Effects of Organic and Conventional Growing Systems on the Phenolic Profile of Extra-Virgin Olive Oil
Abstract
1. Introduction
2. Results
2.1. Total Amount of Phenolic Compounds
2.2. Concentrations of Phenolic Groups and Selected Phenolic Compounds
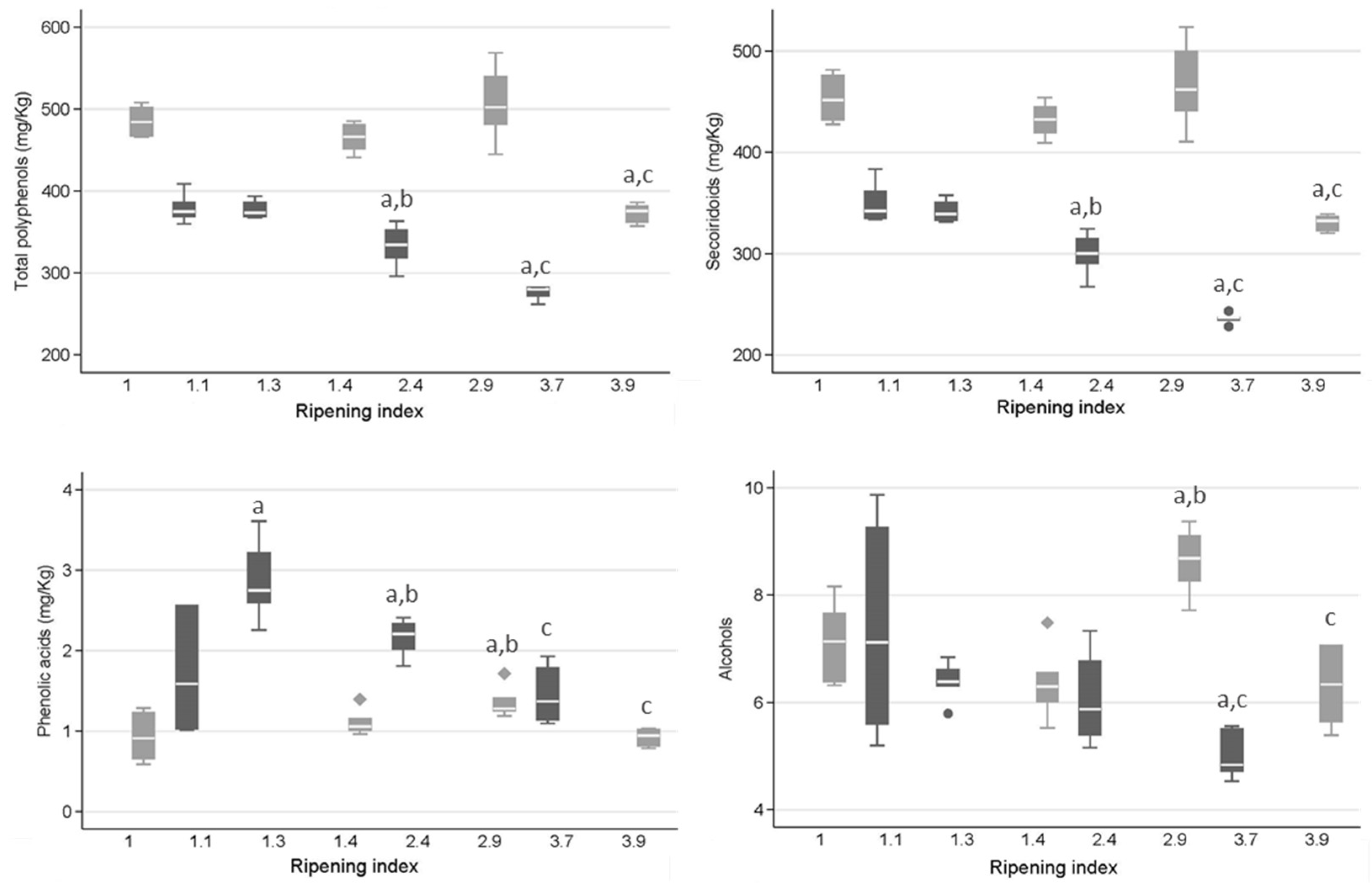
2.3. EVOO Phenolic Profile and Olive Fruit Ripening
2.4. Analysis of Oleocanthal by NMR
3. Discussion
3.1. Total Amount of Phenolic Compounds
3.2. Concentrations of Phenolic Groups and Selected Phenolic Compounds
3.3. EVOO Phenolic Profile and Olive Fruit Ripening
3.4. Analysis of Oleocanthal by NMR
4. Material and Methods
4.1. Chemicals
4.2. Olive Fruit Samples
4.3. Oil Samples
4.4. Polyphenol Analysis by Liquid Chromatography
4.5. Analysis of Oleocanthal by NMR
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Di Maio, I.; Selvaggini, R.; Taticchi, A. Biological Activities of Phenolic Compounds of Extra Virgin Olive Oil. Antioxidants 2013, 3, 1–23. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Koseoglu, O.; Unal, M.K. The effect of phenolic compounds on the quality and stability of virgin olive oil. In Proceedings of the VI International Symposium on Olive Growing, Evora, Portugal, 9–13 September 2008; Volume 791, pp. 655–663. [Google Scholar]
- Di Giovacchino, L.; Costantini, N.; Serraiocco, A.; Surricchio, G.; Basti, C. Natural antioxidants and volatile compounds of virgin olive oils obtained by two or three-phases centrifugal decanters. Eur. J. Lipid Sci. Technol. 2001, 103, 279–285. [Google Scholar] [CrossRef]
- El Riachy, M.; Priego-Capote, F.; León, L.; Rallo, L.; Luque de Castro, M.D. Hydrophilic antioxidants of virgin olive oil. Part 2: Biosynthesis and biotransformation of phenolic compounds in virgin olive oil as affected by agronomic and processing factors. Eur. J. Lipid Sci. Technol. 2011, 113, 692–707. [Google Scholar] [CrossRef]
- Clodoveo, M.L. Malaxation: Influence on virgin olive oil quality. Past, present and future—An overview. Trends Food Sci. Technol. 2012, 25, 13–23. [Google Scholar] [CrossRef]
- Lozano-Sánchez, J.; Cerretani, L.; Bendini, A.; Gallina-Toschi, T.; Segura-Carretero, A.; Fernández-Gutiérrez, A. New filtration systems for extra-virgin olive oil: Effect on antioxidant compounds, oxidative stability, and physicochemical and sensory properties. J. Agric. Food Chem. 2012, 60, 3754–3762. [Google Scholar] [CrossRef]
- Ninfali, P.; Bacchiocca, M.; Biagiotti, E.; Esposto, S.; Servili, M.; Rosati, A.; Montedoro, G. A 3-year study on quality, nutritional and organoleptic evaluation of organic and conventional extra-virgin olive oils. JAOCS J. Am. Oil Chem. Soc. 2008, 85, 151–158. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Foodomics: A new tool to differentiate between organic and conventional foods. Electrophoresis 2016, 37, 1784–1794. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Barroso, S.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Organic food and impact on human health. Int. J. PharmTech Res. 2016, 9, 316–324. [Google Scholar] [CrossRef]
- González, N.; Marquès, M.; Nadal, M.; Domingo, J.L. Occurrence of environmental pollutants in foodstu ff s: A review of organic vs. conventional food. Food Chem. Toxicol. 2019, 125, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M. Nutritional quality of organic food: Shades of grey or shades of green? Proc. Nutr. Soc. 2008, 61, 19–24. [Google Scholar] [CrossRef]
- Worthington, V. Effect of Agricultural Methods on Nutritional Quality: A Comparison of Organic with Conventional Crops. Altern. Ther. Health Med. 1998, 4, 58–69. [Google Scholar]
- Häkkinen, S.H.; Törrönen, A.R. Content of flavonols and selected phenolic acids in strawberries and Vaccinium species: Influence of cultivar, cultivation site and technique. Food Res. Int. 2000, 33, 517–524. [Google Scholar] [CrossRef]
- Romero-Pérez, A.I.; Lamuela-Raventós, R.M.; Andrés-Lacueva, C.; De La Carmen Torre-Boronat, M. Method for the quantitative extraction of resveratrol and piceid isomers in grape berry skins. Effect of powdery mildew on the stilbene content. J. Agric. Food Chem. 2001, 49, 210–215. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Medina-Remón, A.; Casals-Ribes, I.; Amat, M.; Lamuela-Raventós, R.M. A metabolomic approach differentiates between conventional and organic ketchups. J. Agric. Food Chem. 2011, 59, 11703–11710. [Google Scholar] [CrossRef]
- Martí, R.; Leiva-Brondo, M.; Lahoz, I.; Campillo, C.; Cebolla-Cornejo, J.; Roselló, S. Polyphenol and L-ascorbic acid content in tomato as influenced by high lycopene genotypes and organic farming at different environments. Food Chem. 2018, 239, 148–156. [Google Scholar] [CrossRef]
- Cuevas, F.J.; Pradas, I.; Ruiz-Moreno, M.J.; Arroyo, F.T.; Perez-Romero, L.F.; Montenegro, J.C.; Moreno-Rojas, J.M. Effect of organic and conventional management on bio-functional quality of thirteen plum cultivars (Prunus salicina Lindl.). PLoS ONE 2015, 10, e0136596. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef]
- Barbieri, S.; Bendini, A.; Valli, E.; Gallina Toschi, T. Do consumers recognize the positive sensorial attributes of extra virgin olive oils related with their composition? A case study on conventional and organic products. J. Food Compos. Anal. 2015, 44, 186–195. [Google Scholar] [CrossRef]
- Rosati, A.; Cafiero, C.; Paoletti, A.; Alfei, B.; Caporali, S.; Casciani, L.; Valentini, M. Effect of agronomical practices on carpology, fruit and oil composition, and oil sensory properties, in olive (Olea europaea L.). Food Chem. 2014, 159, 236–243. [Google Scholar] [CrossRef]
- Trombetta, D.; Smeriglio, A.; Marcoccia, D.; Giofrè, S.; Toscano, G.; Mazzotti, F.; Giovanazzi, A.; Lorenzetti, S. Analytical evaluation and antioxidant properties of some secondary metabolites in northern Italian mono-and multi-varietal extra virgin olive oils (EVOOs) from early and late harvested olives. Int. J. Mol. Sci. 2017, 18, 797. [Google Scholar] [CrossRef]
- Alowaiesh, B.; Singh, Z.; Fang, Z.; Kailis, S.G. Harvest time impacts the fatty acid compositions, phenolic compounds and sensory attributes of Frantoio and Manzanilla olive oil. Sci. Hortic. (Amsterdam) 2018, 234, 74–80. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Medina-Remón, A.; Casals-Ribes, I.; Lamuela-Raventos, R.M. Is there any difference between the phenolic content of organic and conventional tomato juices? Food Chem. 2012, 130, 222–227. [Google Scholar] [CrossRef]
- Hallmann, E.; Rembial kowska, E. Characterisation of antioxidant compounds in sweet bell pepper (Capsicum annuum L.) under organic and conventional growing systems. J. Sci. Food Agric. 2012, 92, 2409–2415. [Google Scholar] [CrossRef]
- Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z.; Mbili, N. Postharvest quality and composition of organically and conventionally produced fruits: A review. Sci. Hortic. (Amsterdam) 2017, 216, 148–159. [Google Scholar] [CrossRef]
- Assumpção, C.F.; Nunes, I.L.; Mendonça, T.A.; Bortolin, R.C.; Jablonski, A.; Flôres, S.H.; De Oliveira Rios, A. Bioactive Compounds and Stability of Organic and Conventional Vitis labrusca Grape Seed Oils. JAOCS J. Am. Oil Chem. Soc. 2016, 93, 115–124. [Google Scholar] [CrossRef]
- Hunter, D.; Foster, M.; Mcarthur, J.O.; Ojha, R.; Petocz, P.; Samman, S. Evaluation of the micronutrient composition of plant foods produced by organic and conventional agricultural methods. Crit. Rev. Food Sci. Nutr. 2011, 51, 571–582. [Google Scholar] [CrossRef]
- Miho, H.; Díez, C.M.; Mena-Bravo, A.; Sánchez de Medina, V.; Moral, J.; Melliou, E.; Magiatis, P.; Rallo, L.; Barranco, D.; Priego-Capote, F. Cultivar influence on variability in olive oil phenolic profiles determined through an extensive germplasm survey. Food Chem. 2018, 266, 192–199. [Google Scholar] [CrossRef]
- Karkoula, E.; Skantzari, A.; Melliou, E.; Magiatis, P. Quantitative measurement of major secoiridoid derivatives in olive oil using qNMR. Proof of the artificial formation of aldehydic oleuropein and ligstroside aglycon isomers. J. Agric. Food Chem. 2014, 62, 600–607. [Google Scholar] [CrossRef]
- García-Rodríguez, R.; Belaj, A.; Romero-Segura, C.; Sanz, C.; Pérez, A.G. Exploration of genetic resources to improve the functional quality of virgin olive oil. J. Funct. Foods 2017, 38, 1–8. [Google Scholar] [CrossRef]
- Campestre, C.; Angelini, G.; Gasbarri, C.; Angerosa, F. The compounds responsible for the sensory profile in monovarietal virgin olive oils. Molecules 2017, 22, 1833. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.R.; Franzyk, H.; Wallander, E. Chemotaxonomy of the oleaceae: Iridoids as taxonomic markers. Phytochemistry 2002, 60, 213–231. [Google Scholar] [CrossRef]
- Dabbaghi, O.; Tekaya, M.; Flamini, G.; Zouari, I.; El-Gharbi, S.; M’barki, N.; Laabidi, F.; Cheheb, H.; Attia, F.; Aïachi Mezghani, M.; et al. Modification of Phenolic Compounds and Volatile Profiles of Chemlali Variety Olive Oil in Response to Foliar Biofertilization. J. Am. Oil Chem. Soc. 2019, 96, 585–593. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Reboredo-Rodríguez, P.; González-Barreiro, C.; Carrasco-Pancorbo, A.; Cancho-Grande, B.; Simal-Gándara, J. The involvement of phenolic-rich extracts from Galician autochthonous extra-virgin olive oils against the α-glucosidase and α-amylase inhibition. Food Res. Int. 2019, 116, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Kelebek, H.; Kesen, S.; Selli, S. Comparative study of bioactive constituents in Turkish olive oils by LC-ESI/MS/MS. Int. J. Food Prop. 2015, 18, 2231–2245. [Google Scholar] [CrossRef]
- Arslan, D.; Karabekir, Y.; Schreiner, M. Variations of phenolic compounds, fatty acids and some qualitative characteristics of Sariulak olive oil as induced by growing area. Food Res. Int. 2013, 54, 1897–1906. [Google Scholar] [CrossRef]
- de Torres, A.; Espínola, F.; Moya, M.; Alcalá, S.; Vidal, A.M.; Castro, E. Assessment of phenolic compounds in virgin olive oil by response surface methodology with particular focus on flavonoids and lignans. LWT Food Sci. Technol. 2018, 90, 22–30. [Google Scholar] [CrossRef]
- Suárez, M.; Macià, A.; Romero, M.P.; Motilva, M.J. Improved liquid chromatography tandem mass spectrometry method for the determination of phenolic compounds in virgin olive oil. J. Chromatogr. A 2008, 1214, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Murkovic, M.; Lechner, S.; Pietzka, A.; Bratacos, M.; Katzogiannos, E. Analysis of minor components in olive oil. J. Biochem. Biophys. Methods 2004, 61, 155–160. [Google Scholar] [CrossRef]
- Ye, J.H.; Wijesundera, C.; Shi, M. Effects of Agronomic and Oil Processing Conditions on Natural Antioxidative Phenolics in Olive (Oleaeuropaea L.). Austin J. Nutr. Food Sci. 2014, 2, 1050. [Google Scholar]
- Collado-González, J.; Grosso, C.; Valentão, P.; Andrade, P.B.; Ferreres, F.; Durand, T.; Guy, A.; Galano, J.M.; Torrecillas, A.; Gil-Izquierdo, Á. Inhibition of α-glucosidase and α-amylase by Spanish extra virgin olive oils: The involvement of bioactive compounds other than oleuropein and hydroxytyrosol. Food Chem. 2017, 235, 298–307. [Google Scholar] [CrossRef]
- Jimenez, B.; Sánchez-Ortiz, A.; Lorenzo, M.L.; Rivas, A. Effect of organic cultivation of Picual and Hojiblanca olive varieties on the quality of virgin olive oil at four ripening stages. Eur. J. Lipid Sci. Technol. 2014, 116, 1634–1646. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Aalizadeh, R.; Thomaidis, N.S. Investigating the organic and conventional production type of olive oil with target and suspect screening by LC-QTOF-MS, a novel semi-quantification method using chemical similarity and advanced chemometrics. Anal. Bioanal. Chem. 2017, 409, 5413–5426. [Google Scholar] [CrossRef]
- Dean, J.F.D.; LaFayette, P.R.; Rugh, C.; Tristram, A.H.; Hoopes, J.T.; Eriksson, K.-E.L.; Merkle, S.A. Laccases Associated with Lignifying Vascular Tissues. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2009; Volume 78, pp. 96–108. ISBN 9780841235663. [Google Scholar]
- Ali Ghasemzadeh Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011, 5, 6697–6703. [CrossRef]
- Fraser, C.M.; Chapple, C. The Phenylpropanoid Pathway in Arabidopsis. Am. Soc. Plant Biol. 2011, 9, e0152. [Google Scholar] [CrossRef]
- Ben Brahim, S.; Kelebek, H.; Ammar, S.; Abichou, M.; Bouaziz, M. LC–MS phenolic profiling combined with multivariate analysis as an approach for the characterization of extra virgin olive oils of four rare Tunisian cultivars during ripening. Food Chem. 2017, 229, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Clodoveo, M.L.; Hbaieb, R.H.; Kotti, F.; Mugnozza, G.S.; Gargouri, M. Mechanical strategies to increase nutritional and sensory quality of virgin olive oil by modulating the endogenous enzyme activities. Compr. Rev. Food Sci. Food Saf. 2014, 13, 135–154. [Google Scholar] [CrossRef]
- Amanpour, A.; Kelebek, H.; Selli, S. Characterization of aroma, aroma-active compounds and fatty acids profiles of cv. Nizip Yaglik oils as affected by three maturity periods of olives. J. Sci. Food Agric. 2019, 99, 726–740. [Google Scholar] [CrossRef] [PubMed]
- Peres, F.; Martins, L.L.; Ferreira-Dias, S. Influence of enzymes and technology on virgin olive oil composition. Crit. Rev. Food Sci. Nutr. 2017, 57, 3104–3126. [Google Scholar] [CrossRef]
- Bilušić, T.; Žanetić, M.; Ljubenkov, I.; Generalić Mekinić, I.; Štambuk, S.; Bojović, V.; Soldo, B.; Magiatis, P. Molecular characterization of Dalmatian cultivars and the influence of the olive fruit harvest period on chemical profile, sensory characteristics and oil oxidative stability. Eur. Food Res. Technol. 2018, 244, 281–289. [Google Scholar] [CrossRef]
- Amanpour, A.; Kelebek, H.; Selli, S. LC-DAD-ESI-MS/MS-based phenolic profiling and antioxidant activity in Turkish cv. Nizip Yaglik olive oils from different maturity olives. J. Mass Spectrom. 2018, 227–238. [Google Scholar] [CrossRef]
- Lukić, I.; Krapac, M.; Horvat, I.; Godena, S.; Kosić, U.; Brkić Bubola, K. Three-factor approach for balancing the concentrations of phenols and volatiles in virgin olive oil from a late-ripening olive cultivar. LWT Food Sci. Technol. 2018, 87, 194–202. [Google Scholar] [CrossRef]
- Lozano-Sánchez, J.; Amir, Y.; Fernández-Gutiérrez, A.; Bakhouche, A.; Bengana, M.; Segura-Carretero, A.; Youyou, A. Influence of olive ripeness on chemical properties and phenolic composition of Chemlal extra-virgin olive oil. Food Res. Int. 2013, 54, 1868–1875. [Google Scholar] [CrossRef]
- Gutierrez-Rosales, F.; Romero, M.P.; Casanovas, M.; Motilva, M.J.; Mínguez-Mosquera, M.I. Metabolites involved in oleuropein accumulation and degradation in fruits of Olea europaea L.: Hojiblanca and Arbequina varieties. J. Agric. Food Chem. 2010, 58, 12924–12933. [Google Scholar] [CrossRef]
- Jiménez, B.; Sánchez-Ortiz, A.; Rivas, A. Influence of the malaxation time and olive ripening stage on oil quality and phenolic compounds of virgin olive oils. Int. J. Food Sci. Technol. 2014, 49, 2521–2527. [Google Scholar] [CrossRef]
- Artajo, L.S.; Romero, M.P.; Motilva, M.J. Transfer of phenolic compounds during olive oil extraction in relation to ripening stage of the fruit. J. Sci. Food Agric. 2006, 86, 518–527. [Google Scholar] [CrossRef]
- Jiménez, B.; Sánchez-Ortiz, A.; Lorenzo, M.L.; Rivas, A. Influence of fruit ripening on agronomic parameters, quality indices, sensory attributes and phenolic compounds of Picudo olive oils. Food Res. Int. 2013, 54, 1860–1867. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, P.; Termentzi, A.; Michel, T.; Gikas, E.; Halabalaki, M.; Skaltsounis, A.L. From olive drupes to olive Oil. An HPLC-orbitrap-based qualitative and quantitative exploration of olive key metabolites. Planta Med. 2013, 79, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Di Donna, L.; Benabdelkamel, H.; Mazzotti, F.; Napoli, A.; Nardi, M.; Sindona, G. High-throughput assay of oleopentanedialdheydes in extra virgin olive oil by the UHPLC-ESI-MS/MS and isotope dilution methods. Anal. Chem. 2011, 83, 1990–1995. [Google Scholar] [CrossRef] [PubMed]
- Sánchez de Medina, V.; Miho, H.; Melliou, E.; Magiatis, P.; Priego-Capote, F.; Luque de Castro, M.D. Quantitative method for determination of oleocanthal and oleacein in virgin olive oils by liquid chromatography–tandem mass spectrometry. Talanta 2017, 162, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Karkoula, E.; Skantzari, A.; Melliou, E.; Magiatis, P. Direct measurement of oleocanthal and oleacein levels in olive oil by quantitative 1H NMR. Establishment of a new index for the characterization of extra virgin olive oils. J. Agric. Food Chem. 2012, 60, 11696–11703. [Google Scholar] [CrossRef] [PubMed]
- Godelmann, R.; Kost, C.; Patz, C.D.; Ristow, R.; Wachter, H. Quantitation of compounds in wine using 1H NMR spectroscopy: Description of the method and collaborative study. J. AOAC Int. 2016, 99, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Uceda, M.; Frías, L. Epocas de recolección. Evolución del fruto y de la composición y calidad del aceite. (Seasons of harvest. Changes on fruit oil content, oil composition and oil quality). In Proceeding of II Seminario Olícola Internacional; International Olive-Oil Council: Cordoba, Spain, 1975. [Google Scholar]
- Capriotti, A.L.; Cavaliere, C.; Crescenzi, C.; Foglia, P.; Nescatelli, R.; Samperi, R.; Laganà, A. Comparison of extraction methods for the identification and quantification of polyphenols in virgin olive oil by ultra-HPLC-QToF mass spectrometry. Food Chem. 2014, 158, 392–400. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


| Organic | Conventional | p * | |
|---|---|---|---|
| Total Phenols | 456.89 ± 56.74 | 338.19 ± 42.96 | <0.001 |
| Secoiridoids | 420.72 ± 59.42 | 306.48 ± 48.09 | <0.001 |
| Oleuropein | 0.82 ± 0.02 | 0.81 ± 0.02 | 0.2 |
| Oleuropein derivatives | |||
| Oleuropein der I | 22.77 ± 3.01 | 34.83 ± 4.44 | <0.001 |
| Oleuropein der II | 3.21 ± 0.54 | 1.67 ± 0.25 | <0.001 |
| Oleuropein der III | 3.63 ± 0.58 | 2.33 ± 0.33 | <0.001 |
| me-3,4-DHPEA-EA | 1.46 ± 0.25 | 0.97 ± 0.07 | <0.001 |
| Hydroxy oleuropein aglycone I (HOA I) | 1.44 ± 0.34 | 1.17 ± 0.18 | 0.007 |
| Hydroxy oleuropein aglycone II (HOA II) | 2.18 ± 0.77 | 1.67 ± 0.41 | 0.02 |
| HDCM OA | 9.13 ± 3.53 | 6.37 ± 1.94 | 0.01 |
| 3,4-DHPEA-EA I | 7.18 ± 0.98 | 4.74 ± 0.42 | <0.001 |
| 3,4-DHPEA-EA II | 5.82 ± 0.94 | 3.02 ± 0.74 | <0.001 |
| Lactone | 0.19 ± 0.04 | 0.33 ± 0.20 | <0.001 |
| Ligstroside derivatives | |||
| Ligstroside I | 20.45 ± 2.77 | 12.32 ± 2.47 | <0.001 |
| Ligstroside II | 41.61 ± 3.68 | 24.41 ± 4.02 | <0.001 |
| Ligstroside III | 54.59 ± 10.63 | 34.75 ± 8.00 | <0.001 |
| Oleocanthal | 186.72 ± 40.61 | 132.10 ± 37.02 | <0.001 |
| Elenolic acid | 55.35 ± 8.10 | 40.37 ± 7.39 | <0.001 |
| Elenolic acid derivatives | |||
| Hydroxyelenolic acid | 3.41 ± 1.42 | 3.19 ± 1.70 | 0.5 |
| Flavones | 28.21 ± 5.55 | 25.53 ± 5.85 | 0.09 |
| Luteolin | 22.69 ± 5.09 | 19.35 ± 5.38 | 0.03 |
| Apigenin | 5.51 ± 0.69 | 6.17 ± 0.78 | 0.008 |
| Phenolic alcohols | 7.11 ± 1.15 | 6.21 ± 1.37 | 0.07 |
| Hydroxytyrosol | 4.47 ± 1.10 | 3.65 ± 1.32 | 0.01 |
| Dihydroxytyrosol | 1.73 ± 0.09 | 1.78 ± 0.10 | 0.001 |
| 3,4-DHPEA-AC | 0.91 ± 0.02 | 0.91 ± 0.03 | 0.51 |
| Lignans | 0.47 ± 0.06 | 0.79 ± 0.09 | <0.001 |
| Pinoresinol | 0.47 ± 0.06 | 0.79 ± 0.09 | <0.001 |
| Phenolic acids | 1.08 ± 0.26 | 2.05 ± 0.71 | <0.001 |
| Ferulic acid | 0.05 ± 0.01 | 0.07 ± 0.01 | 0.003 |
| p-coumaric acid | 0.67 ± 0.15 | 1.04 ± 0.36 | <0.001 |
| Vanillic acid | 0.35 ± 0.12 | 0.93 ± 0.37 | <0.001 |
| Organic | Conventional | |||
|---|---|---|---|---|
| Coefficient | p | Coefficient | p | |
| Total phenols | −27.4 | 0.004 | −40.2 | <0.001 |
| Secoiridoids | −31.2 | 0.001 | −44.7 | <0.001 |
| Phenolic alcohols | 0.05 | 0.81 | −0.76 | 0.002 |
| Phenolic acids | 0.02 | 0.74 | −0.29 | 0.04 |
| Flavones | 3.77 | <0.001 | 4.23 | <0.001 |
| Lignans | −0.02 | 0.01 | 0.003 | 0.9 |
| Integration | Concentration (mg·kg−1) | |
|---|---|---|
| Conventional | 0.477 | 118.21 |
| Organic | 0.684 | 168.96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Yerena, A.; Lozano-Castellón, J.; Olmo-Cunillera, A.; Tresserra-Rimbau, A.; Quifer-Rada, P.; Jiménez, B.; Pérez, M.; Vallverdú-Queralt, A. Effects of Organic and Conventional Growing Systems on the Phenolic Profile of Extra-Virgin Olive Oil. Molecules 2019, 24, 1986. https://doi.org/10.3390/molecules24101986
López-Yerena A, Lozano-Castellón J, Olmo-Cunillera A, Tresserra-Rimbau A, Quifer-Rada P, Jiménez B, Pérez M, Vallverdú-Queralt A. Effects of Organic and Conventional Growing Systems on the Phenolic Profile of Extra-Virgin Olive Oil. Molecules. 2019; 24(10):1986. https://doi.org/10.3390/molecules24101986
Chicago/Turabian StyleLópez-Yerena, Anallely, Julián Lozano-Castellón, Alexandra Olmo-Cunillera, Anna Tresserra-Rimbau, Paola Quifer-Rada, Brígida Jiménez, Maria Pérez, and Anna Vallverdú-Queralt. 2019. "Effects of Organic and Conventional Growing Systems on the Phenolic Profile of Extra-Virgin Olive Oil" Molecules 24, no. 10: 1986. https://doi.org/10.3390/molecules24101986
APA StyleLópez-Yerena, A., Lozano-Castellón, J., Olmo-Cunillera, A., Tresserra-Rimbau, A., Quifer-Rada, P., Jiménez, B., Pérez, M., & Vallverdú-Queralt, A. (2019). Effects of Organic and Conventional Growing Systems on the Phenolic Profile of Extra-Virgin Olive Oil. Molecules, 24(10), 1986. https://doi.org/10.3390/molecules24101986








