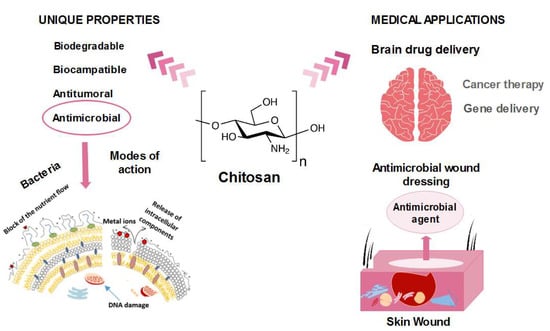
Chitosan-Based (Nano)Materials for Novel Biomedical Applications
Abstract
1. Introduction
2. Antimicrobial Nature of Chitosan
2.1. Mode of Action
2.2. Effect of Factors on the Antimicrobial Activity of Chitosan
2.2.1. Environmental Factors
2.2.2. Fundamental Factors
2.1.3. Type of Microorganism
3. Chitosan in Biomedical Applications
3.1. Wound Dressing
3.2. Tissue Engineering for Bone Regeneration
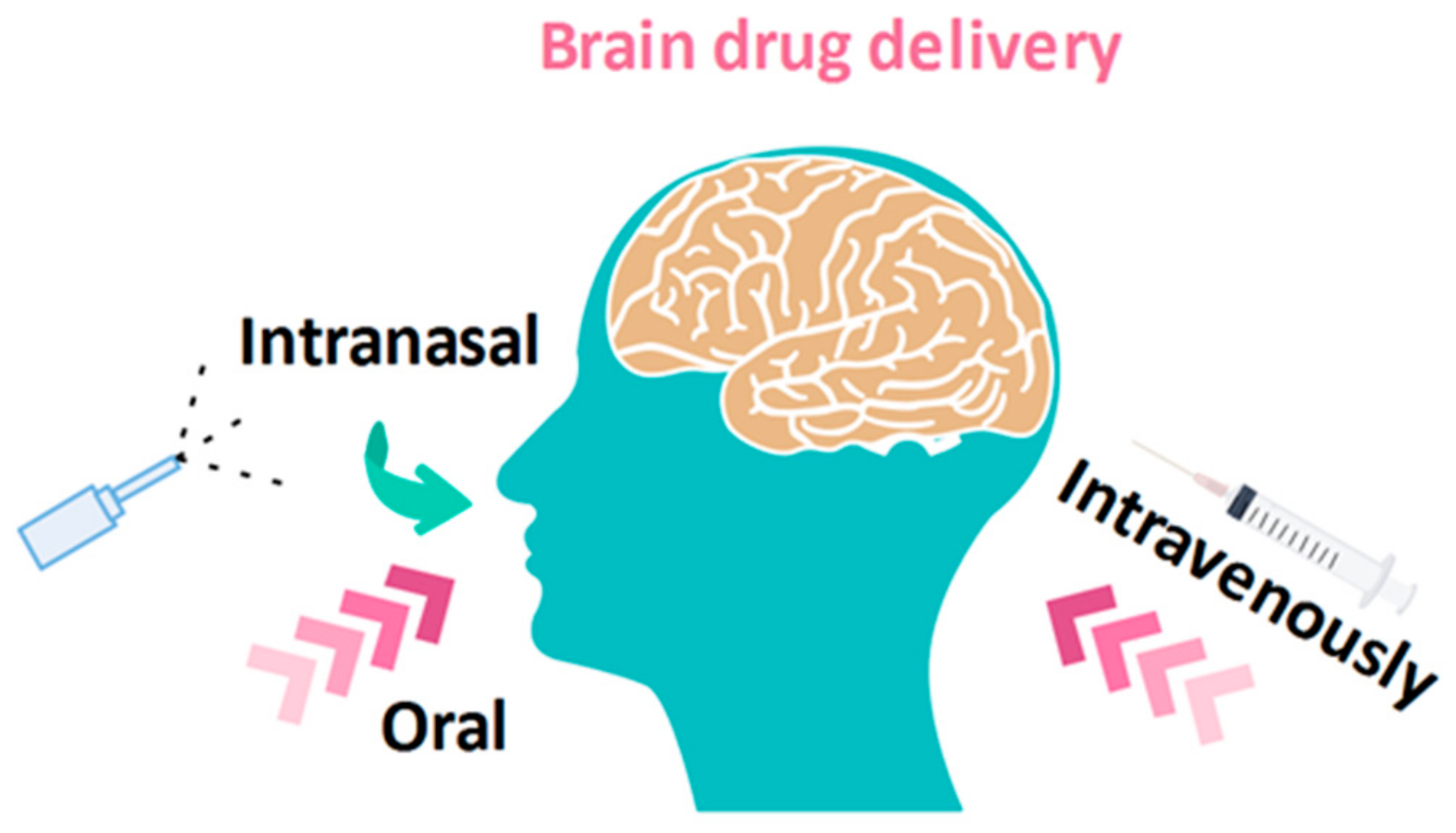
3.3. Chitosan as a Brain Drug Delivery Carrier
3.4. Other Biomedical Applications
4. Conclusions and Future Trends
Author Contributions
Funding
Conflicts of Interest
References
- Elgadir, M.A.; Uddin, M.S.; Ferdosh, S.; Adam, A.; Chowdhury, A.J.K.; Sarker, M.Z.I. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J. Food Drug Anal. 2015, 23, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Qin, A.; Lin, X.; Yang, L.; Wang, Q.; Wang, Z.; Shan, Z.; Li, S.; Wang, J.; Fan, S. Biodegradable and biocompatible high elastic chitosan scaffold is cell-friendly both in vitro and in vivo. Oncotarget 2017, 8, 35583. [Google Scholar] [CrossRef] [PubMed]
- Gallaher, C.M.; Munion, J.; Hesslink Jr, R.; Wise, J.; Gallaher, D.D. Cholesterol reduction by glucomannan and chitosan is mediated by changes in cholesterol absorption and bile acid and fat excretion in rats. J. Nutr. 2000, 130, 2753–2759. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Meng, C.-g.; Liu, S.; Kan, J.; Jin, C.-h. Preparation and characterization of protocatechuic acid grafted chitosan films with antioxidant activity. Food Hydrocoll. 2017, 63, 457–466. [Google Scholar] [CrossRef]
- Goy, R.C.; Britto, D.d.; Assis, O.B. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Fei Liu, X.; Lin Guan, Y.; Zhi Yang, D.; Li, Z.; De Yao, K. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar] [CrossRef]
- Vinsova, J.; Vavrikova, E. Chitosan derivatives with antimicrobial, antitumour and antioxidant activities—A review. Curr. Pharm. Des. 2011, 17, 3596–3607. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef]
- Chuan, D.; Jin, T.; Fan, R.; Zhou, L.; Guo, G. Chitosan for gene delivery: Methods for improvement and applications. Adv. Coll. Int. Sci. 2019, 268, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lu, S.; Cheng, Y.; Kong, S.; Li, S.; Li, C.; Yang, L. Investigation of the Effects of Molecular Parameters on the Hemostatic Properties of Chitosan. Molecules 2018, 23, 3147. [Google Scholar] [CrossRef]
- Husain, S.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Qasim, S.B. Chitosan biomaterials for current and potential dental applications. Materials 2017, 10, 602. [Google Scholar] [CrossRef] [PubMed]
- Wahba, M.I. Sodium bicarbonate-gelled chitosan beads as mechanically stable carriers for the covalent immobilization of enzymes. Biotechnol. Prog. 2018, 34, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, N.; Hoover, D.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Varma, A.; Deshpande, S.; Kennedy, J. Metal complexation by chitosan and its derivatives: A review. Carbohydr. Polym. 2004, 55, 77–93. [Google Scholar] [CrossRef]
- Severino, R.; Ferrari, G.; Vu, K.D.; Donsì, F.; Salmieri, S.; Lacroix, M. Antimicrobial effects of modified chitosan based coating containing nanoemulsion of essential oils, modified atmosphere packaging and gamma irradiation against Escherichia coli O157: H7 and Salmonella Typhimurium on green beans. Food Cont. 2015, 50, 215–222. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Li, Z.; Yang, F.; Yang, R. Synthesis and characterization of chitosan derivatives with dual-antibacterial functional groups. Int. J. Biol. Macromol. 2015, 75, 378–387. [Google Scholar] [CrossRef]
- Roy, K.; Mao, H.-Q.; Huang, S.-K.; Leong, K.W. Oral gene delivery with chitosan–DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat. Med. 1999, 5, 387. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Fan, L.; Liu, H.; Hu, Y. Chitosan-metal complexes as antimicrobial agent: Synthesis, characterization and structure-activity study. Polym. Bull. 2005, 55, 105–113. [Google Scholar] [CrossRef]
- Dutta, P.; Tripathi, S.; Mehrotra, G.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Devlieghere, F.; Vermeulen, A.; Debevere, J. Chitosan: Antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004, 21, 703–714. [Google Scholar] [CrossRef]
- Yuan, G.; Lv, H.; Tang, W.; Zhang, X.; Sun, H. Effect of chitosan coating combined with pomegranate peel extract on the quality of Pacific white shrimp during iced storage. Food Control. 2016, 59, 818–823. [Google Scholar] [CrossRef]
- Aiedeh, K.; Taha, M.O. Synthesis of iron-crosslinked chitosan succinate and iron-crosslinked hydroxamated chitosan succinate and their in vitro evaluation as potential matrix materials for oral theophylline sustained-release beads. Eur. J. Pharm. Sci. 2001, 13, 159–168. [Google Scholar] [CrossRef]
- Sahariah, P.; Maásson, M.r. Antimicrobial chitosan and chitosan derivatives: A review of the structure–activity relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- No, H.K.; Young Park, N.; Ho Lee, S.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Takahashi, T.; Imai, M.; Suzuki, I. Water permeability of chitosan membrane involved in deacetylation degree control. Biochem. Engin. J. 2007, 36, 43–48. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Byun, S.M.; No, H.K.; Hong, J.H.; Lee, S.I.; Prinyawiwatkul, W. Comparison of physicochemical, binding, antioxidant and antibacterial properties of chitosans prepared from ground and entire crab leg shells. Int. J. Food Sci. Technol. 2013, 48, 136–142. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Plapied, L.; Vandermeulen, G.; Vroman, B.; Préat, V.; des Rieux, A. Bioadhesive nanoparticles of fungal chitosan for oral DNA delivery. Int. J. Pharm. 2010, 398, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Ing, L.Y.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal Activity of Chitosan Nanoparticles and Correlation with Their Physical Properties. Int. J. Biomater. 2012, 2012, 9. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Chitosan-based dietary foods. Carbohydr. Polym. 1996, 29, 309–316. [Google Scholar] [CrossRef]
- Roller, S.; Covill, N. The antifungal properties of chitosan in laboratory media and apple juice. Int. J. Food Microbiol. 1999, 47, 67–77. [Google Scholar] [CrossRef]
- No, H.K.; Kim, S.H.; Lee, S.H.; Park, N.Y.; Prinyawiwatkul, W. Stability and antibacterial activity of chitosan solutions affected by storage temperature and time. Carbohydr. Polym. 2006, 65, 174–178. [Google Scholar] [CrossRef]
- Li, X.-f.; Feng, X.-q.; Yang, S.; Fu, G.-q.; Wang, T.-p.; Su, Z.-x. Chitosan kills Escherichia coli through damage to be of cell membrane mechanism. Carbohydr. Polym. 2010, 79, 493–499. [Google Scholar] [CrossRef]
- Kulikov, S.N.; Tikhonov, V.E.; Bezrodnykh, E.A.; Lopatin, S.A.; Varlamov, V.P. Comparative evaluation of antimicrobial activity of oligochitosans against Klebsiella pneumoniae. Rus. J. Bioorg. Chem. 2015, 41, 57–62. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.-G.; Xue, Y.-P.; Liu, C.-S.; Yu, L.-J.; Ji, Q.-X.; Cha, D.S.; Park, H.J. Preparation and antibacterial activity of chitosan microshperes in a solid dispersing system. Front. Mater. Sci. China 2008, 2, 214–220. [Google Scholar] [CrossRef]
- Takahashi, T.; Imai, M.; Suzuki, I.; Sawai, J. Growth inhibitory effect on bacteria of chitosan membranes regulated with deacetylation degree. Biochem. Engin. J. 2008, 40, 485–491. [Google Scholar] [CrossRef]
- Raafat, D.; Von Bargen, K.; Haas, A.; Sahl, H.-G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef]
- Ziani, K.; Fernández-Pan, I.; Royo, M.; Maté, J.I. Antifungal activity of films and solutions based on chitosan against typical seed fungi. Food Hydrocoll. 2009, 23, 2309–2314. [Google Scholar] [CrossRef]
- Mellegård, H.; Strand, S.; Christensen, B.; Granum, P.; Hardy, S. Antibacterial activity of chemically defined chitosans: Influence of molecular weight, degree of acetylation and test organism. Int. J. Food Microbiol. 2011, 148, 48–54. [Google Scholar] [CrossRef]
- Tsai, G.J.; Su, W.H.; Chen, H.C.; Pan, C.L. Antimicrobial activity of shrimp chitin and chitosan from different treatments and applications of fish preservation. Fish. Sci. 2002, 68, 170–177. [Google Scholar] [CrossRef]
- Sivashankari, P.; Prabaharan, M. Prospects of chitosan-based scaffolds for growth factor release in tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1382–1389. [Google Scholar] [CrossRef]
- Misgav, M.; Lubetszki, A.; Brutman-Barazani, T.; Martinowitz, U.; Kenet, G. The hemostatic efficacy of chitosan-pads in hemodialysis patients with significant bleeding tendency. J. Vasc. Access 2017, 18, 220–224. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Y.; Chen, Z.; Geng, Y.; Xie, X.; Shen, X.; Li, T.; Li, S.; Wu, C.; Liu, Y. Chemo-photodynamic combined gene therapy and dual-modal cancer imaging achieved by pH-responsive alginate/chitosan multilayer-modified magnetic mesoporous silica nanocomposites. Biomater. Sci. 2017, 5, 1001–1013. [Google Scholar] [CrossRef]
- Sengiz, C.; Congur, G.; Eksin, E.; Erdem, A. Multiwalled carbon nanotubes-chitosan modified single-use biosensors for electrochemical monitoring of drug-DNA interactions. Electroanalysis 2015, 27, 1855–1863. [Google Scholar] [CrossRef]
- Lee, H.; Kim, M.; Yoon, Y.; Park, W. Fluorescent property of chitosan oligomer and its application as a metal ion sensor. Mar. Drugs 2017, 15, 105. [Google Scholar] [CrossRef]
- Rodrigues, S.V.; John, L.E.; Mitra, D.K.; Shah, R.; Shetty, G.; Prithyani, S.; Vijayakar, H. Evaluation and Comparison of Antimicrobial Effects of Chlorhexidine (CHX) and Chitosan (CHT) Mouthwash in Chronic Periodontitis (CGP) Patients-A Clinico-microbiological Study. JIDA J. Indian Dent. Assoc. 2018, 16, 26–32. [Google Scholar]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A.; Younus, M. Chitosan: A potential biopolymer for wound management. Int. J. Biol. Macromol. 2017, 102, 380–383. [Google Scholar] [CrossRef]
- Dekoninck, S.; Blanpain, C. Stem cell dynamics, migration and plasticity during wound healing. Nat. Cell Biol. 2019, 21, 18. [Google Scholar] [CrossRef]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Yang, L.; Lu, W.; Pang, Y.; Huang, X.; Wang, Z.; Qin, A.; Hu, Q. Fabrication of a novel chitosan scaffold with asymmetric structure for guided tissue regeneration. RSC Adv. 2016, 6, 71567–71573. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1. [Google Scholar]
- Huang, X.; Sun, Y.; Nie, J.; Lu, W.; Yang, L.; Zhang, Z.; Yin, H.; Wang, Z.; Hu, Q. Using absorbable chitosan hemostatic sponges as a promising surgical dressing. Int. J. Biol. Macromol. 2015, 75, 322–329. [Google Scholar] [CrossRef]
- Mi, F.-L.; Shyu, S.-S.; Wu, Y.-B.; Lee, S.-T.; Shyong, J.-Y.; Huang, R.-N. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials 2001, 22, 165–173. [Google Scholar] [CrossRef]
- Coma, V.; Martial-Gros, A.; Garreau, S.; Copinet, A.; Salin, F.; Deschamps, A. Edible antimicrobial films based on chitosan matrix. J. Food Sci. 2002, 67, 1162–1169. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Jones, A.; Vaughan, D. Hydrogel dressings in the management of a variety of wound types: A review. J. Orthop. Nurs. 2005, 9, S1–S11. [Google Scholar] [CrossRef]
- Mori, M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G.; Chlapanidas, T.; Torre, M.L.; Caramella, C. Sponge-like dressings based on the association of chitosan and sericin for the treatment of chronic skin ulcers. I. Design of experiments–assisted development. J. Pharm. Sci. 2016, 105, 1180–1187. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, W.; Wei, B.; Wang, X.; Tang, R.; Nie, J.; Wang, J. Carboxyl-modified poly (vinyl alcohol)-crosslinked chitosan hydrogel films for potential wound dressing. Carbohydr. Polym. 2015, 125, 189–199. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings–a review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Patrick, G.L. An Introduction to Medicinal Chemistry; Oxford University Press: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Bermingham, A.; Derrick, J.P. The folic acid biosynthesis pathway in bacteria: Evaluation of potential for antibacterial drug discovery. Bioessays 2002, 24, 637–648. [Google Scholar] [CrossRef]
- Hong, W.; Zeng, J.; Xie, J. Antibiotic drugs targeting bacterial RNAs. Acta Pharm. Sin. B 2014, 4, 258–265. [Google Scholar] [CrossRef]
- Madhumathi, K.; Kumar, P.S.; Abhilash, S.; Sreeja, V.; Tamura, H.; Manzoor, K.; Nair, S.; Jayakumar, R. Development of novel chitin/nanosilver composite scaffolds for wound dressing applications. J. Mater. Sci. Mater. Med. 2010, 21, 807–813. [Google Scholar] [CrossRef]
- Gliga, A.R.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef]
- Travan, A.; Pelillo, C.; Donati, I.; Marsich, E.; Benincasa, M.; Scarpa, T.; Semeraro, S.; Turco, G.; Gennaro, R.; Paoletti, S. Non-cytotoxic silver nanoparticle-polysaccharide nanocomposites with antimicrobial activity. Biomacromolecules 2009, 10, 1429–1435. [Google Scholar] [CrossRef]
- Zewde, B.; Ambaye, A.; Stubbs III, J.; Dharmara, R. A review of stabilized silver nanoparticles—Synthesis, biological properties, characterization, and potential areas of applications. JSM Nanotechnol. Nanomed. 2016, 4, 1–14. [Google Scholar]
- Anisha, B.S.; Biswas, R.; Chennazhi, K.P.; Jayakumar, R. Chitosan–hyaluronic acid/nano silver composite sponges for drug resistant bacteria infected diabetic wounds. Int. J. Biol. Macromol. 2013, 62, 310–320. [Google Scholar] [CrossRef]
- Mohandas, A.; Deepthi, S.; Biswas, R.; Jayakumar, R. Chitosan based metallic nanocomposite scaffolds as antimicrobial wound dressings. Bioact. Mater. 2018, 3, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Sahnoun, Z.; Bardaa, S.; Rebaï, T. The Effect of Natural Extracts on Laser Burn Wound Healing. In Recent Clinical Techniques, Results, and Research in Wounds; Springer: Cham, Switzerland, 2018; Volume 201, pp. 464–472. [Google Scholar]
- Saleem, M.; Nazir, M.; Ali, M.S.; Hussain, H.; Lee, Y.S.; Riaz, N.; Jabbar, A. Antimicrobial natural products: An update on future antibiotic drug candidates. Nat. Prod. Rep. 2010, 27, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.Y.; Hasan, N.; Malik, M.A. Chitosan and Aloe vera: Two gifts of nature. J. Dispers. Sci. Technol. 2010, 31, 799–811. [Google Scholar] [CrossRef]
- Silva, S.S.; Caridade, S.G.; Mano, J.F.; Reis, R.L. Effect of crosslinking in chitosan/aloe vera-based membranes for biomedical applications. Carbohydr. Polym. 2013, 98, 581–588. [Google Scholar] [CrossRef]
- Nguyen, V.C.; Nguyen, V.B.; Hsieh, M.-F. Curcumin-Loaded Chitosan/Gelatin Composite Sponge for Wound Healing Application. Int. J. Polym. Sci. 2013, 2013, 7. [Google Scholar] [CrossRef]
- Brudzynski, K.; Miotto, D.; Kim, L.; Sjaarda, C.; Maldonado-Alvarez, L.; Fukś, H. Active macromolecules of honey form colloidal particles essential for honey antibacterial activity and hydrogen peroxide production. Sci. Rep. 2017, 7, 7637. [Google Scholar] [CrossRef]
- Qu, Y.; McGiffin, D.; Kure, C.; McLean, J.; Duncan, C.; Peleg, A. A Comprehensive In Vitro Evaluation of Medihoney as an Anti-Biofilm Agent in Preventing Ventricular Assist Device Driveline Infections. J. Heart Lung. Transplant. 2019, 38, S101. [Google Scholar] [CrossRef]
- Morgan, T. The use of Actilite Protect® as an all-round wound care solution. Commun. Nurs. 2015, 29, 61–65. [Google Scholar]
- Stephen-Haynes, J.; Gibson, E.; Greenwood, M. Chitosan: A natural solution for wound healing. J. Community Nurs. 2014, 28, 48–53. [Google Scholar]
- Scagnelli, A.M. Therapeutic review: Manuka honey. J. Exotic Pet. Med. 2016, 25, 168–171. [Google Scholar] [CrossRef]
- Adeli, H.; Khorasani, M.T.; Parvazinia, M. Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: Fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int. J. Biol. Macromol. 2019, 122, 238–254. [Google Scholar] [CrossRef]
- Flores, C.; Lopez, M.; Tabary, N.; Neut, C.; Chai, F.; Betbeder, D.; Herkt, C.; Cazaux, F.; Gaucher, V.; Martel, B.; et al. Preparation and characterization of novel chitosan and β-cyclodextrin polymer sponges for wound dressing applications. Carbohydr. Polym. 2017, 173, 535–546. [Google Scholar] [CrossRef]
- Li, P.; Poon, Y.F.; Li, W.; Zhu, H.-Y.; Yeap, S.H.; Cao, Y.; Qi, X.; Zhou, C.; Lamrani, M.; Beuerman, R.W.; et al. A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat. Mater. 2010, 10, 149. [Google Scholar] [CrossRef]
- Poonguzhali, R.; Basha, S.K.; Kumari, V.S. Synthesis and characterization of chitosan-PVP-nanocellulose composites for in-vitro wound dressing application. Int. J. Biol. Macromol. 2017, 105, 111–120. [Google Scholar] [CrossRef]
- Smith, J.K.; Bumgardner, J.D.; Courtney, H.S.; Smeltzer, M.S.; Haggard, W.O. Antibiotic-loaded chitosan film for infection prevention: A preliminary in vitro characterization. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2010, 94, 203–211. [Google Scholar] [CrossRef]
- Monteiro, N.; Martins, M.; Martins, A.; Fonseca, N.A.; Moreira, J.N.; Reis, R.L.; Neves, N.M. Antibacterial activity of chitosan nanofiber meshes with liposomes immobilized releasing gentamicin. Acta Biomater. 2015, 18, 196–205. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Zisi, A.P.; Exindari, M.K.; Karantas, I.D.; Bikiaris, D.N. Porous dressings of modified chitosan with poly(2-hydroxyethyl acrylate) for topical wound delivery of levofloxacin. Carbohydr. Polym. 2016, 143, 90–99. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; García-González, C.A. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef]
- Shao, W.; Wu, J.; Wang, S.; Huang, M.; Liu, X.; Zhang, R. Construction of silver sulfadiazine loaded chitosan composite sponges as potential wound dressings. Carbohydr. Polym. 2017, 157, 1963–1970. [Google Scholar] [CrossRef]
- Wichai, S.; Chuysinuan, P.; Chaiarwut, S.; Ekabutr, P.; Supaphol, P. Development of bacterial cellulose/alginate/chitosan composites incorporating copper (II) sulfate as an antibacterial wound dressing. J. Drug Deliv. Sci. Technol. 2019, 51, 662–671. [Google Scholar] [CrossRef]
- Rahimi, M.; Ahmadi, R.; Samadi Kafil, H.; Shafiei-Irannejad, V. A novel bioactive quaternized chitosan and its silver-containing nanocomposites as a potent antimicrobial wound dressing: Structural and biological properties. Mater. Sci. Eng. C 2019, 101, 360–369. [Google Scholar] [CrossRef]
- Regiel-Futyra, A.; Liskiewicz, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Stochel, G.; Kyziol, A. Development of non cytotoxic chitosan-gold nanocomposites as efficient antibacterial materials. ACS Appl. Mater. Interfaces 2015, 7, e1099. [Google Scholar]
- Bal-Ozturk, A.; Karal-Yilmaz, O.; Akguner, Z.P.; Aksu, S.; Tas, A.; Olmez, H. Sponge-like chitosan-based nanostructured antibacterial material as a topical hemostat. J. Appl. Polym. Sci. 2019, 136, 47522. [Google Scholar] [CrossRef]
- Cai, N.; Li, C.; Han, C.; Luo, X.; Shen, L.; Xue, Y.; Yu, F. Tailoring mechanical and antibacterial properties of chitosan/gelatin nanofiber membranes with Fe3O4 nanoparticles for potential wound dressing application. Appl. Surface Sci. 2016, 369, 492–500. [Google Scholar] [CrossRef]
- Woo, C.H.; Choi, Y.C.; Choi, J.S.; Lee, H.Y.; Cho, Y.W. A bilayer composite composed of TiO2-incorporated electrospun chitosan membrane and human extracellular matrix sheet as a wound dressing. J. Biomater. Sci. Polym. Ed. 2015, 26, 841–854. [Google Scholar] [CrossRef]
- Gomes Neto, R.J.; Genevro, G.M.; Paulo, L.d.A.; Lopes, P.S.; de Moraes, M.A.; Beppu, M.M. Characterization and in vitro evaluation of chitosan/konjac glucomannan bilayer film as a wound dressing. Carbohydr. Polym. 2019, 212, 59–66. [Google Scholar] [CrossRef]
- Güneş, S.; Tıhmınlıoğlu, F. Hypericum perforatum incorporated chitosan films as potential bioactive wound dressing material. Int. J. Biol. Macromol. 2017, 102, 933–943. [Google Scholar] [CrossRef]
- Silva, S.S.; Popa, E.G.; Gomes, M.E.; Cerqueira, M.; Marques, A.P.; Caridade, S.G.; Teixeira, P.; Sousa, C.; Mano, J.F.; Reis, R.L. An investigation of the potential application of chitosan/aloe-based membranes for regenerative medicine. Acta Biomater. 2013, 9, 6790–6797. [Google Scholar] [CrossRef]
- Altiok, D.; Altiok, E.; Tihminlioglu, F. Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. J. Mat. Sci. Mater. Med. 2010, 21, 2227–2236. [Google Scholar] [CrossRef]
- Noori, S.; Kokabi, M.; Hassan, Z.M. Poly(vinyl alcohol)/chitosan/honey/clay responsive nanocomposite hydrogel wound dressing. J. Appl. Polym. Sci. 2018, 135, 46311. [Google Scholar] [CrossRef]
- Cheng, B.; Pei, B.; Wang, Z.; Hu, Q. Advances in chitosan-based superabsorbent hydrogels. RSC Adv. 2017, 7, 42036–42046. [Google Scholar] [CrossRef]
- Cui, Z.; Zheng, Z.; Lin, L.; Si, J.; Wang, Q.; Peng, X.; Chen, W. Electrospinning and crosslinking of polyvinyl alcohol/chitosan composite nanofiber for transdermal drug delivery. Adv. Polym. Technol. 2018, 37, 1917–1928. [Google Scholar] [CrossRef]
- Rogina, A.; Ivanković, M.; Ivanković, H. Preparation and characterization of nano-hydroxyapatite within chitosan matrix. Mater. Sci. Eng. C 2013, 33, 4539–4544. [Google Scholar] [CrossRef]
- Ressler, A.; Ródenas-Rochina, J.; Ivanković, M.; Ivanković, H.; Rogina, A.; Ferrer, G.G. Injectable chitosan-hydroxyapatite hydrogels promote the osteogenic differentiation of mesenchymal stem cells. Carbohydr. Polym. 2018, 197, 469–477. [Google Scholar] [CrossRef]
- Zhou, P.; Xia, Y.; Cheng, X.; Wang, P.; Xie, Y.; Xu, S. Enhanced bone tissue regeneration by antibacterial and osteoinductive silica-HACC-zein composite scaffolds loaded with rhBMP-2. Biomaterials 2014, 35, 10033–10045. [Google Scholar] [CrossRef]
- García-González, C.A.; Barros, J.; Rey-Rico, A.; Redondo, P.; Gómez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C.; Monteiro, F.J. Antimicrobial Properties and Osteogenicity of Vancomycin-Loaded Synthetic Scaffolds Obtained by Supercritical Foaming. ACS Appl. Mater. Interfaces 2018, 10, 3349–3360. [Google Scholar] [CrossRef]
- Park, J.H.; Cho, Y.W.; Chung, H.; Kwon, I.C.; Jeong, S.Y. Synthesis and characterization of sugar-bearing chitosan derivatives: Aqueous solubility and biodegradability. Biomacromolecules 2003, 4, 1087–1091. [Google Scholar] [CrossRef]
- Salar, S.; Mehrnejad, F.; Sajedi, R.H.; Arough, J.M. Chitosan nanoparticles-trypsin interactions: Bio-physicochemical and molecular dynamics simulation studies. Int. J. Biol. Macromol. 2017, 103, 902–909. [Google Scholar] [CrossRef]
- Rassu, G.; Soddu, E.; Cossu, M.; Gavini, E.; Giunchedi, P.; Dalpiaz, A. Particulate formulations based on chitosan for nose-to-brain delivery of drugs. A review. J. Drug Deliv. Sci. Technol. 2016, 32, 77–87. [Google Scholar] [CrossRef]
- Yu, S.; Xu, X.; Feng, J.; Liu, M.; Hu, K. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int. J. Pharm. 2019, 560, 282–293. [Google Scholar] [CrossRef]
- Santander-Ortega, M.J.; Plaza-Oliver, M.; Rodríguez-Robledo, V.; Castro-Vázquez, L.; Villaseca-González, N.; González-Fuentes, J.; Marcos, P.; Arroyo-Jiménez, M.M.; Lozano, M.V. Colloids for drug delivery to the brain. J. Drug Deliv. Sci. Technol. 2017, 42, 193–206. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Patel, H.M.; Surana, S.J.; Vanjari, Y.H.; Belgamwar, V.S.; Pardeshi, C.V. N,N,N-Trimethyl chitosan: An advanced polymer with myriad of opportunities in nanomedicine. Carbohydr. Polym. 2017, 157, 875–902. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C.V.; Belgamwar, V.S. Controlled synthesis of N,N,N-trimethyl chitosan for modulated bioadhesion and nasal membrane permeability. Int. J. Biol. Macromol. 2016, 82, 933–944. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Belgamwar, V.S. N,N,N-trimethyl chitosan modified flaxseed oil based mucoadhesive neuronanoemulsions for direct nose to brain drug delivery. Int. J. Biol. Macromol. 2018, 120, 2560–2571. [Google Scholar] [CrossRef]
- Mooney, R.; Weng, Y.; Garcia, E.; Bhojane, S.; Smith-Powell, L.; Kim, S.U.; Annala, A.J.; Aboody, K.S.; Berlin, J.M. Conjugation of pH-responsive nanoparticles to neural stem cells improves intratumoral therapy. J. Controll Release 2014, 191, 82–89. [Google Scholar] [CrossRef]
- Turabee, M.H.; Jeong, T.H.; Ramalingam, P.; Kang, J.H.; Ko, Y.T. N,N,N-trimethyl chitosan embedded in situ Pluronic F127 hydrogel for the treatment of brain tumor. Carbohydr. Polym. 2019, 203, 302–309. [Google Scholar] [CrossRef]
- Raj, R.; Wairkar, S.; Sridhar, V.; Gaud, R. Pramipexole dihydrochloride loaded chitosan nanoparticles for nose to brain delivery: Development, characterization and in vivo anti-Parkinson activity. Int. J. Biol. Macromol. 2018, 109, 27–35. [Google Scholar] [CrossRef]
- Ray, S.; Sinha, P.; Laha, B.; Maiti, S.; Bhattacharyya, U.K.; Nayak, A.K. Polysorbate 80 coated crosslinked chitosan nanoparticles of ropinirole hydrochloride for brain targeting. J. Drug Deliv. Sci. Technol. 2018, 48, 21–29. [Google Scholar] [CrossRef]
- Kosaraju, J.; Gali, C.C.; Khatwal, R.B.; Dubala, A.; Chinni, S.; Holsinger, R.M.D.; Madhunapantula, V.S.R.; Muthureddy Nataraj, S.K.; Basavan, D. Saxagliptin: A dipeptidyl peptidase-4 inhibitor ameliorates streptozotocin induced Alzheimer’s disease. Neuropharmacology 2013, 72, 291–300. [Google Scholar] [CrossRef]
- Fernandes, J.; Ghate, M.V.; Basu Mallik, S.; Lewis, S.A. Amino acid conjugated chitosan nanoparticles for the brain targeting of a model dipeptidyl peptidase-4 inhibitor. Int. J. Pharm. 2018, 547, 563–571. [Google Scholar] [CrossRef]
- Kaur, S.; Manhas, P.; Swami, A.; Bhandari, R.; Sharma, K.K.; Jain, R.; Kumar, R.; Pandey, S.K.; Kuhad, A.; Sharma, R.K.; et al. Bioengineered PLGA-chitosan nanoparticles for brain targeted intranasal delivery of antiepileptic TRH analogues. Chem. Eng. J. 2018, 346, 630–639. [Google Scholar] [CrossRef]
- Fisher, R.S.; Boas, W.v.E.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J., Jr. Epileptic Seizures and Epilepsy: Definitions Proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Kaye, C.M.; Nicholls, B. Clinical Pharmacokinetics of Ropinirole. Clin. Pharmacokinet 2000, 39, 243–254. [Google Scholar] [CrossRef]
- Liu, S.; Yang, S.; Ho, P.C. Intranasal administration of carbamazepine-loaded carboxymethyl chitosan nanoparticles for drug delivery to the brain. Asian J. Pharm. Sci. 2018, 13, 72–81. [Google Scholar] [CrossRef]
- Trotta, V.; Pavan, B.; Ferraro, L.; Beggiato, S.; Traini, D.; Des Reis, L.G.; Scalia, S.; Dalpiaz, A. Brain targeting of resveratrol by nasal administration of chitosan-coated lipid microparticles. Eur. J. Pharm. Biopharm. 2018, 127, 250–259. [Google Scholar] [CrossRef]
- Jeong, Y.-I.; Jin, S.-G.; Kim, I.-Y.; Pei, J.; Wen, M.; Jung, T.-Y.; Moon, K.-S.; Jung, S. Doxorubicin-incorporated nanoparticles composed of poly(ethylene glycol)-grafted carboxymethyl chitosan and antitumor activity against glioma cells in vitro. Colloid Surf. B Biointerfaces 2010, 79, 149–155. [Google Scholar] [CrossRef]
- Bruinsmann, F.A.; Pigana, S.; Aguirre, T.; Souto, D.G.; Pereira, G.G.; Bianchera, A.; Fasiolo, L.T.; Colombo, G.; Marques, M.; Pohlmann, A.R.; et al. Chitosan-Coated Nanoparticles: Effect of Chitosan Molecular Weight on Nasal Transmucosal Delivery. Pharmaceutics 2019, 11, 86. [Google Scholar] [CrossRef]
- Ramreddy, S.; Janapareddi, K. Brain targeting of chitosan-based diazepam mucoadhesive microemulsions via nasal route: Formulation optimization, characterization, pharmacokinetic and pharmacodynamic evaluation. Drug Dev. Ind. Pharm. 2019, 45, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Tzeyung, A.S.; Shadab, M.L.; Bhattamisra, S.K.; Madheswaren, T.; Alhakamy, N.A.; Aldawsari, H.M.; Radhakrishnan, A.K. Fabrication, Optimization, and Evaluation of Rotigotine-Loaded Chitosan Nanoparticles for Nose-To-Brain Delivery. Pharmaceutics 2019, 11, 26. [Google Scholar] [CrossRef]
- Rassu, G.; Porcu, E.; Fancello, S.; Obinu, A.; Senes, N.; Galleri, G.; Migheli, R.; Gavini, E.; Giunchedi, P. Intranasal Delivery of Genistein-Loaded Nanoparticles as a Potential Preventive System against Neurodegenerative Disorders. Pharmaceutics 2018, 11, 8. [Google Scholar] [CrossRef]
- Qureshi, M.; Aqil, M.; Imam, S.S.; Ahad, A.; Sultana, Y. Formulation and Evaluation of Neuroactive Drug Loaded Chitosan Nanoparticle for Nose to Brain Delivery: In-vitro Characterization and In-vivo Behavior Study. Curr. Drug Deliv. 2019, 16, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Aqil, M.; Imam, S.S.; Ahad, A.; Sultana, Y.; Ali, A.; Khan, K. Temozolomide loaded nano lipid based chitosan hydrogel for nose to brain delivery: Characterization, nasal absorption, histopathology and cell line study. Int. J. Biol. Macromol. 2018, 116, 1260–1267. [Google Scholar] [CrossRef]
- Salade, L.; Wauthoz, N.; Vermeersch, M.; Amighi, K.; Goole, J. Chitosan-coated liposome dry-powder formulations loaded with ghrelin for nose-to-brain delivery. Eur. J. Pharm. Biopharm. 2018, 129, 257–266. [Google Scholar] [CrossRef]
- Wei, H.; Lai, S.; Wei, J.; Yang, L.; Jiang, N.; Wang, Q.; Yu, Y. A Novel Delivery Method of Cyclovirobuxine D for Brain-Targeting: Chitosan Coated Nanoparticles Loading Cyclovirobuxine D by Intranasal Administration. J. Nanosci. Nanotechnol. 2018, 18, 5274–5282. [Google Scholar] [CrossRef]
- Rinaldi, F.; Hanieh, P.; Chan, L.; Angeloni, L.; Passeri, D.; Rossi, M.; Wang, J.; Imbriano, A.; Carafa, M.; Marianecci, C. Chitosan Glutamate-Coated Niosomes: A Proposal for Nose-to-Brain Delivery. Pharmaceutics 2018, 10, 38. [Google Scholar] [CrossRef]
- Singh, S.K.; Hidau, M.K.; Gautam, S.; Gupta, K.; Singh, K.P.; Singh, S.K.; Singh, S. Glycol chitosan functionalized asenapine nanostructured lipid carriers for targeted brain delivery: Pharmacokinetic and teratogenic assessment. Int. J. Biol. Macromol. 2018, 108, 1092–1100. [Google Scholar] [CrossRef]
- Rassu Gavini, E.; Catra, A.; Obinu, A.; Porcu, E.P.; Guinchedi, P. Hydroxypropyl-β-Cyclodextrin Formulated in Nasal Chitosan Microspheres as Candidate Therapeutic Agent in Alzheimers Disease. Curr. Drug. Deliv. 2018, 15, 746–748. [Google Scholar] [CrossRef] [PubMed]
- Margret, A.A.; Ganesh Kumar, A.A. Therapeutic Paradigm to Appraise the Competence of Chitosan Oligosaccharide Lactate Targeting Monoamine Oxidase-A and PGlycoprotein to Contest Depression by Channeling the Blood Brain Barrier. Curr. Bioinform. 2018, 13, 273–279. [Google Scholar] [CrossRef]
- Belgamwar, A.; Khan, S.; Yeole, P. Intranasal chitosan-g-HPβCD nanoparticles of efavirenz for the CNS targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Park, J.H.; Chung, H.; Kwon, I.C.; Jeong, S.Y.; Kim, I.-S. Physicochemical characteristics of self-assembled nanoparticles based on glycol chitosan bearing 5β-cholanic acid. Langmuir 2003, 19, 10188–10193. [Google Scholar] [CrossRef]
- Mansur, A.A.; Mansur, H.S. Quantum dot/glycol chitosan fluorescent nanoconjugates. Nanoscale Res. Lett. 2015, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Kohgo, O.; Kurita, K.; Kuzuhara, H. Chemospecific manipulations of a rigid polysaccharide: Syntheses of novel chitosan derivatives with excellent solubility in common organic solvents by regioselective chemical modifications. Macromolecules 1991, 24, 4745–4748. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J. Controll Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Amidi, M.; Mastrobattista, E.; Jiskoot, W.; Hennink, W.E. Chitosan-based delivery systems for protein therapeutics and antigens. Adv. Drug Deliv. Rev. 2010, 62, 59–82. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Zhang, H.; Shen, Z.; Bi, J.; Dai, S. Developing a chitosan supported imidazole Schiff-base for high-efficiency gene delivery. Polym. Chem. 2013, 4, 840–850. [Google Scholar] [CrossRef]
- MacLaughlin, F.C.; Mumper, R.J.; Wang, J.; Tagliaferri, J.M.; Gill, I.; Hinchcliffe, M.; Rolland, A.P. Chitosan and depolymerized chitosan oligomers as condensing carriers for in vivo plasmid delivery. J. Controll Release 1998, 56, 259–272. [Google Scholar] [CrossRef]
- Morris, V.B.; Sharma, C.P. Folate mediated histidine derivative of quaternised chitosan as a gene delivery vector. Int. J. Pharm. 2010, 389, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Raftery, R.; O’Brien, F.; Cryan, S.-A. Chitosan for gene delivery and orthopedic tissue engineering applications. Molecules 2013, 18, 5611–5647. [Google Scholar] [CrossRef]
- Shim, G.; Kim, D.; Park, G.T.; Jin, H.; Suh, S.-K.; Oh, Y.-K. Therapeutic gene editing: Delivery and regulatory perspectives. Acta Pharmacol. Sin. 2017, 38, 738. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, H.; Yan, J.; Bryers, J.D. Scaffold-mediated delivery for non-viral mRNA vaccines. Gene Ther. 2018, 25, 556. [Google Scholar] [CrossRef]
- O’Rorke, S.; Keeney, M.; Pandit, A. Non-viral polyplexes: Scaffold mediated delivery for gene therapy. Progr. Polym. Sci. 2010, 35, 441–458. [Google Scholar] [CrossRef]
- Darder, M.; Colilla, M.; Ruiz-Hitzky, E. Chitosan–clay nanocomposites: Application as electrochemical sensors. Appl. Clay Sci. 2005, 28, 199–208. [Google Scholar] [CrossRef]
- Shan, C.; Yang, H.; Han, D.; Zhang, Q.; Ivaska, A.; Niu, L. Graphene/AuNPs/chitosan nanocomposites film for glucose biosensing. Biosens. Bioelectr. 2010, 25, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yao, J.; Russel, M.; Chen, H.; Chen, K.; Zhou, Y.; Ceccanti, B.; Zaray, G.; Choi, M.M. Development and analytical application of a glucose biosensor based on glucose oxidase/O-(2-hydroxyl) propyl-3-trimethylammonium chitosan chloride nanoparticle-immobilized onion inner epidermis. Biosens. Bioelectr. 2010, 25, 2238–2243. [Google Scholar] [CrossRef]
- Shakya, A.K.; Nandakumar, K.S. An update on smart biocatalysts for industrial and biomedical applications. J. R. Soc. Interface 2018, 15, 20180062. [Google Scholar] [CrossRef]
- Bharathiraja, S.; Bui, N.Q.; Manivasagan, P.; Moorthy, M.S.; Mondal, S.; Seo, H.; Phuoc, N.T.; Phan, T.T.V.; Kim, H.; Lee, K.D. Multimodal tumor-homing chitosan oligosaccharide-coated biocompatible palladium nanoparticles for photo-based imaging and therapy. Sci. Rep. 2018, 8, 500. [Google Scholar] [CrossRef]
- Karagozlu, M.Z.; Karadeniz, F.; Kim, S.-K. Anti-HIV activities of novel synthetic peptide conjugated chitosan oligomers. Int. J. Biol. Macromol. 2014, 66, 260–266. [Google Scholar] [CrossRef]
- Ramana, L.N.; Sharma, S.; Sethuraman, S.; Ranga, U.; Krishnan, U.M. Evaluation of chitosan nanoformulations as potent anti-HIV therapeutic systems. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Jeevithan, E.; Chelliah, R.; Kathiresan, K.; Wen-Hui, W.; Oh, D.-H.; Wang, M.-H. Zinc-chitosan nanoparticles induced apoptosis in human acute T-lymphocyte leukemia through activation of tumor necrosis factor receptor CD95 and apoptosis-related genes. Int. J. Biol. Macromol. 2018, 119, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Adebisi, A.O.; Laity, P.R.; Conway, B.R. Formulation and evaluation of floating mucoadhesive alginate beads for targeting Helicobacter pylori. J. Pharm. Pharmacol. 2015, 67, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Li, J.; Wang, J.; Yin, Z.; Zhu, Y.; Liu, W. Development of Timolol-Loaded Galactosylated Chitosan Nanoparticles and Evaluation of Their Potential for Ocular Drug Delivery. AAPS PharmSciTech 2017, 18, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Park, K.; Kim, Y.-S.; Bae, S.M.; Lee, S.; Jo, H.G.; Park, R.-W.; Kim, I.-S.; Jeong, S.Y.; Kim, K. Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy. J. Controll Release 2008, 127, 208–218. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, C.; Xia, X.; Liu, Y. Self-assembled lecithin/chitosan nanoparticles for oral insulin delivery: Preparation and functional evaluation. Int. J. Nanomed. 2016, 11, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Savin, C.-L.; Popa, M.; Delaite, C.; Costuleanu, M.; Costin, D.; Peptu, C.A. Chitosan grafted-poly(ethylene glycol) methacrylate nanoparticles as carrier for controlled release of bevacizumab. Mater. Sci. Eng. C 2019, 98, 843–860. [Google Scholar] [CrossRef]
- Tammam, S.N.; Khalil, M.A.; Gawad, E.A.; Althani, A.; Zaghloul, H.; Azzazy, H.M. Chitosan gold nanoparticles for detection of amplified nucleic acids isolated from sputum. Carbohydr. Polym. 2017, 164, 57–63. [Google Scholar] [CrossRef]
- Huang, W.-C.; Wang, W.; Xue, C.; Mao, X. Effective Enzyme Immobilization onto a Magnetic Chitin Nanofiber Composite. ACS Sustain. Chem. Eng. 2018, 6, 8118–8124. [Google Scholar] [CrossRef]

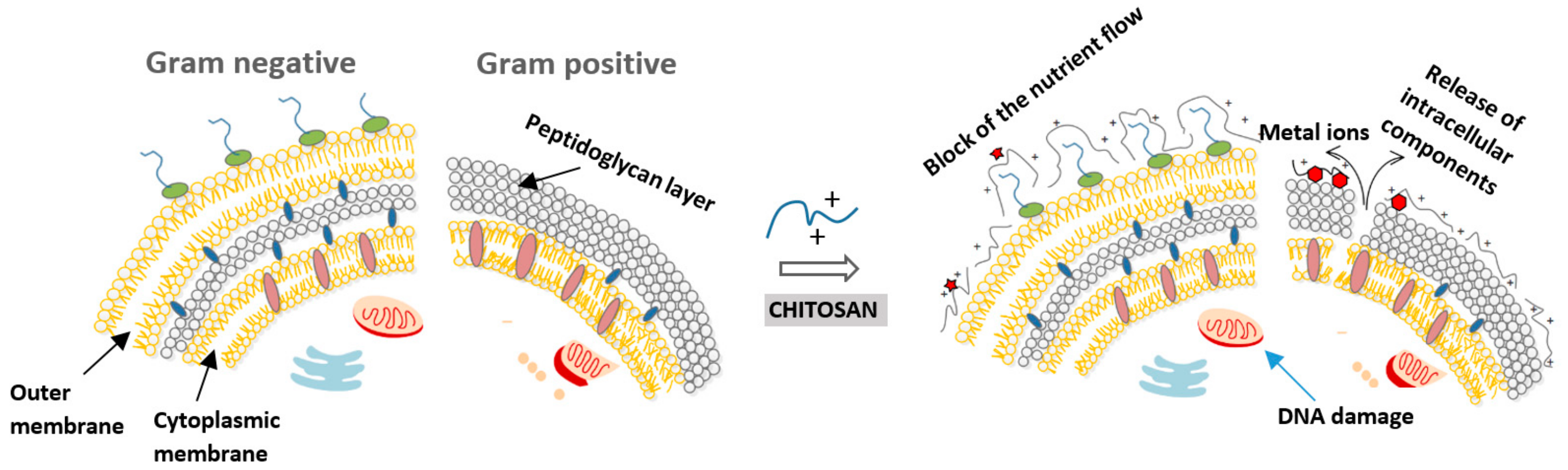
| Antimicrobial Mechanism | Findings |
|---|---|
| Polycationic nature of chitosan |
|
| Binding to bacterial DNA (inhibition of mRNA) |
|
| Chelation agent (nutrients and essential metals) |
|
| Blocking agent |
| Factors Influencing Antimicrobial Activity | Findings |
|---|---|
| Environmental Factors | |
| pH | |
| Temperature | |
| Fundamental Factors | |
| Molecular weight | |
| Degree of acetylation | |
| Type of Microorganism | |
| Gram-positive bacteria |
|
| Gram-negative bacteria | |
| Fungi |
| Type of Microorganism | pH | Mw (kDa) | Degree of Acetylation (%) | MIC (µg/mL) | Ref. |
|---|---|---|---|---|---|
| Gram-positive | |||||
| Bacillus cereus | 5.5 | 43 | 6 | 60 | [22] |
| Bacillus cereus | 6 | 2.3–224 | 16–48 | 80–2000 | [43] |
| Bacillus megaterium | 5.9 | 28–1670 | - | 500–800 | [26] |
| Lactobacillus brevis | 5.9 | 224–1106 | - | 500–1000 | [26] |
| Lactobacillus bulgaricus | 5.9 | 28–1670 | - | up to 1000 | [26] |
| Listeria monocytogenes | 6 | 49–1100 | 2–53 | 150 | [44] |
| Staphylococcus aureus | 5.9 | 28–1106 | - | 800–10000 | [26] |
| Gram-negative | |||||
| Escherichia coli | 6 | 49–1100 | - | 100–500 | [44] |
| Escherichia coli | 5.9 | 28–1670 | 2–53 | 800–1000 | [26] |
| Escherichia coli | 6 | 3–224 | 16–48 | 30–2000 | [43] |
| Enterobacter aerogenes | 5.5 | 43 | 6 | 60 | [22] |
| Pseudomonas aeruginosa | 6 | 49–1100 | 2–53 | 150–200 | [44] |
| Pseudomonas fluorescens | 5.5 | 43 | 6 | 80 | [22] |
| Salmonella typhimurium | 6 | 49–1670 | 2–53 | 1500–2000 | [44] |
| Vibrio cholera | 6 | 49–1100 | 2–53 | 200 | [44] |
| Fungi | |||||
| Botrytis cinerea | - | - | - | 10 | [6] |
| Candida lambica | 5.5 | 43 | 6 | 400 | [22] |
| Drechstera sorokiana | - | - | - | 10 | [6] |
| Fusarium oxysporum | 6 | 49–1100 | 2–57 | 500–2000 | [44] |
| Microsporum canis | - | - | - | 1000 | [6] |
| Micronectriella nivalis | - | - | - | 10 | [6] |
| Trichophyton equinum | - | - | - | 2500 | [6] |
| Type | Findings | Tested Microorganisms | Ref. | |
|---|---|---|---|---|
| Native Chitosan-Based Biomaterials | ||||
| Chitosan/PVA/starch | Membrane | Excellent cell growth and proliferation | Escherichia coli, Staphylococcus aureus | [84] |
| Chitosan/β-cyclodextrin polymer | Sponge | Controlled swelling and drug delivery | Staphylococcus aureus, Escherichia coli | [85] |
| Chitosan | Hydrogel | Superb antifungal and antimicrobial effects | Pseudomonas aeruginosa, Escherichia coli, Fusarium solani | [86] |
| Chitosan | Membrane | Epithelialization rate was increased | Pseudomonas aeruginosa, Staphylococcus aureus | [58] |
| Chitosan/PVP/nano-cellulose | Film | In vitro wound dressing application was significant | Staphylococcus aureus | [87] |
| Chitosan-distamycin and vancomycin | Films | 80% degrees of deacetylation were optimal for eluting antibiotics | Staphylococcus aureus | [88] |
| Chitosan and Antibiotics | ||||
| Chitosan nanofiber mesh-gentamicin-loaded liposomes | Membrane | Antibacterial activity | Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus | [89] |
| Chitosan/poly(2-hydroxyethyl acrylate)-levofloxacin | Sponge | The prepared dressing shows a significant inhibition zone of bacteria strains | Methicillin- susceptible Staphylococcus aureus, Methicillin- resistant Staphylococcus aureus | [90] |
| Chitosan-vancomycin | Aerogel | Low-density, large surface area | Staphylococcus aureus | [91] |
| Chitosan/sulfadiazine | Sponge | Antibacterial activity | Escherichia coli,Staphylococcus aureus, Bacillus subtilis | [92] |
| Chitosan-Entrapped Metallic Nanoparticles | ||||
| Chitosan/sodium alginate-Cu | Hydrogel | Safe to use in contact with living cells | Methicillin-resistant Staphylococcus aureus, Escherichia coli | [93] |
| Quaternized chitosan-nAg | Film | Property with antibacterial effects | Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Candida albicans | [94] |
| Chitosan-nAu | Film | nAu interacts with cell wall and inhibits mitochondrial membrane | Staphylococcus aureus, Pseudomonas aeruginosa | [95] |
| Chitosan/algetic acid-nZnO | Sponge | Potential to be an antibacterial topical hemostat | Staphylococcus aureus | [96] |
| Chitosan/gelatin- nFe3O4 | Fe3O4 enhanced mechanical and antibacterial properties | Escherichia coli, Staphylococcus aureus | [97] | |
| Chitosan/ECM-nTiO2 | Composite | Faster regeneration of granulation tissue | Escherichia coli, Staphylococcus aureus | [98] |
| Chitosan Entrapped with Plant Extracts | ||||
| Chitosan- amorphophallus konjac plant | Film | Low cytotoxicity and inhibition of microbial penetration. | Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa | [99] |
| Chitosan- Hypericum perforatum | Film | The highest strain value was obtained in 0.25% oil content films | Escherichia coli, Staphylococcus aureus | [100] |
| Chitosan- Aloe vera | Membrane | Promising wound dressing material | Escherichia coli, Staphylococcus aureus | [101] |
| Chitosan-thyme oil | Films | Antibacterial activity on all studied microorganisms | Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae | [102] |
| Poly(vinyl alcohol)/chitosan-honey | Hydrogel | Faster honey release rate at higher pH values | Staphylococcus aureus | [103] |
| Chitosan/gelatin-curcumin | Sponge | Enhances the formation of collagen and wound closure in vivo | Pseudomonas aeruginosa | [78] |
| Drug | Disease | Delivery | Ref. | |
|---|---|---|---|---|
| Chitosan nanoparticles | Chlorotoxin and transferrin | Brain tumors | Intranasal | [113] |
| Chitosan nanoparticles | Pramipexole | Parkinson’s disease | Intranasal | [120] |
| Flaxseed oil/N,N,N-trimethyl chitosan neuronanoemulsion | Ropinirole-dextran sulfate | Parkinson’s disease | Intranasal | [117] |
| Pluronic F127/N,N,N-trimethyl chitosan hydrogel system | Docetaxel | Malignant glioma | Intracranial injection | [119] |
| Chitosan/L-valine -based nanoparticles | Saxagliptin | Alzheimer’s disease | Intraperitoneal route | [123] |
| Chitosan coated lipid microparticles | Resveratrol | Central nervous system diseases | Nasal administration | [128] |
| Poly-lactide-co-glycolide/chitosan nanoparticles | L-pGlu-(1-benzyl)–L-His–LProNH2 and L-pGlu-(2-propyl)–L-His–L-ProNH2 | Epilepsy | Intranasal | [124] |
| Chitosan nanoparticles | Ropinirole hydrochloride | Parkinson’s disease | Intravenously via the dorsal tail vein | [121] |
| Carboxymethyl chitosan nanoparticles | Carbamazepine | Epilepsy | Intranasal | [127] |
| Methoxy poly(ethylene glycol)-grafted Carboxymethyl chitosan nanoparticles | Doxorubicin | Malignant glioma | [129] | |
| Poly-ε-caprolactone nanocapsules coated with chitosan | Simvastatin | Brain tumors | Intranasal | [130] |
| Chitosan-based mucoadhesive microemulsions | Diazepam | Epilepsy | Intranasal | [131] |
| Chitosan nanoparticles | Rotigotine | Parkinson’s disease | Intranasal | [132] |
| Chitosan nanoparticles | Genistein | Neurodegenerative diseases | Intranasal | [133] |
| Chitosan lipid nanoparticles | Risperidone | Schizophrenia | Intranasal | [134] |
| Nano lipid Vit E mixed with melted Gelucire 44/ 14/chitosan hydrogel formulation | Temozolomide | Metastatic melanoma and glioma | Intranasal | [135] |
| Chitosan-coated liposome dry-powder formulations | Ghrelin | Cachexia | Intranasal | [136] |
| Chitosan nanoparticles | Cyclovirobuxine D | Cardiovascular disease | Intranasal | [137] |
| Chitosan glutamate coated niosomes | Pentamidine | Alzheimer’s disease | Intranasal | [138] |
| Glycol chitosan coated nanostructured lipid carrier | Asenapine maleate | Schizophrenia and bipolar disorders | Intranasal | [139] |
| Nasal chitosan microspheres | Hydroxypropyl--cyclodextrin | Alzheimer’s disease | Nasal route | [140] |
| Chitosan oligosaccharide | Chitosan oligosaccharide lactate | Depression | [141] | |
| Chitosan-grafted HPbCD intranasal EFV nanoparticles | Efavirenz | Neuro-AIDS | Intranasal | [142] |
| Matrix | Biomedical Application | Findings | Ref. |
|---|---|---|---|
| Zinc-chitosan nanoparticles | Treatment of acute lymphoblastic leukemia | Induced apoptosis in human acute T-lymphocyte leukemia through activation of tumor necrosis factor receptor CD95 | [163] |
| Sodium alginate beads with olive oil and coating with chitosan | Helicobacter pylori infections | Controlled release of active Clarithromycin | [164] |
| Timolol maleate-loaded galactosylated chitosan nanoparticles | Ocular delivery of timolol maleate | In vitro transcorneal permeation study and confocal microscopy showed enhanced penetration, and retention in the cornea | [165] |
| Modified glycol chitosan nanoparticles encapsulated camptothecin | Cancer therapy | Stable and effective drug delivery system in cancer therapy | [166] |
| Insulin-loaded lecithin/chitosan nanoparticles | Drug delivery system to the deep lung | Improved oral bioavailability, time-dependent release, and therapeutic activity | [167] |
| Chitosan grafted poly(ethylene glycol) methacrylate | Posterior eye diseases | Particles were found suitable from the cytotoxicity and hemocompatibility points of view | [168] |
| Palladium nanoparticles chitosan oligosaccharide (COS) functionalized with RGD peptide | Breast cancer therapy by imaging | Matrix acts as an ideal nanotheranostic agent for enhanced imaging and tumor therapy, using a non-invasive near-infrared laser | [160] |
| Graphene/AuNPs/chitosan electrode | Construction of a glucose biosensor | High electrocatalytic activity toward H2O2 and O2 | [157] |
| Chitosan-RNAi complexes | Gene therapy | Transfection of CHO-K1, HEK293, H1299, HepG2 cells | [152] |
| Chitosan–montmorillonite nanocomposites | Biomedical sensors | Bulk-modified potentiometric sensors for anionic detection in aqueous samples | [156] |
| Chitosan-Au particles | Biomedical sensors to detect DNA | Low cost of preparation | [169] |
| Saquinavir-loaded chitosan nanoparticles | Effective anti-HIV system | Strains of HIV – NL4-3 and Indie-C1 were found to respond to delivery system | [162] |
| Magnetic chitin nanofiber composite | Immobilization of therapeutic enzyme | Immobilized chymotrypsin could be easily separated and recycled from the reaction system by magnetic force | [170] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Chitosan-Based (Nano)Materials for Novel Biomedical Applications. Molecules 2019, 24, 1960. https://doi.org/10.3390/molecules24101960
Kravanja G, Primožič M, Knez Ž, Leitgeb M. Chitosan-Based (Nano)Materials for Novel Biomedical Applications. Molecules. 2019; 24(10):1960. https://doi.org/10.3390/molecules24101960
Chicago/Turabian StyleKravanja, Gregor, Mateja Primožič, Željko Knez, and Maja Leitgeb. 2019. "Chitosan-Based (Nano)Materials for Novel Biomedical Applications" Molecules 24, no. 10: 1960. https://doi.org/10.3390/molecules24101960
APA StyleKravanja, G., Primožič, M., Knez, Ž., & Leitgeb, M. (2019). Chitosan-Based (Nano)Materials for Novel Biomedical Applications. Molecules, 24(10), 1960. https://doi.org/10.3390/molecules24101960