced hyperplasia of the prostate of sprague-dawley rats by pumpkin seed oil. J. Med. Food 2006, 9, 284–286. [Google Scholar] [CrossRef]
- PDR for Herbal Medicines, 4th ed.; Thomson Healthcare: Montvale, NJ, USA, 2007.
- Hong, H.; Kim, C.S.; Maeng, S. Effects of pumpkin seed oil and saw palmetto oil in Korean men with symptomatic benign prostatic hyperplasia. Nutr. Res. Pract. 2009, 3, 323. [Google Scholar] [CrossRef] [PubMed]
- Ramak, P.; Mahboubi, M. The beneficial effects of pumpkin (Cucurbita pepo L.) seed oil for health condition of men. Food Rev. Int. 2018, 1–11. [Google Scholar] [CrossRef]
- Rezig, L.; Chouaibi, M.; Msaada, K.; Hamdi, S. Chemical composition and profile characterisation of pumpkin (Cucurbita maxima) seed oil. Ind. Crop. Prod. 2012, 37, 82–87. [Google Scholar] [CrossRef]
- Sogabe, H.; Terado, T. Open clinical study of effects of pumpkin seed extract/soybean germ extract vixture-containing processed food on nocturia. Jpn. J. Med. Pharm. Sci. 2001, 46, 727–737. [Google Scholar]
- Shim, B.; Jeong, H.; Lee, S.; Hwang, S.; Moon, B.; Storni, C. A randomized double-blind placebo-controlled clinical trial of a product containing pumpkin seed extract and soy germ extract to improve overactive bladder-related voiding dysfunction and quality of life. J. Funct. Foods 2014, 8, 111–117. [Google Scholar] [CrossRef]
- Nishimura, M.; Ohkawara, T.; Sato, H.; Takeda, H.; Nishihira, J. Pumpkin seed oil extracted from Cucurbita maxima improves urinary disorder in human overactive bladder. J. Tradit. Complement. Med. 2014, 4, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Alenazi, B.; Deeb, A.; Alrowaili, A.; Alkhaldi, A.; Alanazi, A. Does pumpkin affect glycemic control in diabetic patient. Case report and literature review. Eur. J. Pharm. Med. Res. 2017, 4, 42–45. [Google Scholar]
- Jain, A.; Mishra, M.; Yadav, D.; Khatarker, D.; Jadaun, P.; Tiwari, A.; Prasad, G. Evaluation of the antihyperglycemic, antilipidemic and antioxidant potential of Cucurbita ficifolia in human type 2 diabetes. Prog. Nutr. 2018, 20, 191–198. [Google Scholar]
- Shi, Y.; Xiong, X.; Cao, J.; Kang, M. Effect of pumpkin polysaccharide granules on glycemic control in type 2 diabetes. Cent. South Pharm. 2003, 1, 275–276. [Google Scholar]
- Cândido, F.G.; de Oliveira, F.C.E.; Lima, M.F.C.; Pinto, C.A.; da Silva, L.L.; Martino, H.S.D.; Alfenas, R.C.G. Addition of pooled pumpkin seed to mixed meals reduced postprandial glycemia: A randomized placebo-controlled clinical trial. Nutr. Res. 2018, 56, 90–97. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Zhang, Y.; Jin, H.; Zhu, L.; Li, J.; Yao, H. Effects of polysaccharide from pumpkin on biochemical indicator and pancreatic tissue of the diabetic rabbits. Int. J. Biol. Macromol. 2013, 62, 574–581. [Google Scholar] [CrossRef]
- Li, Q.; Fu, C.; Rui, Y.; Hu, G.; Cai, T. Effects of protein-bound polysaccharide isolated from pumpkin on insulin in diabetic rats. Plant Foods Hum. Nutr. 2005, 60, 13–16. [Google Scholar]
- Allkanjari, O.; Vitalone, A. What do we know about phytotherapy of benign prostatic hyperplasia? Life Sci. 2015, 126, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Schiebel-Schlosser, G.; Friederich, M. Phytotherapy of BPH with pumpkin seeds-a multicenter clinical trial. Phytotherapy 1998, 19, 71–76. [Google Scholar]
- Coulson, S.; Rao, A.; Beck, S.L.; Steels, E.; Gramotnev, H.; Vitetta, L. A phase II randomised double-blind placebo-controlled clinical trial investigating the efficacy and safety of ProstateEZE Max: A herbal medicine preparation for the management of symptoms of benign prostatic hypertrophy. Complementary Ther. Med. 2013, 21, 172–179. [Google Scholar] [CrossRef]
- Assessment Report on Cucurbita pepo L. Semen; European Medicines Agency: Amsterdam, The Netherlands, 2013.
- Gažová, A.; Valášková, S.; Žufková, V.; Castejon, A.M.; Kyselovič, J. Clinical study of effectiveness and safety of CELcomplex®containing Cucurbita pepo seed extract and flax and casuarina on stress urinary incontinence in women. J. Tradit. Complement. Med. 2018, 9, 138–142. [Google Scholar] [CrossRef]

| Scientific Name (Common Name) | Location | Local Name | Parts Used | Administration | Disease(s) Treatment | References |
|---|---|---|---|---|---|---|
| Cucurbita maxima Duchesne (Squash) | Basque Country, Iberian Peninsula | Kalabazea | Seeds | Oral | Digestive (Intestinal worms, Constipation) | [23] |
| Mkuranga District, Tanzania | Maboga | Leaves | Oral | Anemia | [24] | |
| Polish people in Misiones, Argentina | Zapallo | Seeds | Oral | Intestinal parasites | [25] | |
| Nelliyampathy hills of Kerala, India | Parangi | Seeds | Oral | Vomiting blood, Blood bile | [26] | |
| Fruits | Oral | Urinal disorders | ||||
| Mauritius | Giromon | Flowers | Dermal | Cataract | [27] | |
| Seeds | Oral | Renal failure | ||||
| Fruits | Dermal | Wound | ||||
| Agro Nocerino Sarnese, Campania, Southern Italy | Cocozza | Seeds | Oral | Prostatitis | [28] | |
| India | UNSP | Flowers | UNSP | Osteosarcoma | [29] | |
| Pakistani descent in Copenhagen, Denmark | Kadoo | Fruits | Oral | Blood pressure, constipation | [30] | |
| Ashanti region, Ghana | UNSP | Leaves | Oral | Cancer (lung, head) | [31] | |
| Cucurbita pepo L. (Pumpkin) | Ghimbi District, Southwest Ethiopia | Buqqee | Seeds | Oral | Gonorrhea | [32] |
| Mexico, Central America, Caribbean | Calabaza | Whole plant | Oral | Obesity | [33] | |
| Ripollès district, Pyrenees, Catalonia, Iberian Peninsula | Carbassa | Flowers | Dermal | Acne, Dermatitis, Ecchymosis, Fever, Toxicity, Wound | [34] | |
| Fruits | Dermal | Infection | ||||
| Nkonkobe Municipality, Eastern Cape, South Africa | Imithwane | Leaves | Oral | Arthritis, Blood booster | [35] | |
| West Bank, Palestine | Kare’a | Seeds | Oral | Breast cancer | [36] | |
| Delanta, Northwestern Wello, Northern Ethiopia | UNSP | Fruits | Oral | Gastritis, Stomachache | [37] | |
| Leaves | Dermal | Dandruff | ||||
| Local Government Area, south-eastern Nigeria | Okeugu | Leaves | Oral | Malaria | [38] | |
| Cucurbita galeottii Cogn. (Pumpkin) | Mauritius | Giraumon | Seeds | Oral | Mucous discharge | [39] |
| Compound Name | Synonym(s) | Empirical Formula | Structure | References |
|---|---|---|---|---|
| Protocatechuic acid | 3,4-Dihydroxybenzoic acid | C7H6O4 |  | [52] http://phenol-explorer.eu/compounds/412 |
| p-Hydroxybenzoic acid | 4-Hydroxybenzoic acid | C7H6O3 |  | [52] http://phenol-explorer.eu/compounds/418 |
| p-Hydroxybenzaldehyde | 4-Hydroxybenzaldehyde | C7H6O2 |  | [52] http://phenol-explorer.eu/compounds/725 |
| Vanillic acid | 4-Hydroxy-3-methoxybenzoic acid; p-Vanillic acid | C8H8O4 |  | [52] http://phenol-explorer.eu/compounds/414 |
| Caffeic acid | 3,4-Dihydroxycinnamic acid | C9H8O4 |  | [52] http://phenol-explorer.eu/compounds/457 |
| Syringic acid | 3,5-Dimethoxy-4-hydroxybenzoic acid | C9H10O5 |  | [52] http://phenol-explorer.eu/metabolites/420 |
| trans-p-coumaric acid | trans-4-Hydroxycinnamic acid | C9H8O3 |  | [52] http://phenol-explorer.eu/compounds/454 |
| Ferulic acid | 3-Methoxy-4-Hydroxycinnamic acid; 3-Methylcaffeic acid; Coniferic acid | C10H10O4 | 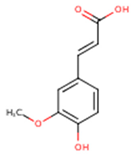 | [52] http://phenol-explorer.eu/compounds/459 |
| trans-sinapic acid | trans-4-Hydroxy-3,5-dimethoxy-cinnamic acid; trans-Sinapinic acid | C11H12O5 |  | [52] http://phenol-explorer.eu/compounds/464 |
| Tyrosol | p-HPEA; 4-(2-Hydroxyethyl)phenol; 2-(4-Hydroxyphenyl)ethanol; 2,4-Hydroxyphenyl-ethyl-alcohol; 4-Hydroxyphenylethanol | C8H10O2 |  | [52] http://phenol-explorer.eu/compounds/673 |
| Vanillin | 4-Hydroxy-3-methoxy-benzoic aldehyde; Methylprotocatechuic aldehyde; Vanillic aldehyde; p-Vanillin | C8H8O3 |  | [52] http://phenol-explorer.eu/compounds/724 |
| Luteolin | 5,7,3′,4′-Tetrahydroxyflavone | C15H10O6 | 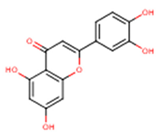 | [52] http://phenol-explorer.eu/compounds/229 |
| Kaempferol | 3,5,7,4′-Tetrahydroxyflavone | C15H10O6 |  | [52] http://phenol-explorer.eu/compounds/290 |
| Cucurbita spp./Plant Part | Extract | Microbial | References |
|---|---|---|---|
| Cucurbita pepo L. fruits | Water | Escherichia coli | [53] |
| Cucurbita pepo L. fruits | Methanol | Bacillus cereus Bacillus subtilis Escherichia coli Enterobacter aerogenes Enterobacter agglomerans Salmonella enteritidis Salmonella choleraesuis Staphylococcus aureus Pseudomonas aeruginosa Enterobacter faecalis Klebsiella pneumoniae Bacillus sphericus Bacillus thruengenesis Cryptococcus meningitis Penicillium chrysogenum | [54] |
| Cucurbita pepo L. | Phosphate buffered saline (PBS) | Serratia marcescens Escherichia coli Streptococcus thermophilous Fusarium oxysporium Trichoderma reesei Aspergillus niger | [55] |
| Cucurbita pepo L. fruits | Ethanol extract | Heligmosoides bakeri (worm) | [56] |
| Cucurbita pepo L. cortex | Water, methanol | Staphylococcus aureus Escherichia coli Proteus mirabilis Klebsiella pneumoniae | [57] |
| Cucurbita pepo L. seeds, backpeel | Methanol, ethanol | Staphylococcus aureus Salmonella typhi | [58] |
| Cucurbita pepo L. leaves | Ethanol | Serratia sp. Escherichia coli Klebsiella pneumoniae Bacillus subtilis | [59] |
| Cucurbita pepo L. leaves | Methanol | Providencia stuartii Pseudomonas aeruginosa Klebsiella pneumoniae Escherichia coli Enterobacter aerogenes Enterobacter cloacae | [60] |
| Cucurbita pepo L. leaves | Ethyl acetate, n-butanol, water | Bacillus subtilis Pseudomonas aeruginosa Staphylococcus aureus | [61] |
| Cucurbita moschata Duchesne seeds oil extract | Methanol | Candida albicans Rhodotorula rubra Trichoderma viride Penicillium chrysogenum Rhizopus oligosporus | [62] |
| Cucurbita moschata Duchesne crude protein from rinds, seeds and pulp | Acetone | Aspergillus fumigatus Aspergillus parasiticus Aspergillus niger Staphylococcus aureus Bacillus subtilis Klebsiella pneumoniae Pseudomonas aeruginosa Escherichia coli | [62] |
| Cucurbita maxima Duchesne fruit | Petroleum ether and methanol | Giardia lamblia | [63] |
| Cucurbita maxima Duchesne flowers | Alcohol | Salmonella typhi, Escherichia coli Enterobacter faecalis, Bacillus cereus Curvularia lunata Candida albicans | [64] |
| Cucurbita maxima Duchesne peels | Water | Escherichia coli Pseudomonas sp. Vibrio cholerae | [65] |
| Cucurbita maxima Duchesne seeds | Ethanol | Entamoeba histolytica Staphylococcus aureus Bacillus subtilis Pseudomonas aeruginosa Escherichia coli Candida albicans Aspergillus niger | [66] |
| Cucurbita maxima Duchesne seeds | Ethanol | Staphylococcus aureus Bacillus subtilis Staphylococcus werneri Pseudomonas putida Pseudomonas aeruginosa Proteus mirabilis Escherichia coli Kleibsella pneumoniae | [67] |
| Cucurbita spp. Proteins | Microbial | References |
|---|---|---|
| Cucurbita maxima Duchesne seeds proteins | Fusarium oxysporum, Verticillium dahliae Saccharomyces cerevisiae | [68] |
| Cucurbita maxima Duchesne seeds protein RIP | Phytophora infestans, Erwinia amylovora, Pseudomonas solanacearum | [70] |
| Pumpkin leaves protein PR-5 | Candida albicans | [71] |
| Pumpkin rind protein Pr-1 | Botrytis cinerea, Fusarium oxysporum, Fusarium solani Rhizoctonia solani, Candida albicans | [72] |
| Black pumpkin seeds protein cucurmoschin | Botrytis cinerea, Fusarium oxysporum Mycosphaerella oxysporum | [69] |
| Antimicrobial Property | References | |
|---|---|---|
| Cucurbita pepo L. seeds | Wounds healing | [74] |
| Pumpkin seeds | Anthelmintic, treatment of bladder functional disorders | [78] |
| Pumpkin seed oil | Arthritis prevention | [75] |
| Pumpkin fruits | Control of gastrointestinal nematode infections | [56] |
| Cucurbita pepo L. cortex extract | Effective treatment of bacterial urinary tract infections | [57] |
| Part of the Plant with Active Compounds | Cucurbita spp. | References | |
|---|---|---|---|
| Hypoglycemic | Polysaccharides from pulp fruit | Cucurbita maxima Duchesne Cucurbita ficifolia Bouché | [105,106] |
| Non-pectines polysaccharides and pectines from pulp; proteins and oil from seeds | Cucurbita ficifolia Bouché | [107,108,109] | |
| Reduced clinical symptoms of benign prostatic hyperplasia | Δ5-Δ7-Δ8-Phytosterols, unsaturated fatty acids from seeds extracts, lignans | Cucurbita pepo L. | [110,111,112,113] |
| Positive effects in stress urinary incontinence in female | Oil, sterols from seeds | Cucurbita pepo L. | [114,115] |
| Improved urinary symptoms in human overactive bladder | Seeds oil (sterols) blended with soy germ extract (phenols, isoflavones) | Cucurbita pepo L. | [116] |
| Cucurbita maxima Duchesne | [117] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehi, B.; Capanoglu, E.; Adrar, N.; Catalkaya, G.; Shaheen, S.; Jaffer, M.; Giri, L.; Suyal, R.; Jugran, A.K.; Calina, D.; et al. Cucurbits Plants: A Key Emphasis to Its Pharmacological Potential. Molecules 2019, 24, 1854. https://doi.org/10.3390/molecules24101854
Salehi B, Capanoglu E, Adrar N, Catalkaya G, Shaheen S, Jaffer M, Giri L, Suyal R, Jugran AK, Calina D, et al. Cucurbits Plants: A Key Emphasis to Its Pharmacological Potential. Molecules. 2019; 24(10):1854. https://doi.org/10.3390/molecules24101854
Chicago/Turabian StyleSalehi, Bahare, Esra Capanoglu, Nabil Adrar, Gizem Catalkaya, Shabnum Shaheen, Mehwish Jaffer, Lalit Giri, Renu Suyal, Arun K Jugran, Daniela Calina, and et al. 2019. "Cucurbits Plants: A Key Emphasis to Its Pharmacological Potential" Molecules 24, no. 10: 1854. https://doi.org/10.3390/molecules24101854
















