Efficacy of Obcordata A from Aspidopterys obcordata on Kidney Stones by Inhibiting NOX4 Expression
Abstract
:1. Introduction
2. Results
2.1. Cytotoxicity of OA on HK-2 Cells
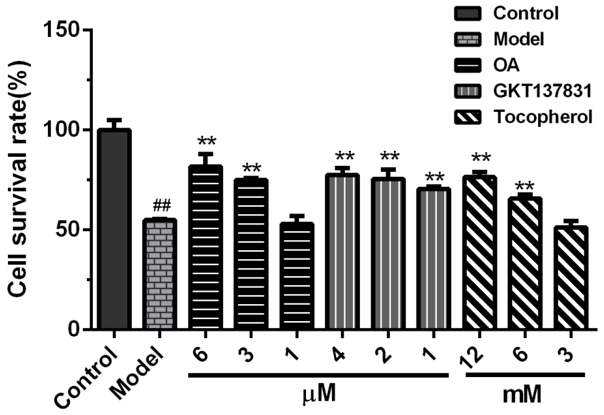
2.2. Protective Effects of OA on the Viability of HK-2 Cells Exposed to Calcium Oxalate Crystals
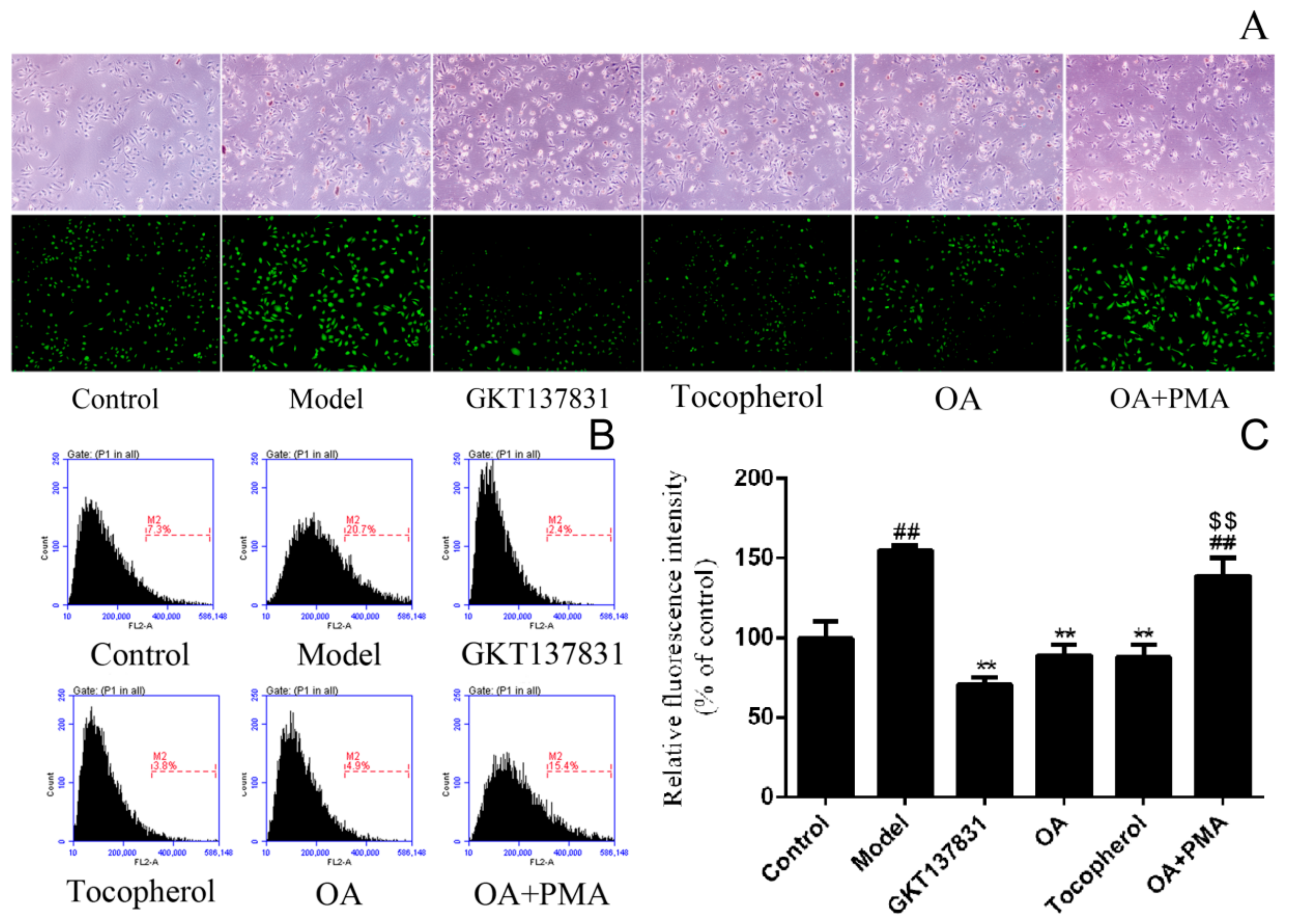
2.3. Inhibition of OA on the Intracellular Reactive Oxygen Species Production
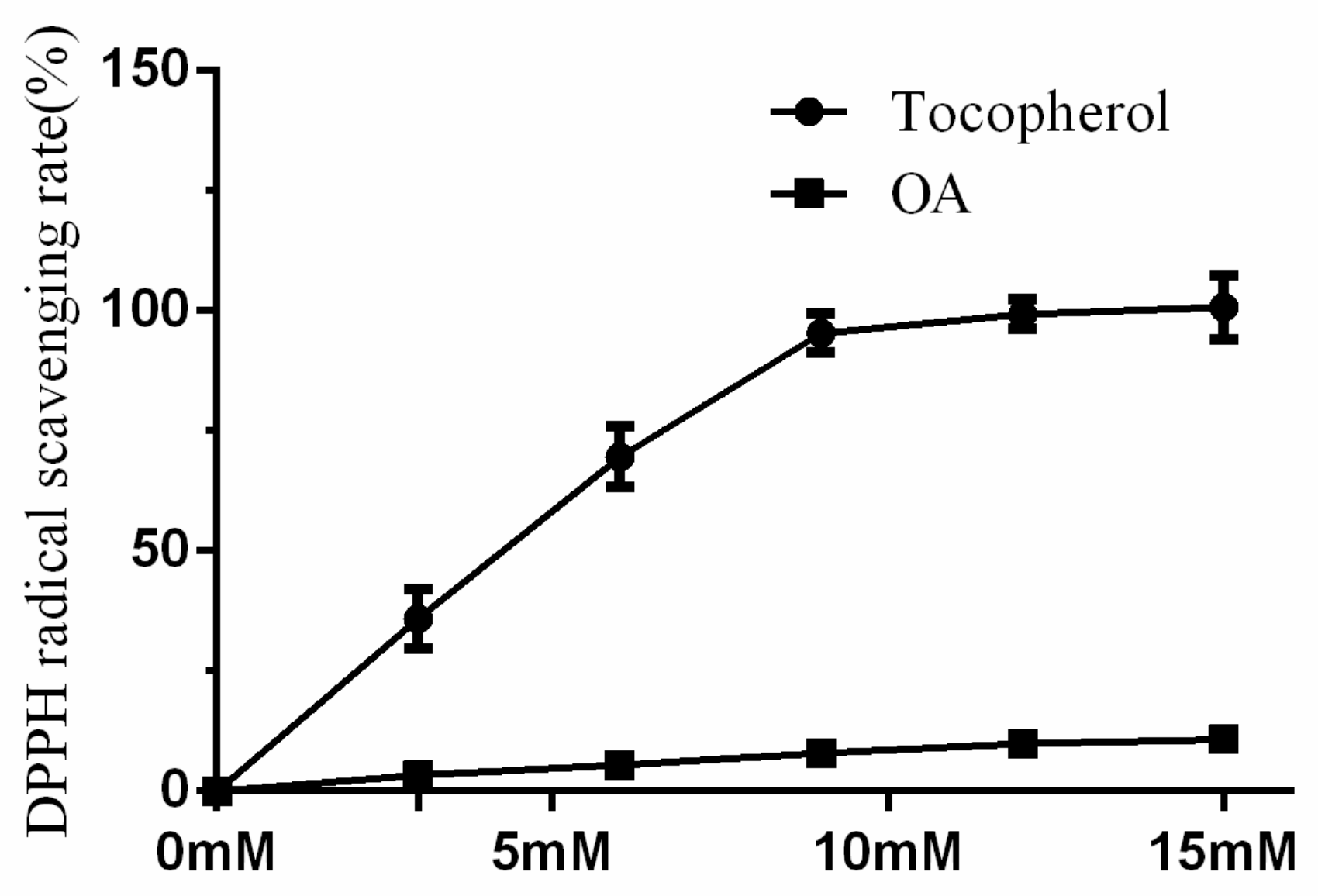
2.4. DPPH Radical Scavenging Activity of OA Was Low
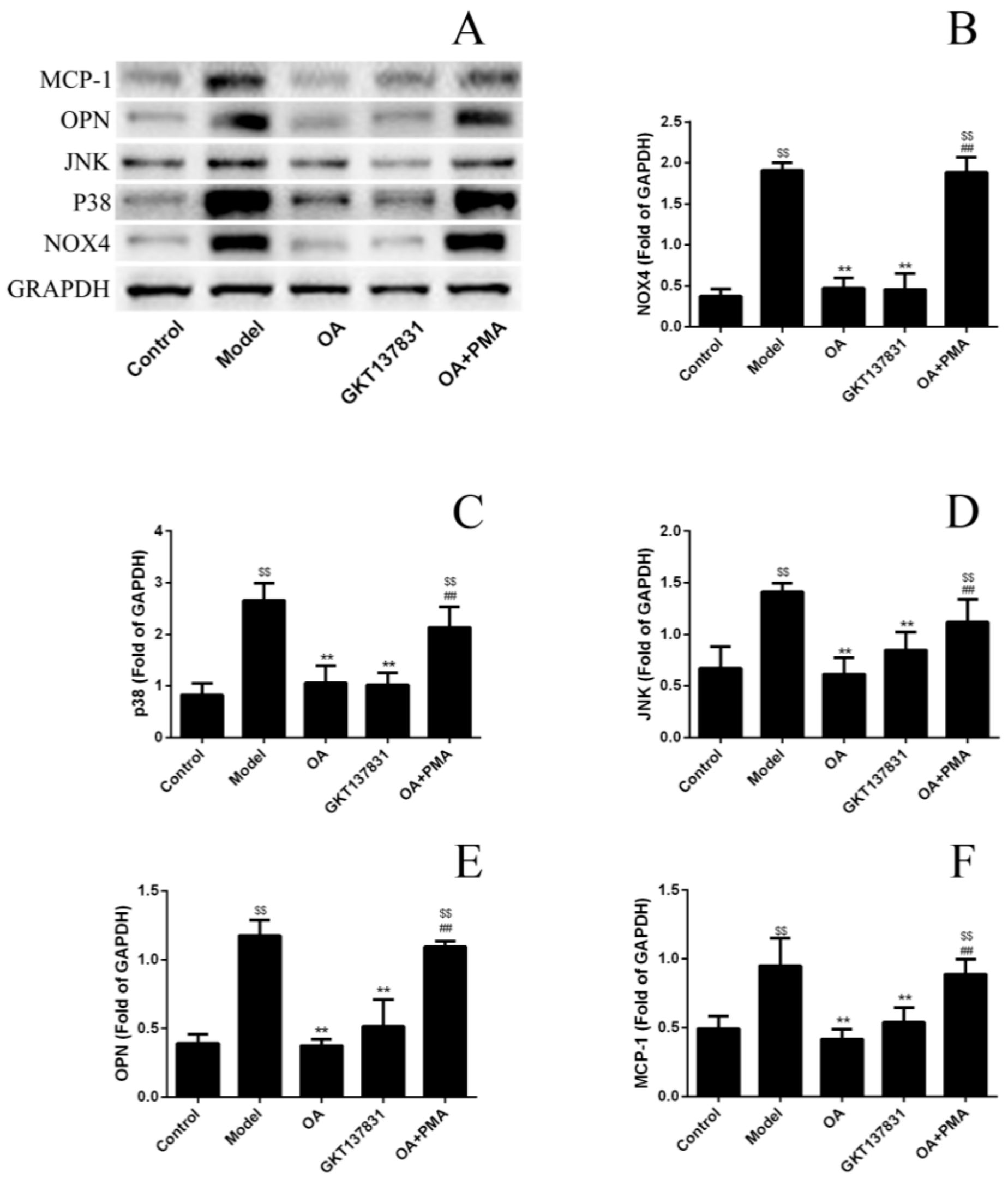
2.5. OA Inhibits the Expression Level of Proteins in NOX4/ROS/p38 MAPK Signaling and Decreases ROS Levels
3. Discussion
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. Cell Culture
4.3. Cytotoxicity Assay of OA
4.4. Calcium Oxalate Crystals Exposure and Treatment
4.5. Intracellular Reactive Oxygen Species Production by DCFH-DA Assay, Fluorescence Microscopy, and Flow Cytometry
4.6. DPPH Radical Scavenging Test
4.7. Western Blots Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, W.; Fan, J.; Huang, G.; Li, J.; Zhu, X.; Tian, Y.; Su, L. Prevalence of kidney stones in mainland China: A systematic review. Sci. Rep. 2017, 7, 41630. [Google Scholar] [CrossRef] [PubMed]
- Januchowski, R.; Dabecco, R.; Verdone, C. Nephrolithiasis. Osteopath. Fam. Physician 2014, 6, 8–12. [Google Scholar]
- Sorokin, I.; Mamoulakis, C.; Miyazawa, K.; Rodgers, A.; Talati, J.; Lotan, Y. Epidemiology of stone disease across the world. World J. Urol. 2017, 35, 1301–1320. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.D.; Ellison, J.; Lendvay, T.S.; Hernandez, M.J.D.; Ellison, M.J.S.; Lendvay, M.T.S. Current Trends, Evaluation, and Management of Pediatric Nephrolithiasis. JAMA Pediatr. 2015, 169, 964. [Google Scholar] [CrossRef]
- Jiang, Z.; Asplin, J.R.; Evan, A.P.; Rajendran, V.M.; Velazquez, H.; Nottoli, T.P.; Binder, H.J.; Aronson, P.S. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat. Genet. 2006, 38, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Indridason, O.S.; Birgisson, S.; Edvardsson, V.O.; Sigvaldason, H.; Sigfusson, N.; Palsson, R. Epidemiology of kidney stones in Iceland A population-based study. Scand. J. Urol. Nephrol. 2006, 40, 215–220. [Google Scholar] [CrossRef]
- Lotan, Y.; Cadeddu, J.A.; Pearle, M.S. International comparison of cost effectiveness of medical management strategies for nephrolithiasis. Urol. Res. 2005, 33, 223–230. [Google Scholar] [CrossRef]
- Bushinsky, D.; Michalenka, A.; Strutz, K.; Donahue, S.; Asplin, J. Effect of bolus and divided feeding on urine ions and supersaturation in genetic hypercalciuric stone-forming rats. Kidney Int. 2008, 73, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Dhayat, N.A.; Faller, N.; Bonny, O.; Mohebbi, N.; Ritter, A.; Pellegrini, L.; Bedino, G.; Schönholzer, C.; Venzin, R.M.; Hüsler, C.; et al. Efficacy of standard and low dose hydrochlorothiazide in the recurrence prevention of calcium nephrolithiasis (NOSTONE trial): Protocol for a randomized double-blind placebo-controlled trial. BMC Nephrol. 2018, 19, 349. [Google Scholar] [CrossRef]
- Khan, S.R. Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome? Urol. Res. 2012, 40, 95–112. [Google Scholar] [CrossRef]
- Koul, H.K.; Menon, M.; Chaturvedi, L.S.; Sekhon, A.; Bhandari, A.; Huang, M.; Koul, S. COM Crystals Activate the p38 Mitogen-activated Protein Kinase Signal Transduction Pathway in Renal Epithelial Cells. J. Boil. Chem. 2002, 277, 36845–36852. [Google Scholar] [CrossRef] [Green Version]
- Zuo, J.; Khan, A.; Glenton, P.A.; Khan, S.R. Effect of NADPH oxidase inhibition on the expression of kidney injury molecule and calcium oxalate crystal deposition in hydroxy-l-proline-induced hyperoxaluria in the male Sprague–Dawley rats. Nephrol. Dial. Transplant. 2011, 26, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Wang, Q.; Lu, Y.; Li, C.; Hu, H.; Zhang, J.; Wang, Y.; Zhu, J.; Zhu, Y.; Xun, Y.; et al. Losartan Ameliorates Calcium Oxalate-Induced Elevation of Stone-Related Proteins in Renal Tubular Cells by Inhibiting NADPH Oxidase and Oxidative Stress. Oxid. Med. Cell. Longev. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Yihang, L.; Guang, L.; Meifang, S.; Xuelan, L.; Xia, Z.; Juan, L.; Xi, C. Acute toxicity study of Aspidopterys obcordata aqueous extract in Sprague-Dawley rats. J. Tradit. Chin. Med. 2016, 36, 377–381. [Google Scholar] [CrossRef]
- Song, M.-F.; Li, Y.-H.; Zhang, Z.-L.; Lü, Y.-N.; Li, X.-L.; Li, G. Inhibiting effect of Aspidopterys obcordata Hemsl on renal calculus. Chin. J. New Drugs 2015, 24, 1047–1052. [Google Scholar]
- Hu, M.; Li, Y.; Sun, Z.; Huo, X.; Zhu, N.; Sun, Z.; Liu, Y.; Wu, H.; Xu, X.; Ma, G.; et al. New polyoxypregnane glycosides from Aspidopterys obcordata vines with antitumor activity. Fitoterapia 2018, 129, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Healy, K.; Ogan, K. Nonsurgical Management of Urolithiasis: An Overview of Expulsive Therapy. J. Endourol. 2005, 19, 759–767. [Google Scholar] [CrossRef]
- Alelign, T.; Petros, B. Kidney Stone Disease: An Update on Current Concepts. Adv. Urol. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Leveridge, M.; D’Arcy, F.T.; O’Kane, D.; Ischia, J.J.; Webb, D.R.; Bolton, D.M.; Lawrentschuk, N. Renal colic: Current protocols for emergency presentations. Eur. J. Emerg. Med. 2016, 23, 2–7. [Google Scholar] [CrossRef]
- Wang, L.; Feng, C.; Ding, G.; Lin, X.; Gao, P.; Jiang, H.; Xu, J.; Ding, Q.; Wu, Z. Association Study of Reported Significant Loci at 5q35.3, 7p14.3, 13q14.1 and 16p12.3 with Urolithiasis in Chinese Han Ethnicity. Sci. Rep. 2017, 7, 45766. [Google Scholar] [CrossRef]
- Rimer, J.D.; An, Z.; Zhu, Z.; Lee, M.H.; Goldfarb, D.S.; Wesson, J.A.; Ward, M.D. Crystal Growth Inhibitors for the Prevention of L-Cystine Kidney Stones through Molecular Design. Science 2010, 330, 337–341. [Google Scholar] [CrossRef]
- Khan, S.R. Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl. Androl. Urol. 2014, 3, 256–276. [Google Scholar]
- Tsujihata, M. Mechanism of calcium oxalate renal stone formation and renal tubular cell injury. Int. J. Urol. 2008, 15, 115–120. [Google Scholar] [CrossRef]
- Dörrenhaus, A.; Müller, J.I.; Golka, K.; Jedrusik, P.; Schulze, H.; Föllmann, W. Cultures of exfoliated epithelial cells from different locations of the human urinary tract and the renal tubular system. Arch. Toxicol. 2000, 74, 618–626. [Google Scholar] [CrossRef]
- Lee, H.-J.; Jeong, S.-J.; Park, M.N.; Linnes, M.; Han, H.J.; Kim, J.H.; Lieske, J.C.; Kim, S.-H. Gallotannin Suppresses Calcium Oxalate Crystal Binding and Oxalate-Induced Oxidative Stress in Renal Epithelial Cells. Boil. Pharm. 2012, 35, 539–544. [Google Scholar] [CrossRef] [Green Version]
- Devkota, K.P.; Lenta, B.N.; Choudhary, M.I.; Naz, Q.; Fekam, F.B.; Rosenthal, P.J.; Sewald, N. Cholinesterase Inhibiting and Antiplasmodial Steroidal Alkaloids from Sarcococca hookeriana. Chem. Pharm. 2007, 55, 1397–1401. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.-Y.; Shao, C.-L.; Li, Z.-Y.; Han, L.; Cao, F.; Wang, C.-Y. Bioactive Pregnane Steroids from a South China Sea Gorgonian Carijoa sp. Molecules 2013, 18, 3458. [Google Scholar] [CrossRef] [PubMed]
- Orans, J.; Teotico, D.G.; Redinbo, M.R. The Nuclear Xenobiotic Receptor Pregnane X Receptor: Recent Insights and New Challenges. Mol. Endocrinol. 2005, 19, 2891–2900. [Google Scholar] [CrossRef] [Green Version]
- Hussain, H.; Raees, M.A.; Rehman, N.U.; Al-Rawahi, A.; Csuk, R.; Khan, H.Y.; Abbas, G.; Al-Broumi, M.A.; Green, I.R.; Elyassi, A.; et al. Nizwaside: A new anticancer pregnane glycoside from the sap of Desmidorchis flava. Arch. Pharmacal 2015, 38, 2137–2142. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Ouyang, J.-M. New view in cell death mode: Effect of crystal size in renal epithelial cells. Cell Death 2015, 6, e2013. [Google Scholar] [CrossRef] [PubMed]
- Grohm, J.; Kim, S.-W.; Mamrak, U.; Tobaben, S.; Cassidy-Stone, A.; Nunnari, J.; Plesnila, N.; Culmsee, C. Inhibition of Drp1 provides neuroprotection in vitro and in vivo. Cell Death Differ. 2012, 19, 1446–1458. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Xing, X.; Luo, Y.; Deng, X.; Lu, S.; Tang, S.; Sun, G.; Sun, X. Notoginsenoside R1 prevents H9c2 cardiomyocytes apoptosis against hypoxia/reoxygenation via the ERs/PI3K/Akt pathway. RSC Adv. 2018, 8, 13871–13878. [Google Scholar] [CrossRef] [Green Version]
- Wiessner, J.H.; Hung, L.Y.; Mandel, N.S. Crystal attachment to injured renal collecting duct cells: Influence of urine proteins and pH. Kidney Int. 2003, 63, 1313–1320. [Google Scholar] [CrossRef] [Green Version]
- Jeong, B.Y.; Lee, H.Y.; Park, C.G.; Kang, J.; Yu, S.L.; Choi, D.; Han, S.Y.; Park, M.H.; Cho, S.; Lee, S.Y.; et al. Oxidative stress caused by activation of NADPH oxidase 4 promotes contrast-induced acute kidney injury. PLoS ONE 2018, 2018. [Google Scholar] [CrossRef]
- Von Lohneysen, K.; Noack, D.; Wood, M.R.; Friedman, J.S.; Knaus, U.G. Structural Insights into Nox4 and Nox2: Motifs Involved in Function and Cellular Localization. Mol. Cell. Biol. 2010, 30, 961–975. [Google Scholar] [CrossRef] [Green Version]
- Serrander, L.; Cartier, L.; Bedard, K.; Bánfi, B.; Lardy, B.; Plastre, O.; Sienkiewicz, A.; Forró, L.; Schlegel, W.; Krause, K.-H. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem. J. 2007, 406, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Villaño, D.; Fernández-Pachón, M.; Moyá, M.L.; Troncoso, A.M.; Garcia-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound Obcordata A are available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ma, G.; Lv, Y.; Su, J.; Li, G.; Chen, X. Efficacy of Obcordata A from Aspidopterys obcordata on Kidney Stones by Inhibiting NOX4 Expression. Molecules 2019, 24, 1957. https://doi.org/10.3390/molecules24101957
Li Y, Ma G, Lv Y, Su J, Li G, Chen X. Efficacy of Obcordata A from Aspidopterys obcordata on Kidney Stones by Inhibiting NOX4 Expression. Molecules. 2019; 24(10):1957. https://doi.org/10.3390/molecules24101957
Chicago/Turabian StyleLi, Yihang, Guoxu Ma, Yana Lv, Jing Su, Guang Li, and Xi Chen. 2019. "Efficacy of Obcordata A from Aspidopterys obcordata on Kidney Stones by Inhibiting NOX4 Expression" Molecules 24, no. 10: 1957. https://doi.org/10.3390/molecules24101957





