Reconsidering the Role of Cyclooxygenase Inhibition in the Chemotherapeutic Value of NO-Releasing Aspirins for Lung Cancer
Abstract
:1. Introduction
2. Results
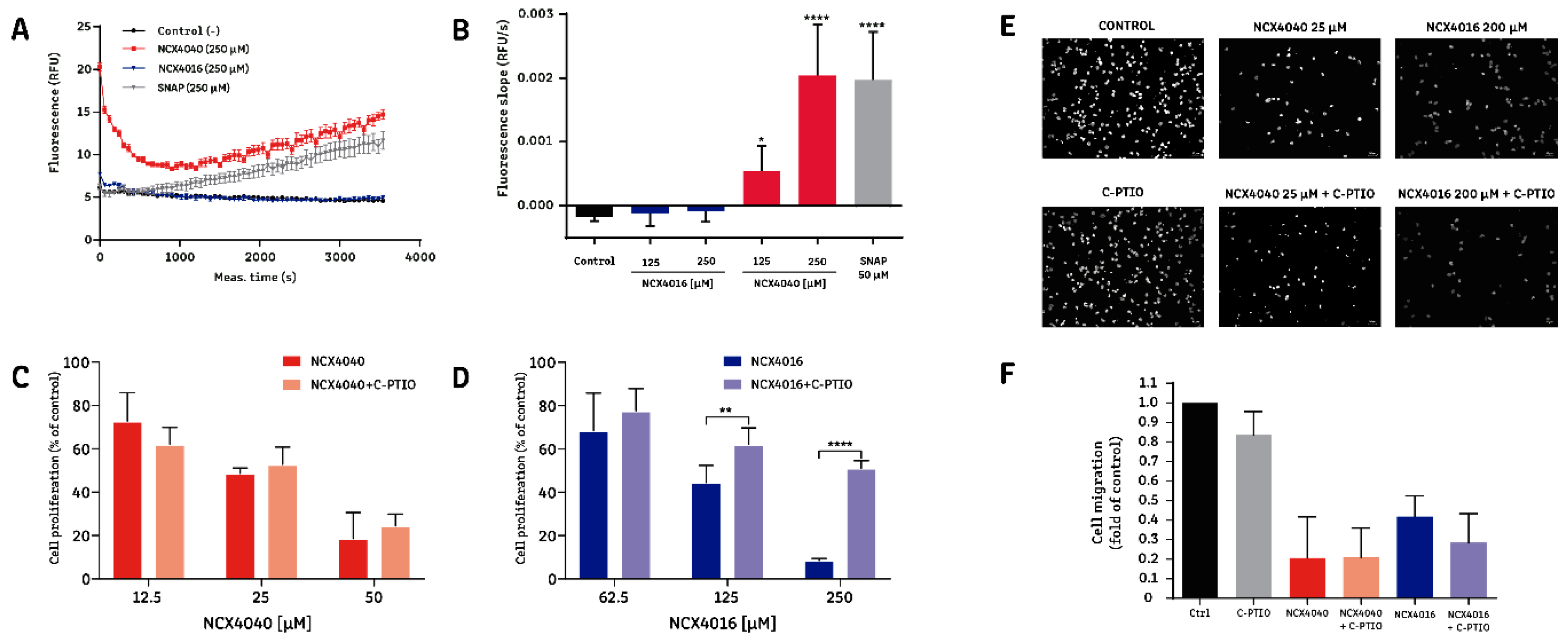
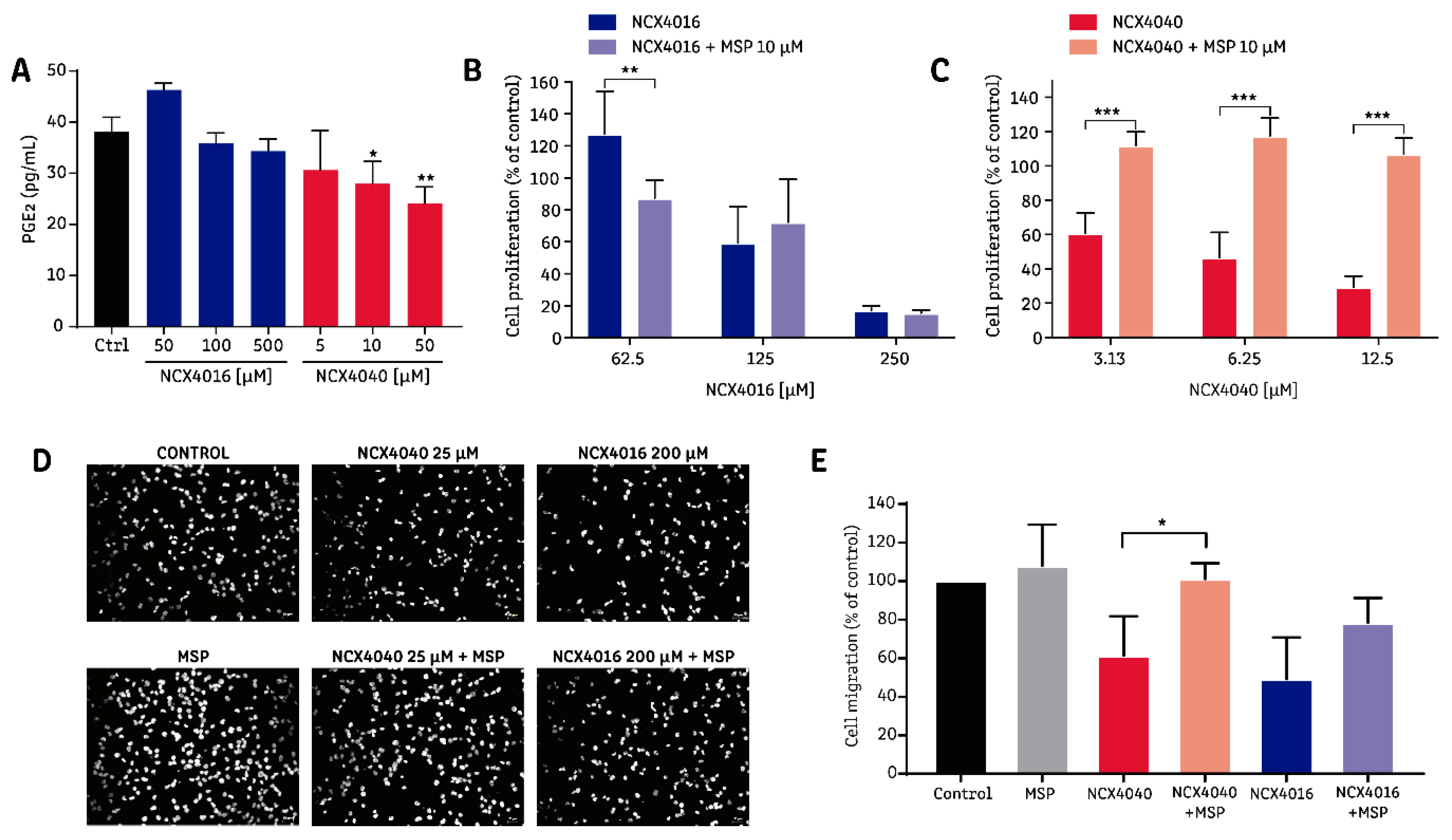
2.1. NO-Aspirins Reduce NSCLC Cell Viability and Migration with Different Potencies
2.2. NCX4040 and Erlotinib Have Synergistic Effects on NSCLC Cell Lines
2.3. The Effect of NCX4016, but Not That of NCX4040, Is Related to Its Ability to Release NO•
2.4. The Effect of NCX4040 is Related to Its Ability to Inhibit PGE2 Synthesis
3. Discussion
4. Materials and Methods
4.1. NSCLC Cell Culture
4.2. Drugs and Experimental Design
4.3. Evaluation of Cell Viability by MTT Reduction
4.4. Evaluation of Cell Proliferation by Uptake of 5-Bromo-2’-Deoxyuridine (BrdU)
4.5. Measurement of Nitric Oxide (NO•) Release
4.6. Prostaglandin E2 Measurement
4.7. Cell Migration
4.8. Drug Combination Studies
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Santana-Davila, R.; Szabo, A.; Arce-Lara, C.; Williams, C.D.; Kelley, M.J.; Whittle, J. Cisplatin versus carboplatin-based regimens for the treatment of patients with metastatic lung cancer. An analysis of Veterans Health Administration data. J. Thorac. Oncol. 2014, 9, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Alama, A.; Genova, C.; Rijavec, E.; Tagliamento, M.; Biello, F.; Coco, S.; Dal Bello, M.G.; Boccardo, S.; Grossi, F. The evolving role of pemetrexed disodium for the treatment of non-small cell lung cancer. Expert Opin. Pharm. 2018, 19, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Bruckl, W.; Tufman, A.; Huber, R.M. Advanced non-small cell lung cancer (NSCLC) with activating EGFR mutations: First-line treatment with afatinib and other EGFR TKIs. Expert Rev. Anticancer Ther. 2017, 17, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef]
- Huang, X.Z.; Chen, Y.; Wu, J.; Zhang, X.; Wu, C.C.; Zhang, C.Y.; Sun, S.S.; Chen, W.J. Aspirin and non-steroidal anti-inflammatory drugs use reduce gastric cancer risk: A dose-response meta-analysis. Oncotarget 2017, 8, 4781–4795. [Google Scholar] [PubMed]
- Khuder, S.A.; Herial, N.A.; Mutgi, A.B.; Federman, D.J. Nonsteroidal antiinflammatory drug use and lung cancer: A metaanalysis. Chest 2005, 127, 748–754. [Google Scholar] [CrossRef]
- Ali, A.T. Towards prevention of ovarian cancer. Curr. Cancer Drug Targets 2018, 18, 522–537. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, Z.; Li, H. NSAIDs Use and reduced metastasis in cancer patients: Results from a meta-analysis. Sci. Rep. 2017, 7, 1875. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Chan, F.K.; McColl, K.E. Peptic ulcer disease. Lancet 2009, 374, 1449–1461. [Google Scholar] [CrossRef]
- Grosser, T.; Yu, Y.; Fitzgerald, G.A. Emotion recollected in tranquility: Lessons learned from the COX-2 saga. Annu. Rev. Med. 2010, 61, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Reuter, B.; Cicala, C.; McKnight, W.; Grisham, M.B.; Cirino, G. Novel nonsteroidal anti-inflammatory drug derivatives with markedly reduced ulcerogenic properties in the rat. Gastroenterology 1994, 107, 173–179. [Google Scholar] [CrossRef]
- Wallace, J.L.; Reuter, B.; Cicala, C.; McKnight, W.; Grisham, M.; Cirino, G. A diclofenac derivative without ulcerogenic properties. Eur. J. Pharmacol. 1994, 257, 249–255. [Google Scholar] [CrossRef]
- Gresele, P.; Momi, S. Pharmacologic profile and therapeutic potential of NCX 4016, a nitric oxide-releasing aspirin, for cardiovascular disorders. Cardiovasc. Drug Rev. 2006, 24, 148–168. [Google Scholar] [CrossRef] [PubMed]
- Kashfi, K.; Rayyan, Y.; Qiao, L.L.; Williams, J.L.; Chen, J.; Del Soldato, P.; Traganos, F.; Rigas, B.; Ryann, Y. Nitric oxide-donating nonsteroidal anti-inflammatory drugs inhibit the growth of various cultured human cancer cells: Evidence of a tissue type-independent effect. J. Pharmacol. Exp. Ther. 2002, 303, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.C.; Singh, T.; Kapur, P.; Weng, Z.; Arumugam, A.; Elmets, C.A.; Kopelovich, L.; Athar, M. Nitric oxide-releasing sulindac is a novel skin cancer chemopreventive agent for UVB-induced photocarcinogenesis. Toxicol. Appl. Pharmacol. 2013, 268, 249–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huguenin, S.; Vacherot, F.; Kheuang, L.; Fleury-Feith, J.; Jaurand, M.C.; Bolla, M.; Riffaud, J.P.; Chopin, D.K. Antiproliferative effect of nitrosulindac (NCX 1102), a new nitric oxide-donating non-steroidal anti-inflammatory drug, on human bladder carcinoma cell lines. Mol. Cancer Ther. 2004, 3, 291–298. [Google Scholar]
- Rao, C.V.; Reddy, B.S.; Steele, V.E.; Wang, C.X.; Liu, X.; Ouyang, N.; Patlolla, J.M.; Simi, B.; Kopelovich, L.; Rigas, B. Nitric oxide-releasing aspirin and indomethacin are potent inhibitors against colon cancer in azoxymethane-treated rats: Effects on molecular targets. Mol. Cancer Ther. 2006, 5, 1530–1538. [Google Scholar] [CrossRef]
- Royle, J.S.; Ross, J.A.; Ansell, I.; Bollina, P.; Tulloch, D.N.; Habib, F.K. Nitric oxide donating nonsteroidal anti-inflammatory drugs induce apoptosis in human prostate cancer cell systems and human prostatic stroma via caspase-3. J. Urol. 2004, 172, 338–344. [Google Scholar] [CrossRef]
- Gao, J.; Kashfi, K.; Rigas, B. In vitro metabolism of nitric oxide-donating aspirin: The effect of positional isomerism. J. Pharmacol. Exp. Ther. 2005, 312, 989–997. [Google Scholar] [CrossRef]
- Razavi, R.; Gehrke, I.; Gandhirajan, R.K.; Poll-Wolbeck, S.J.; Hallek, M.; Kreuzer, K.A. Nitric oxide-donating acetylsalicylic acid induces apoptosis in chronic lymphocytic leukemia cells and shows strong antitumor efficacy in vivo. Clin. Cancer Res. 2011, 17, 286–293. [Google Scholar] [CrossRef]
- Selvendiran, K.; Bratasz, A.; Tong, L.; Ignarro, L.J.; Kuppusamy, P. NCX-4016, a nitro-derivative of aspirin, inhibits EGFR and STAT3 signaling and modulates Bcl-2 proteins in cisplatin-resistant human ovarian cancer cells and xenografts. Cell Cycle 2008, 7, 81–88. [Google Scholar] [CrossRef]
- Bratasz, A.; Weir, N.M.; Parinandi, N.L.; Zweier, J.L.; Sridhar, R.; Ignarro, L.J.; Kuppusamy, P. Reversal to cisplatin sensitivity in recurrent human ovarian cancer cells by NCX-4016, a nitro derivative of aspirin. Proc. Natl. Acad. Sci. USA 2006, 103, 3914–3919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesei, A.; Ricotti, L.; Ulivi, P.; Medri, L.; Amadori, D.; Zoli, W. NCX 4016, a nitric oxide-releasing aspirin derivative, exhibits a significant antiproliferative effect and alters cell cycle progression in human colon adenocarcinoma cell lines. Int. J. Oncol. 2003, 22, 1297–1302. [Google Scholar] [CrossRef]
- Tesei, A.; Ulivi, P.; Fabbri, F.; Rosetti, M.; Leonetti, C.; Scarsella, M.; Zupi, G.; Amadori, D.; Bolla, M.; Zoli, W. In vitro and in vivo evaluation of NCX 4040 cytotoxic activity in human colon cancer cell lines. J. Transl. Med. 2005, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Tesei, A.; Rosetti, M.; Ulivi, P.; Fabbri, F.; Medri, L.; Vannini, I.; Bolla, M.; Amadori, D.; Zoli, W. Study of molecular mechanisms of pro-apoptotic activity of NCX 4040, a novel nitric oxide-releasing aspirin, in colon cancer cell lines. J. Transl. Med. 2007, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, C.; Scarsella, M.; Zupi, G.; Zoli, W.; Amadori, D.; Medri, L.; Fabbri, F.; Rosetti, M.; Ulivi, P.; Cecconetto, L.; et al. Efficacy of a nitric oxide-releasing nonsteroidal anti-inflammatory drug and cytotoxic drugs in human colon cancer cell lines in vitro and xenografts. Mol. Cancer Ther. 2006, 5, 919–926. [Google Scholar] [CrossRef] [Green Version]
- Rosetti, M.; Tesei, A.; Ulivi, P.; Fabbri, F.; Vannini, I.; Brigliadori, G.; Amadori, D.; Bolla, M.; Zoli, W. Molecular characterization of cytotoxic and resistance mechanisms induced by NCX 4040, a novel NO-NSAID, in pancreatic cancer cell lines. Apoptosis 2006, 11, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Upadhyaya, P.; Kassie, F. Nitric oxide-donating aspirin (NO-Aspirin) suppresses lung tumorigenesis in vitro and in vivo and these effects are associated with modulation of the EGFR signaling pathway. Carcinogenesis 2018, 39, 911–920. [Google Scholar] [CrossRef]
- Dunlap, T.; Abdul-Hay, S.O.; Chandrasena, R.E.; Hagos, G.K.; Sinha, V.; Wang, Z.; Wang, H.; Thatcher, G.R. Nitrates and NO-NSAIDs in cancer chemoprevention and therapy: In vitro evidence querying the NO donor functionality. Nitric Oxide 2008, 19, 115–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashfi, K.; Rigas, B. The mechanism of action of nitric oxide-donating aspirin. Biochem. Biophys. Res. Commun. 2007, 358, 1096–1101. [Google Scholar] [CrossRef]
- Di Veroli, G.Y.; Fornari, C.; Wang, D.; Mollard, S.; Bramhall, J.L.; Richards, F.M.; Jodrell, D.I. Combenefit: An interactive platform for the analysis and visualization of drug combinations. Bioinformatics 2016, 32, 2866–2868. [Google Scholar] [CrossRef]
- Hu, J.; Han, Y.; Wang, J.; Liu, Y.; Zhao, Y.; Liu, Y.; Gong, P. Discovery of selective EGFR modulator to inhibit L858R/T790M double mutants bearing a N-9-Diphenyl-9H-purin-2-amine scaffold. Bioorg. Med. Chem. 2018, 26, 1810–1822. [Google Scholar] [CrossRef]
- Williams, J.L.; Borgo, S.; Hasan, I.; Castillo, E.; Traganos, F.; Rigas, B. Nitric oxide-releasing nonsteroidal anti-inflammatory drugs (NSAIDs) alter the kinetics of human colon cancer cell lines more effectively than traditional NSAIDs: Implications for colon cancer chemoprevention. Cancer Res. 2001, 61, 3285–3289. [Google Scholar]
- Kashfi, K.; Borgo, S.; Williams, J.L.; Chen, J.; Gao, J.; Glekas, A.; Benedini, F.; Del Soldato, P.; Rigas, B. Positional isomerism markedly affects the growth inhibition of colon cancer cells by nitric oxide-donating aspirin in vitro and in vivo. J. Pharmacol. Exp. Ther. 2005, 312, 978–988. [Google Scholar] [CrossRef]
- Kang, H.N.; Kim, S.H.; Yun, M.R.; Kim, H.R.; Lim, S.M.; Kim, M.S.; Hong, K.W.; Kim, S.M.; Kim, H.; Pyo, K.H.; et al. ER2, a novel human anti-EGFR monoclonal antibody inhibit tumor activity in non-small cell lung cancer models. Lung Cancer 2016, 95, 57–64. [Google Scholar] [CrossRef]
- Hulsman, N.; Medema, J.P.; Bos, C.; Jongejan, A.; Leurs, R.; Smit, M.J.; de Esch, I.J.; Richel, D.; Wijtmans, M. Chemical insights in the concept of hybrid drugs: The antitumor effect of nitric oxide-donating aspirin involves a quinone methide but not nitric oxide nor aspirin. J. Med. Chem. 2007, 50, 2424–2431. [Google Scholar] [CrossRef]
- Corazzi, T.; Leone, M.; Roberti, R.; Del Soldato, P.; Gresele, P. Effect of nitric oxide-donating agents on human monocyte cyclooxygenase-2. Biochem. Biophys. Res. Commun. 2003, 311, 897–903. [Google Scholar] [CrossRef]
- Carini, M.; Aldini, G.; Orioli, M.; Maffei Facino, R. In vitro metabolism of a nitroderivative of acetylsalicylic acid (NCX4016) by rat liver: LC and LC-MS studies. J. Pharm. Biomed. Anal. 2002, 29, 1061–1071. [Google Scholar] [CrossRef]
- Fiorucci, S.; Santucci, L.; Wallace, J.L.; Sardina, M.; Romano, M.; del Soldato, P.; Morelli, A. Interaction of a selective cyclooxygenase-2 inhibitor with aspirin and NO-releasing aspirin in the human gastric mucosa. Proc. Natl. Acad. Sci. USA 2003, 100, 10937–10941. [Google Scholar] [CrossRef] [Green Version]
- Ricciotti, E.; Dovizio, M.; Di Francesco, L.; Anzellotti, P.; Salvatore, T.; Di Francesco, A.; Sciulli, M.G.; Pistritto, G.; Monopoli, A.; Patrignani, P. NCX 4040, a nitric oxide-donating aspirin, exerts anti-inflammatory effects through inhibition of I kappa B-alpha degradation in human monocytes. J. Immunol. 2010, 184, 2140–2147. [Google Scholar] [CrossRef]
- Sharma, S.D.; Meeran, S.M.; Katiyar, S.K. Proanthocyanidins inhibit in vitro and in vivo growth of human non-small cell lung cancer cells by inhibiting the prostaglandin E(2) and prostaglandin E(2) receptors. Mol. Cancer Ther. 2010, 9, 569–580. [Google Scholar] [CrossRef]
- Allaj, V.; Guo, C.; Nie, D. Non-steroid anti-inflammatory drugs, prostaglandins, and cancer. Cell Biosci. 2013, 3, 8. [Google Scholar] [CrossRef]
- Tada, H.; Shiho, O.; Kuroshima, K.; Koyama, M.; Tsukamoto, K. An improved colorimetric assay for interleukin 2. J. Immunol. Methods 1986, 93, 157–165. [Google Scholar] [CrossRef]
Sample Availability: Not Available. |


| EC50 (µM) 1 | ||
|---|---|---|
| MTT | BrdU | |
| Aspirin | 937.7 ± 162.3 | 2,534 ± 142.1 |
| NCX4016 | 83.0 ± 5.4 **** | 101.0 ± 15.6 **** |
| NCX4040 | 13.5 ± 2.6 ***** | 7.8 ± 1.3 **** |
| NSCLC Cell Line (EC50) 1 | |||
|---|---|---|---|
| A549 | H1299 | H1975 | |
| Erlotinib | 8.22 µM | 12.1 µM | 4.77 µM |
| Aspirin | 0.993 mM | 1.14 mM | 1.11 mM |
| NCX4040 | 237 µM | 93.1 µM | 202 µM |
| NCX4016 | 23.8 µM | 13.8 µM | 7.0 µM |
| Max Synergy Score by Cell Line 1 | |||
|---|---|---|---|
| A549 | H1299 | H1975 | |
| Erlotinib + aspirin | 7 ± 5 * | 3 ± 0 * | 5 ± 1 * |
| Erlotinib + NCX4040 | 20 ± 3 * | 13 ± 3 * | 4 ± 1 * |
| Erlotinib + NCX4016 | 9 ± 2 * | 6 ± 6 | 6 ± 1 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Martin, A.; Rivera-Dictter, A.; Muñoz-Uribe, M.; López-Contreras, F.; Pérez-Laines, J.; Molina-Berríos, A.; López-Muñoz, R. Reconsidering the Role of Cyclooxygenase Inhibition in the Chemotherapeutic Value of NO-Releasing Aspirins for Lung Cancer. Molecules 2019, 24, 1924. https://doi.org/10.3390/molecules24101924
Martin-Martin A, Rivera-Dictter A, Muñoz-Uribe M, López-Contreras F, Pérez-Laines J, Molina-Berríos A, López-Muñoz R. Reconsidering the Role of Cyclooxygenase Inhibition in the Chemotherapeutic Value of NO-Releasing Aspirins for Lung Cancer. Molecules. 2019; 24(10):1924. https://doi.org/10.3390/molecules24101924
Chicago/Turabian StyleMartin-Martin, Antonia, Andrés Rivera-Dictter, Matías Muñoz-Uribe, Freddy López-Contreras, Jorge Pérez-Laines, Alfredo Molina-Berríos, and Rodrigo López-Muñoz. 2019. "Reconsidering the Role of Cyclooxygenase Inhibition in the Chemotherapeutic Value of NO-Releasing Aspirins for Lung Cancer" Molecules 24, no. 10: 1924. https://doi.org/10.3390/molecules24101924
APA StyleMartin-Martin, A., Rivera-Dictter, A., Muñoz-Uribe, M., López-Contreras, F., Pérez-Laines, J., Molina-Berríos, A., & López-Muñoz, R. (2019). Reconsidering the Role of Cyclooxygenase Inhibition in the Chemotherapeutic Value of NO-Releasing Aspirins for Lung Cancer. Molecules, 24(10), 1924. https://doi.org/10.3390/molecules24101924





