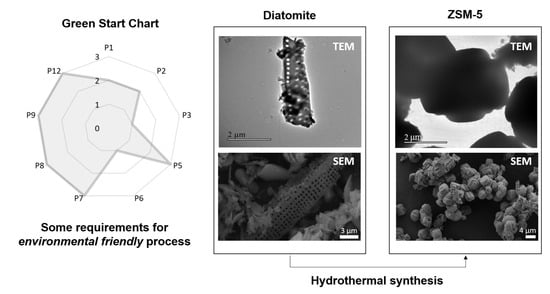
Incorporation of Brazilian Diatomite in the Synthesis of An MFI Zeolite
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vinaches, P.; Bernardo-Gusmão, K.; Pergher, S.B.C. An Introduction to Zeolite Synthesis Using Imidazolium-Based Cations as Organic Structure-Directing Agents. Molecules 2017, 22, 1307. [Google Scholar] [CrossRef]
- Corma, A. From Microporous to Mesoporous Molecular Sieve Materials and Their Use in Catalysis. Chem. Rev. 1997, 97, 2373–2420. [Google Scholar] [PubMed]
- Shi, J.; Wang, Y.; Yang, W.; Tang, Y.; Xie, Z. Recent advances of pore system construction in zeolite-catalyzed chemical industry processes. Chem. Soc. Rev. 2015, 44, 8877–8903. [Google Scholar]
- Tanabe, K.; Hölderich, W.F. Industrial application of solid acid–base catalysts. Appl. Catal. A Gener. 1999, 181, 399–434. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813. [Google Scholar] [CrossRef] [PubMed]
- Mintova, S.; Jaber, M.; Valtchev, V. Nanosized microporous crystals: Emerging applications. Chem. Soc. Rev. 2015, 44, 7207–7233. [Google Scholar] [PubMed]
- Zones, S.I. Translating new materials discoveries in zeolite research to commercial manufacture. Micropor. Mesopor. Mater. 2011, 144, 1–8. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 978-0-08-055434-1. [Google Scholar]
- Kokotailo, G.T.; Lawton, S.L.; Olson, D.H.; Meier, W.M. Structure of synthetic zeolite ZSM-5. Nature 1978, 272, 437–438. [Google Scholar]
- Verheyen, E.; Jo, C.; Kurttepeli, M.; Vanbutsele, G.; Gobechiya, E.; Korányi, T.I.; Bals, S.; Van Tendeloo, G.; Ryoo, R.; Kirschhock, C.E.A.; et al. Molecular shape-selectivity of MFI zeolite nanosheets in n-decane isomerization and hydrocracking. J. Catal. 2013, 300, 70–80. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallog. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Ng, E.-P.; Zou, X.; Mintova, S. New and Future Developments in Catalysis: Chapter 12. Environmental Synthesis Concerns of Zeolites; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 0-12-808181-3. [Google Scholar]
- Li, X.-Y.; Jiang, Y.; Liu, X.-Q.; Shi, L.-Y.; Zhang, D.-Y.; Sun, L.-B. Direct Synthesis of Zeolites from a Natural Clay, Attapulgite. ACS Sustain. Chem. Eng. 2017, 5, 6124–6130. [Google Scholar] [CrossRef]
- Schwanke, A.J.; Balzer, R.; Pergher, S. Microporous and Mesoporous Materials from Natural and Inexpensive Sources. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B., Eds.; Springer: Berlin, Germany, 2017; pp. 1–22. ISBN 978-3-319-48281-1. [Google Scholar]
- Vinaches, P.; Alves, J.A.B.L.R.; Melo, D.M.A.; Pergher, S.B.C. Raw powder glass as a silica source in the synthesis of colloidal MEL zeolite. Mater. Lett. 2016, 178, 217–220. [Google Scholar] [CrossRef]
- da Silva Filho, S.H.; Vinaches, P.; Pergher, S.B.C. Zeolite synthesis in basic media using expanded perlite and its application in Rhodamine B adsorption. Mater. Lett. 2018, 227, 258–260. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, J.; Jiang, Y.; Jiang, X.; Zhang, D. Preparation of biosilica structures from frustules of diatoms and their applications: Current state and perspectives. Appl. Microbiol. Biotechnol. 2013, 97, 453–460. [Google Scholar] [CrossRef]
- Lamastra, F.R.; Mori, S.; Cherubini, V.; Scarselli, M.; Nanni, F. A new green methodology for surface modification of diatomite filler in elastomers. Mater. Chem. Phys. 2017, 194, 253–260. [Google Scholar] [CrossRef]
- Qi, X.; Liu, M.; Chen, Z.; Liang, R. Preparation and properties of diatomite composite superabsorbent. Polym. Adv. Technol. 2007, 18, 184–193. [Google Scholar] [CrossRef]
- Khraisheh, M.A.M.; Al-Ghouti, M.A.; Allen, S.J.; Ahmad, M.N. Effect of OH and silanol groups in the removal of dyes from aqueous solution using diatomite. Water Res. 2005, 39, 922–932. [Google Scholar] [CrossRef]
- Rea, I.; Martucci, N.M.; De Stefano, L.; Ruggiero, I.; Terracciano, M.; Dardano, P.; Migliaccio, N.; Arcari, P.; Taté, R.; Rendina, I.; et al. Diatomite biosilica nanocarriers for siRNA transport inside cancer cells. Biochim. Biophys. Acta (BBA) 2014, 1840, 3393–3403. [Google Scholar] [CrossRef]
- Ruggiero, I.; Terracciano, M.; Martucci, N.M.; De Stefano, L.; Migliaccio, N.; Tatè, R.; Rendina, I.; Arcari, P.; Lamberti, A.; Rea, I. Diatomite silica nanoparticles for drug delivery. Nanoscale Res. Lett. 2014, 9, 329. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.R.; Sobrinho, E.M.O.; Assis, R.B.; Fagundes, R.F.; Bieseki, L.; Pergher, S.B.C. Síntese da zeólita A utilizando diatomita como fonte de sílicio e alumínio. Cerâmica 2014, 60, 63–68. [Google Scholar] [CrossRef]
- Sanhueza, V.; Kelm, U.; Cid, R. Synthesis of mordenite from diatomite: A case of zeolite synthesis from natural material. J. Chem. Technol. Biotechnol. 2003, 78, 485–488. [Google Scholar] [CrossRef]
- Garcia, G.; Cardenas, E.; Cabrera, S.; Hedlund, J.; Mouzon, J. Synthesis of zeolite Y from diatomite as silica source. Micropor. Mesopor. Mater. 2016, 219, 29–37. [Google Scholar] [CrossRef]
- Ahmad Alyosef, H.; Roggendorf, H.; Schneider, D.; Inayat, A.; Welscher, J.; Schwieger, W.; Münster, T.; Kloess, G.; Ibrahim, S.; Enke, D. MFI-type zeolites from natural materials: A comparative study of MFI-type zeolites generated from different diatomite species (part I). J. Porous Mater. 2016, 23, 1609–1618. [Google Scholar] [CrossRef]
- Song, W.; Justice, R.E.; Jones, C.A.; Grassian, V.H.; Larsen, S.C. Synthesis, Characterization, and Adsorption Properties of Nanocrystalline ZSM-5. Langmuir 2004, 20, 8301–8306. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. CHAPTER 6—Assessment of Surface Area. In Adsorption by Powders and Porous Solids; Academic Press: London, UK, 1999; pp. 165–189. ISBN 978-0-12-598920-6. [Google Scholar]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Particle Technology Series; Springer: Berlin, Germany, 2006; ISBN 978-1-4020-2302-6. [Google Scholar]
- Villarroel-Rocha, J.; Barrera, D.; Sapag, K. Introducing a self-consistent test and the corresponding modification in the Barrett, Joyner and Halenda method for pore-size determination. Micropor. Mesopor. Materials 2014, 200, 68–78. [Google Scholar] [CrossRef]
- Chen, S.G.; Yang, R.T. Theoretical Basis for the Potential Theory Adsorption Isotherms. The Dubinin-Radushkevich and Dubinin-Astakhov Equations. Langmuir 1994, 10, 4244–4249. [Google Scholar] [CrossRef]
- Ribeiro, M.G.T.C.; Costa, D.A.; Machado, A.A.S.C. “Green Star”: A holistic Green Chemistry metric for evaluation of teaching laboratory experiments. Green Chem. Lett. Rev. 2010, 3, 149–159. [Google Scholar] [CrossRef]
- Van Grieken, R.; Sotelo, J.L.; Menéndez, J.M.; Melero, J.A. Anomalous crystallization mechanism in the synthesis of nanocrystalline ZSM-5. Micropor. Mesopor. Mater. 2000, 39, 135–147. [Google Scholar] [CrossRef]
- Vinaches, P.; Rebitski, E.P.; Alves, J.A.B.L.R.; Melo, D.M.A.; Pergher, S.B.C. Unconventional silica source employment in zeolite synthesis: Raw powder glass in MFI synthesis case study. Mater. Lett. 2015, 159, 233–236. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |









| Parameter | Score | Comments |
|---|---|---|
| P1 | 2 | This score implies that the waste generated involved a moderate risk to human health and environment. Even though we did not study the reuse and recycle of the mother liquors in this research work, it is possible to perform it as demonstrated elsewhere. This fact reduced this score to the former value of 2. |
| P2 | 2 | We considered the mother liquors as by-products. |
| P3 | 1 | The presence of tetraethylorthosilicate and sodium hydroxide decreased the score. To perform basic media synthesis of zeolite, it is compulsory to use a hydroxide source, so the score cannot be higher in the obtention of zeolites. |
| P4 | - | The resulting product was not planned for a biological use. |
| P5 | 3 | It is a consequence of choosing distilled water as solvent. |
| P6 | 1 | The synthesis was performed at a temperature higher than 100 °C. Most zeolitic topologies are obtained at this temperature or superior, which means that further study is still needed in this sense. |
| P7 | 3 | This synthesis was performed incorporating diatomite, a natural raw material. |
| P8 | 3 | No derivatization was needed. |
| P9 | 3 | Not using any catalyst for the synthesis. |
| P10 | - | Zeolites are also natural materials. |
| P11 | - | There was no formation of hazardous substances during or after the synthesis. |
| P12 | 3 | The hydrothermal synthesis in basic media is one of the most well-known and most controlled among the zeolite synthesis methods. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinaches, P.; Schwanke, A.J.; Lopes, C.W.; Souza, I.M.S.; Villarroel-Rocha, J.; Sapag, K.; Pergher, S.B.C. Incorporation of Brazilian Diatomite in the Synthesis of An MFI Zeolite. Molecules 2019, 24, 1980. https://doi.org/10.3390/molecules24101980
Vinaches P, Schwanke AJ, Lopes CW, Souza IMS, Villarroel-Rocha J, Sapag K, Pergher SBC. Incorporation of Brazilian Diatomite in the Synthesis of An MFI Zeolite. Molecules. 2019; 24(10):1980. https://doi.org/10.3390/molecules24101980
Chicago/Turabian StyleVinaches, Paloma, Anderson Joel Schwanke, Christian Wittee Lopes, Iane M. S. Souza, Jhonny Villarroel-Rocha, Karim Sapag, and Sibele B. C. Pergher. 2019. "Incorporation of Brazilian Diatomite in the Synthesis of An MFI Zeolite" Molecules 24, no. 10: 1980. https://doi.org/10.3390/molecules24101980
APA StyleVinaches, P., Schwanke, A. J., Lopes, C. W., Souza, I. M. S., Villarroel-Rocha, J., Sapag, K., & Pergher, S. B. C. (2019). Incorporation of Brazilian Diatomite in the Synthesis of An MFI Zeolite. Molecules, 24(10), 1980. https://doi.org/10.3390/molecules24101980