Exploring African Medicinal Plants for Potential Anti-Diabetic Compounds with the DIA-DB Inverse Virtual Screening Web Server
Abstract
1. Introduction
2. Results and Discussion
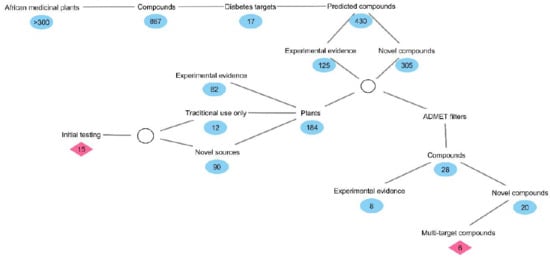
2.1. Inverse Virtual Screening and Identification of Compounds with Potential Anti-Diabetic Activity
2.2. Identification of Potentially Important Scaffolds for Enzyme Activity
2.3. Molecular Similarity Evaluation of Predicted Active Compounds and Known/Experimental Anti-Diabetic Drugs
2.4. Prediction of Oral Bioavailability and Favourable Abosrption, Distribution, Metabolism, Excretion and Toxicity (ADMET) Properties of the Predicted Active Compounds
3. Materials and Methods
3.1. Preparation of Compound Structures and Inverse Virtual Screening of Potential Anti-Diabetic Activity
3.2. Clustering and Maximum Common Substructure Analysis of Predicted Active Compounds
3.3. Similarity Studies with Known/Experimental Anti-Diabetic Drugs
3.4. Studies on Oral Bioavailability and ADMET Properties of the Predicted Active Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Fact Sheet. 15 November 2017. Available online: http://www.who.int/news-room/fact-sheet/detail/diabetes (accessed on 10 April 2019).
- DeFronzo, R.A.; Triplitt, C.L.; Abdul-Ghani, M.; Cersosimo, E. Novel agents for the treatment of type 2 diabetes. Diabetes Spectr. 2014, 27, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Gourgari, E.; Wilhelm, E.E.; Hassanzadeh, H.; Aroda, V.R.; Shoulson, I. A comprehensive review of the FDA-approved labels of diabetes drugs: Indications, safety, and emerging cardiovascular safety data. J. Diabetes Complicat. 2017, 31, 1719–1727. [Google Scholar] [CrossRef]
- Abo, K.; Fred-Jaiyesimi, A.; Jaiyesimi, A. Ethnobotanical studies of medicinal plants used in the management of diabetes mellitus in South Western Nigeria. J. Ethnopharmacol. 2008, 115, 67–71. [Google Scholar] [CrossRef]
- Bahmani, M.; Zargaran, A.; Rafieian-Kopaei, M.; Saki, K. Ethnobotanical study of medicinal plants used in the management of diabetes mellitus in the Urmia, Northwest Iran. Asian Pac. J. Trop. Med. 2014, 7, S348–S354. [Google Scholar] [CrossRef]
- Deutschländer, M.; Lall, N.; Van De Venter, M. Plant species used in the treatment of diabetes by South African traditional healers: An inventory. Pharm. Biol. 2009, 47, 348–365. [Google Scholar] [CrossRef]
- Grover, J.; Yadav, S.; Vats, V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef]
- Li, W.; Zheng, H.; Bukuru, J.; De Kimpe, N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 2004, 92, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ghadge, A.A.; Kuvalekar, A.A. Controversy of oral hypoglycemic agents in type 2 diabetes mellitus: Novel move towards combination therapies. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S5–S13. [Google Scholar] [CrossRef]
- Prabhakar, P.; Kumar, A.; Doble, M. Combination therapy: A new strategy to manage diabetes and its complications. Phytomedicine 2014, 21, 123–130. [Google Scholar] [CrossRef]
- Chen, B.-W.; Li, W.-X.; Wang, G.-H.; Li, G.-H.; Liu, J.-Q.; Zheng, J.-J.; Wang, Q.; Li, H.-J.; Dai, S.-X.; Huang, J.-F. A strategy to find novel candidate anti-Alzheimer’s disease drugs by constructing interaction networks between drug targets and natural compounds in medical plants. PeerJ 2018, 6, e4756. [Google Scholar] [CrossRef]
- Dai, S.-X.; Li, W.-X.; Han, F.-F.; Guo, Y.-C.; Zheng, J.-J.; Liu, J.-Q.; Wang, Q.; Gao, Y.-D.; Li, G.-H.; Huang, J.-F. In silico identification of anti-cancer compounds and plants from traditional Chinese medicine database. Sci. Rep. 2016, 6, 25462. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Dai, S.-X.; Zheng, J.-J.; Guo, Y.-C.; Li, W.-X.; Li, G.-H.; Huang, J.-F. The identification and molecular mechanism of anti-stroke traditional Chinese medicinal compounds. Sci. Rep. 2017, 7, 41406. [Google Scholar] [CrossRef]
- Sánchez-Pérez, A.; Muñoz, A.; Peña-García, J.; den-Haan, H.; Bekas, N.; Katsikoudi, A.; Tzakos, A.G.; Péréz-Sánchez, H. DIA-DB: A Web-Accessible Database for the Prediction of Diabetes Drugs. In International Conference on Bioinformatics and Biomedical Engineering, 2015; Springer: New York, NY, USA, 2015; pp. 655–663. [Google Scholar]
- Aronoff, S.L.; Berkowitz, K.; Shreiner, B.; Want, L. Glucose metabolism and regulation: Beyond insulin and glucagon. Diabetes Spect. 2004, 17, 183–190. [Google Scholar] [CrossRef]
- Wagner, R.; Kaiser, G.; Gerst, F.; Christiansen, E.; Due-Hansen, M.E.; Grundmann, M.; Machicao, F.; Peter, A.; Kostenis, E.; Ulven, T. Reevaluation of fatty acid receptor 1 (FFAR1/GPR40) as drug target for the stimulation of insulin secretion in humans. Diabetes 2013, 62, 2106–2111. [Google Scholar] [CrossRef]
- Stulnig, T.; Waldhäusl, W. 11β-hydroxysteroid dehydrogenase type 1 in obesity and type 2 diabetes. Diabetologia 2004, 47, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Herman, M.A.; Kahn, B.B. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J. Clin. Invest. 2006, 116, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Yabe-Nishimura, C. Aldose reductase in glucose toxicity: A potential target for the prevention of diabetic complications. Pharmacol. Rev. 1998, 50, 21–34. [Google Scholar]
- Etxeberria, U.; de la Garza, A.L.; Campión, J.; Martinez, J.A.; Milagro, F.I. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef]
- Jeoung, N.H. Pyruvate dehydrogenase kinases: Therapeutic targets for diabetes and cancers. Diabetes Metab. J. 2015, 39, 188–197. [Google Scholar] [CrossRef]
- Martin, J.; Veluraja, K.; Ross, K.; Johnson, L.; Fleet, G.; Ramsden, N.; Bruce, I.; Orchard, M.; Oikonomakos, N. Glucose analog inhibitors of glycogen phosphorylase: The design of potential drugs for diabetes. Biochemistry 1991, 30, 10101–10116. [Google Scholar] [CrossRef]
- Mellado-Gil, J.M.; Cobo-Vuilleumier, N.; Gauthier, B.R. Islet β-cell mass preservation and regeneration in diabetes mellitus: Four factors with potential therapeutic interest. J. Transplant. 2012, 2012, 230870. [Google Scholar] [CrossRef]
- Monsalve, F.A.; Pyarasani, R.D.; Delgado-Lopez, F.; Moore-Carrasco, R. Peroxisome proliferator-activated receptor targets for the treatment of metabolic diseases. Mediat. Inflamm. 2013, 2013, 549627. [Google Scholar] [CrossRef]
- Moller, D.E. New drug targets for type 2 diabetes and the metabolic syndrome. Nature 2001, 414, 821–827. [Google Scholar] [CrossRef]
- Csermely, P.; Agoston, V.; Pongor, S. The efficiency of multi-target drugs: The network approach might help drug design. Trends Pharmacol. Sci. 2005, 26, 178–182. [Google Scholar] [CrossRef]
- Peters, J.-U. Polypharmacology–foe or friend? J. Med. Chem. 2013, 56, 8955–8971. [Google Scholar] [CrossRef]
- Reddy, A.S.; Zhang, S. Polypharmacology: Drug discovery for the future. Expert Rev. Clin. Pharmacol. 2013, 6, 41–47. [Google Scholar] [CrossRef]
- Cho, Y.; Kang, Y.; Lee, S.; Lee, J.; Park, J.-Y.; Lee, W.; Kim, Y.-J.; Jung, C. Efficacy and safety of combination therapy with SGLT2 and DPP4 inhibitors in the treatment of type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 2018, 44, 393–401. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Heerden, F.v.; Oudtshoorn, B.v. Poisonous Plants of South Africa; Briza Publications: Pretoria, South Africa, 2002. [Google Scholar]
- Beltrán-Debón, R.; Rull, A.; Rodríguez-Sanabria, F.; Iswaldi, I.; Herranz-López, M.; Aragonès, G.; Camps, J.; Alonso-Villaverde, C.; Menéndez, J.; Micol, V. Continuous administration of polyphenols from aqueous rooibos (Aspalathus linearis) extract ameliorates dietary-induced metabolic disturbances in hyperlipidemic mice. Phytomedicine 2011, 18, 414–424. [Google Scholar] [CrossRef]
- Kamakura, R.; Son, M.J.; de Beer, D.; Joubert, E.; Miura, Y.; Yagasaki, K. Antidiabetic effect of green rooibos (Aspalathus linearis) extract in cultured cells and type 2 diabetic model KK-A y mice. Cytotechnology 2015, 67, 699–710. [Google Scholar] [CrossRef]
- Muller, C.; Joubert, E.; De Beer, D.; Sanderson, M.; Malherbe, C.; Fey, S.; Louw, J. Acute assessment of an aspalathin-enriched green rooibos (Aspalathus linearis) extract with hypoglycemic potential. Phytomedicine 2012, 20, 32–39. [Google Scholar] [CrossRef]
- Bierer, D.E.; Fort, D.M.; Mendez, C.D.; Luo, J.; Imbach, P.A.; Dubenko, L.G.; Jolad, S.D.; Gerber, R.E.; Litvak, J.; Lu, Q. Ethnobotanical-directed discovery of the antihyperglycemic properties of cryptolepine: Its isolation from Cryptolepis sanguinolenta, synthesis, and in vitro and in vivo activities. J. Med. Chem. 1998, 41, 894–901. [Google Scholar] [CrossRef]
- Adaramoye, O. Antidiabetic effect of kolaviron, a biflavonoid complex isolated from Garcinia kola seeds, in Wistar rats. African Health Sci. 2012, 12, 498–506. [Google Scholar] [CrossRef]
- Adaramoye, O.; Adeyemi, E. Hypoglycaemic and hypolipidaemic effects of fractions from kolaviron, a biflavonoid complex from Garcinia kola in streptozotocin-induced diabetes mellitus rats. J. Pharm. Pharmacol. 2006, 58, 121–128. [Google Scholar] [CrossRef]
- Saxena, S. Glycyrrhiza glabra: Medicine over the millennium. Nat. Prod. Radiance 2005, 4, 358–367. [Google Scholar]
- Vermaak, I.; Hamman, J.H.; Viljoen, A.M. Hoodia gordonii: An up-to-date review of a commercially important anti-obesity plant. Planta Med. 2011, 77, 1149–1160. [Google Scholar] [CrossRef]
- Gao, D.; Li, Q.; Li, Y.; Liu, Z.; Fan, Y.; Liu, Z.; Zhao, H.; Li, J.; Han, Z. Antidiabetic and antioxidant effects of oleanolic acid from Ligustrum lucidum Ait in alloxan-induced diabetic rats. Phytother. Res. 2009, 23, 1257–1262. [Google Scholar] [CrossRef]
- Muhammad, H.I.; Asmawi, M.Z.; Khan, N.A.K. A review on promising phytochemical, nutritional and glycemic control studies on Moringa oleifera Lam. in tropical and sub-tropical regions. Asian Pac. J. Trop. Biomed. 2016, 6, 896–902. [Google Scholar] [CrossRef]
- Sato, H.; Genet, C.; Strehle, A.; Thomas, C.; Lobstein, A.; Wagner, A.; Mioskowski, C.; Auwerx, J.; Saladin, R. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem. Biophys. Res. Commun. 2007, 362, 793–798. [Google Scholar] [CrossRef]
- Bellesia, A.; Verzelloni, E.; Tagliazucchi, D. Pomegranate ellagitannins inhibit α-glucosidase activity in vitro and reduce starch digestibility under simulated gastro-intestinal conditions. Int. J. Food Sci. Nutr. 2015, 66, 85–92. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Moneim, A.A.; Yazid, I.A.; Mahmoud, A.M. Antihyperglycemic, antihyperlipidemic and antioxidant effects and the probable mechanisms of action of Ruta graveolens infusion and rutin in nicotinamide-streptozotocin-induced diabetic rats. Diabetol. Croat. 2010, 39, 15–35. [Google Scholar]
- Wu, C.; Luan, H.; Wang, S.; Zhang, X.; Wang, R.; Jin, L.; Guo, P.; Chen, X. Modulation of lipogenesis and glucose consumption in HepG2 cells and C2C12 myotubes by sophoricoside. Molecules 2013, 18, 15624–15635. [Google Scholar] [CrossRef]
- Musabayane, C.; Tufts, M.; Mapanga, R. Synergistic antihyperglycemic effects between plant-derived oleanolic acid and insulin in streptozotocin-induced diabetic rats. Ren. Fail. 2010, 32, 832–839. [Google Scholar] [CrossRef]
- Ong, K.W.; Hsu, A.; Song, L.; Huang, D.; Tan, B.K.H. Polyphenols-rich Vernonia amygdalina shows anti-diabetic effects in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2011, 133, 598–607. [Google Scholar] [CrossRef]
- Gorelick, J.; Rosenberg, R.; Smotrich, A.; Hanuš, L.; Bernstein, N. Hypoglycemic activity of withanolides and elicitated Withania somnifera. Phytochemistry 2015, 116, 283–289. [Google Scholar] [CrossRef]
- Yin, J.; Ye, J.; Jia, W. Effects and mechanisms of berberine in diabetes treatment. Acta Pharm. Sinica B 2012, 2, 327–334. [Google Scholar] [CrossRef]
- Moser, C.; Vickers, S.P.; Brammer, R.; Cheetham, S.C.; Drewe, J. Antidiabetic effects of the Cimicifuga racemosa extract Ze 450 in vitro and in vivo in ob/ob mice. Phytomedicine 2014, 21, 1382–1389. [Google Scholar] [CrossRef]
- Choi, J.; He, N.; Sung, M.K.; Yang, Y.; Yoon, S. Sanguinarine is an allosteric activator of AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2011, 413, 259–263. [Google Scholar] [CrossRef]
- Ye, X.-P.; Song, C.-Q.; Yuan, P.; Mao, R.-G. α-Glucosidase and α-Amylase Inhibitory Activity of Common Constituents from Traditional Chinese Medicine Used for Diabetes Mellitus. Chin. J. Nat. Med. 2010, 8, 349–352. [Google Scholar] [CrossRef]
- Yu, H.; Zheng, L.; Xu, L.; Yin, L.; Lin, Y.; Li, H.; Liu, K.; Peng, J. Potent effects of the total saponins from Dioscorea nipponica Makino against streptozotocin-induced type 2 diabetes mellitus in rats. Phytother. Res. 2015, 29, 228–240. [Google Scholar] [CrossRef]
- Ghosh, S.; More, P.; Derle, A.; Patil, A.B.; Markad, P.; Asok, A.; Kumbhar, N.; Shaikh, M.L.; Ramanamurthy, B.; Shinde, V.S.; et al. Diosgenin from Dioscorea bulbifera: Novel hit for treatment of type II diabetes mellitus with inhibitory activity against alpha-amylase and alpha-glucosidase. PLoS ONE 2014, 9, e106039. [Google Scholar] [CrossRef] [PubMed]
- Inthongkaew, P.; Chatsumpun, N.; Supasuteekul, C.; Kitisripanya, T.; Putalun, W.; Likhitwitayawuid, K.; Sritularak, B. α-Glucosidase and pancreatic lipase inhibitory activities and glucose uptake stimulatory effect of phenolic compounds from Dendrobium formosum. Revista Brasileira de Farmacogn 2017, 27, 480–487. [Google Scholar] [CrossRef]
- Saeidnia, S.; Manayi, A.; Gohari, A.R.; Abdollahi, M. The story of B-sitosterol—A review. Eur. J. Med. Plants 2014, 4, 590–610. [Google Scholar] [CrossRef]
- Somsak, N.; Peerawit, P.; Chusri, T. Hypoglycemic activity in diabetic rats of stigmasterol and sitosterol-3-O-β-D-glucopyranoside isolated from Pseuderanthemum palatiferum (Nees) Radlk. leaf extract. J. Med. Plants Res. 2015, 9, 629–635. [Google Scholar] [CrossRef]
- Panda, S.; Jafri, M.; Kar, A.; Meheta, B.K. Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma. Fitoterapia 2009, 80, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Sun, H.; Liu, J.; Cheng, K.; Zhang, P.; Zhang, L.; Hao, J.; Zhang, L.; Ni, P.; Zographos, S.E. Naturally Occurring Pentacyclic Triterpenes as Inhibitors of Glycogen Phosphorylase: Synthesis, Structure-Activity Relationships, and X-ray Crystallographic Studies. J. Med. Chem. 2008, 51, 3540–3554. [Google Scholar] [CrossRef]
- Castellano, J.M.; Guinda, A.; Delgado, T.; Rada, M.; Cayuela, J.A. Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes 2013, 62, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Daisy, P.; Balasubramanian, K.; Rajalakshmi, M.; Eliza, J.; Selvaraj, J. Insulin mimetic impact of Catechin isolated from Cassia fistula on the glucose oxidation and molecular mechanisms of glucose uptake on Streptozotocin-induced diabetic Wistar rats. Phytomedicine 2010, 17, 28–36. [Google Scholar] [CrossRef]
- Kamiyama, O.; Sanae, F.; Ikeda, K.; Higashi, Y.; Minami, Y.; Asano, N.; Adachi, I.; Kato, A. In vitro inhibition of α-glucosidases and glycogen phosphorylase by catechin gallates in green tea. Food Chem. 2010, 122, 1061–1066. [Google Scholar] [CrossRef]
- Murase, T.; Misawa, K.; Haramizu, S.; Hase, T. Catechin-induced activation of the LKB1/AMP-activated protein kinase pathway. Biochem. Pharmacol. 2009, 78, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, L.; Arias, N.; Macarulla, M.T.; Gracia, A.; Portillo, M.P. Beneficial Effects of Quercetin on Obesity and Diabetes. Open Nutraceuticals 2011, 4, 189–199. [Google Scholar]
- Bedekar, A.; Shah, K.; Koffas, M. Natural Products for Type II Diabetes Treatment. In Natural Products for Type II Diabetes Treatment: Advances in Applied Microbiology; Laskin, A.I., Gadd, G.M., Sariaslani, S., Eds.; Elsevier Science: San Diego, CA, USA, 2010; Volume 17, pp. 21–73. [Google Scholar]
- Pulbutr, P.; Nualkaew, S.; Rattanakiat, S.; Cushnie, B.; Jaruchotikamol, A. Inhibitory actions of Pseuderanthemum palatiferum (Nees) Radlk. leaf ethanolic extract and its phytochemicals against carbohydrate-digesting enzymes. Asian Pac. J. Trop. Biomed. 2016, 6, 93–99. [Google Scholar] [CrossRef]
- Marles, R.J.; Farnsworth, N.R. Antidiabetic plants and their active constituents. Phytomedicine 1995, 2, 137–189. [Google Scholar] [CrossRef]
- Kpodar, M.S.; Lawson-Evi, P.; Bakoma, B.; Eklu-Gadegbeku, K.; Agbonon, A.; Aklikokou, K.; Gbeassor, M. Ethnopharmacological survey of plants used in the treatment of diabetes mellitus in south of Togo (Maritime Region). J. Herb. Med. 2015, 5, 147–152. [Google Scholar] [CrossRef]
- Bading Taika, B.; Bouckandou, M.; Souza, A.; Bourobou Bourobou, H.P.; MacKenzie, L.S.; Lione, L. An overview of anti-diabetic plants used in Gabon: Pharmacology and toxicology. J. Ethnopharmacol. 2018, 216, 203–228. [Google Scholar] [CrossRef]
- Balogun, F.O.; Tshabalala, N.T.; Ashafa, A.O. Antidiabetic Medicinal Plants Used by the Basotho Tribe of Eastern Free State: A Review. J. Diabetes Res. 2016, 2016, 4602820. [Google Scholar] [CrossRef]
- Zhu, Q.; Ge, F.; Dong, Y.; Sun, W.; Wang, Z.; Shan, Y.; Chen, R.; Sun, J.; Ge, R.S. Comparison of flavonoids and isoflavonoids to inhibit rat and human 11beta-hydroxysteroid dehydrogenase 1 and 2. Steroids 2018, 132, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Mok, S.Y.; Lee, S. Identification of flavonoids and flavonoid rhamnosides from Rhododendron mucronulatum for. albiflorum and their inhibitory activities against aldose reductase. Food Chem. 2013, 136, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.K.; Gacche, R.N. Inhibition of glycation and aldose reductase activity using dietary flavonoids: A lens organ culture studies. Int. J. Biol. Macromol. 2017, 98, 730–738. [Google Scholar] [CrossRef]
- Zhang, B.W.; Xing, Y.; Wen, C.; Yu, X.X.; Sun, W.L.; Xiu, Z.L.; Dong, Y.S. Pentacyclic triterpenes as alpha-glucosidase and alpha-amylase inhibitors: Structure-activity relationships and the synergism with acarbose. Bioorg. Med. Chem. Lett. 2017, 27, 5065–5070. [Google Scholar] [CrossRef]
- Jesus, A.R.; Vila-Vicosa, D.; Machuqueiro, M.; Marques, A.P.; Dore, T.M.; Rauter, A.P. Targeting Type 2 Diabetes with C-Glucosyl Dihydrochalcones as Selective Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: Synthesis and Biological Evaluation. J. Med. Chem. 2017, 60, 568–579. [Google Scholar] [CrossRef]
- Hu, S.; Wang, S.; Fanelli, B.; Bell, P.A.; Dunning, B.E.; Geisse, S.; Schmitz, R.; Boettcher, B.R. Pancreatic b-Cell KATP Channel Activity and Membrane-Binding Studies with Nateglinide: A Comparison with Sulfonylureas and Repaglinide. J. Pharmacol. Exp. Ther. 2000, 293, 444–452. [Google Scholar]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Schrey, A.K.; Nickel-Seeber, J.; Drwal, M.N.; Zwicker, P.; Schultze, N.; Haertel, B.; Preissner, R. Computational prediction of immune cell cytotoxicity. Food Chem. Toxicol. 2017, 107, 150–166. [Google Scholar] [CrossRef]
- Ntie-Kang, F. An in silico evaluation of the ADMET profile of the StreptomeDB database. Springerplus 2013, 2, 353. [Google Scholar] [CrossRef]
- Van Wyk, B.; Oudtshoorn, B.v.; Gericke, N. Medicinal Plants of South Africa; Briza Publications: Pretoria, South Africa, 1997. [Google Scholar]
- Kaneta, H.; Koda, M.; Saito, S.; Imoto, M.; Kawada, M.; Yamazaki, Y.; Momose, I.; Shindo, K. Biological activities of unique isoflavones prepared from Apios americana Medik. Biosci. Biotechnol. Biochem. 2016, 80, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Kar, A. Apigenin (4′,5,7-trihydroxyflavone) regulates hyperglycaemia, thyroid dysfunction and lipid peroxidation in alloxan-induced diabetic mice. J. Pharm. Pharmacol. 2007, 59, 1543–1548. [Google Scholar] [CrossRef]
- Gowd, V.; Jia, Z.; Chen, W. Anthocyanins as promising molecules and dietary bioactive components against diabetes – A review of recent advances. Trends Food Sci. Technol. 2017, 68, 1–13. [Google Scholar] [CrossRef]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Huttemann, M. Molecular Mechanisms and Therapeutic Effects of (-)-Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration. Oxid. Med. Cell. Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Lee, J.J.; Kim, Y.; Kim, I.S.; Han, J.H.; Lee, S.G.; Ahn, M.J.; Jung, S.H.; Myung, C.S. Effect of eriodictyol on glucose uptake and insulin resistance in vitro. J. Agric. Food Chem. 2012, 60, 7652–7658. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.P.; Raghu, K.G.; Nair, M.S.; Padmakumari, K.P. Isolation and identification of alpha-glucosidase and protein glycation inhibitors from Stereospermum colais. Appl. Biochem. Biotechnol. 2014, 173, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Brendler, T.; Eloff, J.N.; Gurib-Fakim, A.; Philips, L. African Herbal Pharmacopoeia; Association for African Medicinal Plants Standards: Port Louis, Mauritius, 2010. [Google Scholar]
- ACD/Chemsketch, version 12.02; Advanced Chemistry Development, Inc.: Toronto, ON, Canada, 2015.
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Cytoscape, version 3.4.0; Cytoscape Consortium: San Diego, CA, USA, 2016.
- NetworkAnalyzer Application, version 2.7; Max-Planck-Institut für Informatik: Saarbrücken, Germany, 2016.
- Schrödinger Canvas Suite, version 3.2.013; Schrödinger, LLC: New York, NY, USA, 2017.
- UCSF ChemViz2 Cheminformatics Application, version 1.1.0 for Cytoscape; Resource for Biocomputing, Visualization and Informatics: San Francisco, CA, USA, 2016.
- Schrödinger Maestro, version 11.2.013; Schrödinger, LLC: New York, NY, USA, 2017.
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

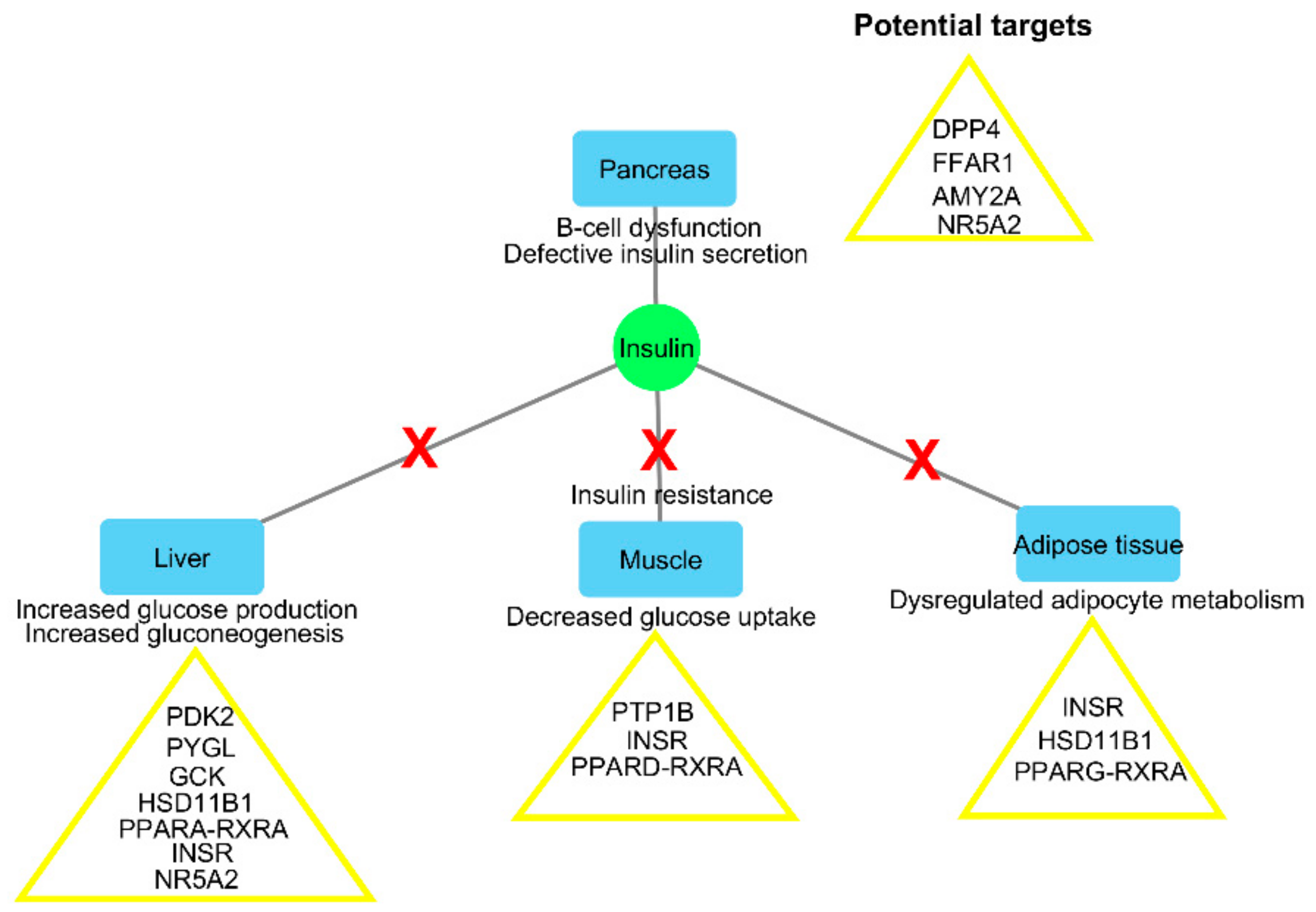




| Mode of Action | Protein Target | Function | PDB Code | Crystallized Ligand–Docking Score (kcal/mol) | Test Compounds–Lowest Energy (kcal/mol) | Test Compound Name |
|---|---|---|---|---|---|---|
| Regulation of insulin secretion and sensitivity | DPP4 | Degrades and inactivates glucagon-like peptide-1 that stimulates insulin secretion from the pancreas [15] | 4A5S | −10.5 | −11.8 | Cryptospirolepine |
| FFAR1 | Binding of free fatty acids to the receptor results in increased glucose-stimulated insulin secretion [16] | 4PHU | −9.8 | −11.6 | Procyanidin C1 | |
| HSD11B1 | Coverts inactive glucocorticoid precursors to active glucocorticoids; glucocorticoids counteract the effects of insulin [17] | 4K1L | −8.3 | −12.8 | Cryptomisrine | |
| INSR | Regulates glucose uptake, as well as glycogen, lipid, and protein synthesis [15] | 3EKN | −8.7 | −10.9 | Typharin | |
| PTPN9 | Dephosphorylates the insulin receptor, thereby reducing insulin sensitivity [18] | 4GE6 | −7.7 | −10.2 | Cryptospirolepine | |
| RBP4 | Secreted as an adipokine that reduces insulin signaling and promotes gluconeogenesis [19] | 2WR6 | −7.9 | −11 | Benzo[c]phenanthridine | |
| Regulation of glucose metabolism | AKR1B1 | Catalyses the reduction of glucose to sorbitol in the polyol pathway, and plays a role in diabetic complications [20] | 3G5E | −11.3 | −11.9 | Pterygospermin |
| AMY2A | Hydrolyses alpha-1,4-glycosidic bonds to starch during digestion of starch to glucose [21] | 4GQR | −7.9 | −11.5 | Clivimine | |
| GCK | Phosphorylates glucose to glucose-6-phosphate for glycolysis or glycogen synthesis [18] | 3IMX | −10.6 | −13 | Cryptomisrine | |
| MGAM | Hydrolyzes 1,4-alpha bonds, the last step in the digestion of starch to glucose [21] | 3L4Y | −5.7 | −10 | Cryptospirolepine | |
| PDK2 | Responsible for inactivating the pyruvate dehydrogenase complex that is involved during glucose oxidation [22] | 4MPC | −7.8 | −11.5 | Clivimine | |
| PYGL | Catalyses the first step of glycogenolysis by the phosphorolysis of glycogen to glucose-1-phosphate [23] | 3DDS | −9.6 | −10.8 | Cryptomisrine | |
| Regulation of lipid metabolism | NR5A2 | Regulates the expression of the genes involved in bile acid synthesis, cholesterol synthesis, and steroidogenesis [24] | 4DOR | −6.5 | −12.2 | Clivimine |
| PPARA | Regulates the expression of the genes involved in lipid metabolism, in particular, the oxidation of fatty acids, as well as lipoprotein assembly and lipid transport [25] | 3FEI | −8.3 | −11.4 | Biscryptolepine | |
| PPARD | Regulates the expression of the genes involved in fatty acid catabolism [25] | 3PEQ | −11.3 | −14.3 | Cryptomisrine | |
| PPARG | Regulates the expression of the genes involved in adipogenesis and lipid metabolism, particularly fatty acid transport, lipid droplet formation, triacyglycerol metabolism, and lipolysis of triglycerides [25] | 2FVJ | −10 | −11.9 | Cryptoquindoline | |
| RXRA | Heterodimerizes with PPARs, thereby initiating gene transcription [25] | 1FM9 | −10.6 | −10.9 | Crinasiatine |
| Plant Name | Family | Compounds |
|---|---|---|
| Acokanthera oppositifolia | Apocynaceae | Acolongifloroside K31, acovenoside A32, ouabain304 |
| Adenium multiflorum | Apocynaceae | Obebioside294 |
| Agapanthus africanus | Amaryllidaceae | Agapanthagenin36 |
| Amaryllis belladonna | Amaryllidaceae | Acetylcaranine30, caranine*97, lycorine277 |
| Anagallis arvensis | Primulaceae | Arvenin I60, arvenin II61 |
| Asclepias fruticosa | Apocynaceae | Afroside35, 19-deoxyuscharin20, gomphoside195 |
| Aster bakeranus | Asteraceae | ent-16-Kauren-18-oic-acid162, ent-16-Kauren-19-oic-acid163, friedelin*174 |
| Balanites maughamii | Zygophyllaceae | Cryptogenin127, diosgenin*153 |
| Bersama lucens | Melianthaceae | Melianthugenin282 |
| Boophane disticha | Amaryllidaceae | 3-Acetylnerbowdine16, buphanisin93 |
| Bowiea volubilis | Asparagaceae | Bovogenin A89, bovoside A90 |
| Brabejum stellatifolium | Proteaceae | Amygdalin*51 |
| Cestrum laevigatum | Solanaceae | Parquin310 |
| Chrysanthemum cinerariifolium | Asteraceae | Pyrethrin I330 |
| Clivia miniata | Amaryllidaceae | Cliviamartine112, cliviasine113, clividine114, clivimine115, clivonine116, hippeastrine217, lycorine277 |
| Cotyledon orbiculata | Crassulaceae | Orbicuside A302, tyledoside C397 |
| Crinum bulbispermum | Amaryllidaceae | Acetylcaranine30, bulbispermine92, crinamine122, crinasiadine123, crinasiatine124, galanthamine180, hippeastrine217, lycorine277, pratorimine319 |
| Crinum macowanii | Amaryllidaceae | Crinamine122, lycorine277, pratorimine319 |
| Crotalaria spartioides | Fabaceae | Retrorsine343 |
| Croton gratissimus | Euphorbiaceae | Crotofolin A125, crotonin126 |
| Cucumis africanus | Cucurbitaceae | Cucurbitacin B133 |
| Cyclamen persicum | Primulaceae | Cyclamin137 |
| Cynanchum africanum | Apocynaceae | Cynafoside B139 |
| Danais fragans | Rubiaceae | 1-Hydroxydimethylanthraquinone8, kaempferol-3-O-rhamnodiglucoside250, quercitrin*335, rubiadin348, rubiadin xyloglucoside349 |
| Datura stramonium | Solanaceae | Hyoscyamine220 |
| Delphinium grandiflorum | Ranunculaceae | Nudicauline293 |
| Digitalis purpurea | Plantaginaceae | Digitoxin150 |
| Dioscorea dregeana | Dioscoreaceae | Deltonin145, deltoside146, dioscin*152, diosgenin*153, hircinol*218 |
| Dodonaea angustifolia | Sapindaceae | Beta-sitosterol*70, hautriwaic acid205, stigmasterol*375 |
| Drimia robusta | Hycinthaceae | 12-Beta-hydroxyscillirosidin4, proscillardin A324 |
| Eriocephalus africanus | Asteraceae | Ivangustine246 |
| Erythrina caffra | Fabaceae | Erythraline169 |
| Erythrina lysistemon | Fabaceae | Erythraline169 |
| Erythrophleum lasianthum | Fabaceae | Erythrophleine170 |
| Eschscholzia californica | Papaveraceae | Dihydrosanguinarine*151 |
| Eucomis autumnalis | Asparagaceae | Autumnariniol65, autumnariol66, 3,9-dihydroeucomnalin19, eucosterol171 |
| Euphorbia ingens | Euphorbiaceae | Ingenol231 |
| Ficus salicifolia | Moraceae | Aviprin69 |
| Geigeria ornativa | Asteraceae | Vermeerin407 |
| Geranium incanum | Geraniaceae | Geraniin*189 |
| Gnidia kraussiana | Thymelaeaceae | Gnidicin192, gnidilatin193, gniditrin194, 12-hydroxydaphnetoxin5 |
| Griffonia simplicifolia | Fabaceae | Indole-3-acetyl aspartic acid230 |
| Homeria pallida | Iridaceae | 1,2-Epoxyscillirosidin1 |
| Hyaenanche globosa | Picrodendraceae | Urushiol III402 |
| Hypericum aethiopicum | Hypericaceae | Hypericin222 |
| Ipomoea purpurea | Convolvulaceae | Ergine167 |
| Kalanchoe lanceolata | Crassulaceae | Lanceotoxin A258, hellebrigenin210 |
| Lippia rehmannii | Verbenaceae | Icterogenin229, lantadene A259 |
| Lotononis laxa | Fabaceae | Integerrimine234, senecionine359 |
| Melianthus comosus | Francoaceae | 3-Epioleanolic acid*17, hellebrigenin-3-acetate211, melianthugenin282, oleanolic acid*299 |
| Melilotus alba | Fabaceae | Dicoumarol148 |
| Moraea polystachya | Iridaceae | 16-Beta-formyloxybovogenin A7 |
| Mundulea sericea | Fabaceae | Deguelin142, rotenone347, tephrosin384 |
| Ocotea bullata | Lauraceae | Ocubullenone295 |
| Peddiea africana | Thymelaeaceae | Peddiea factor A1311 |
| Pelargonium sidoides | Geraniaceae | Catechin*100, gallocatechin*181, quercetin*331, sitosterol-3-glucoside*364 |
| Phytolacca dodecandra | Phytolaccaceae | Lemmatoxin262, oleanoglycotoxin298 |
| Plumbago auriculata | Plumbaginaceae | Plumbagin*318 |
| Polygala fruticosa | Polygalaceae | Frutinone A175, presenegenin321 |
| Ptaeroxylon obliquum | Rutaceae | Umtatin22 |
| Quercus robur | Fagaceae | Catalagin*99, digallic acid149 |
| Rapanea melanophloeos | Primulaceae | 3-Oxo-20,24-dammaradien-26-ol18, sakurasosaponin353 |
| Rhododendron indicum | Ericaceae | Grayanotoxin I197 |
| Rhus undulata | Anacardiaceae | Apigenin dimethylether56 |
| Sanseviera hyacinthoides | Asparagaceae | Ruscogenin-(25S)-form350 |
| Sarcostemma viminale | Apocynaceae | Sarcovimiside B356 |
| Scabiosa columbaria | Caprifoliaceae | Chlorogenic acid*106 |
| Scadoxus puniceus | Amaryllidaceae | Haemanthamine206, haemanthidine207 |
| Schotia brachypetala | Fabaceae | 3,3,4,5,5-Pentahydroxystilbene*14 |
| Scilla natalensis | Asparagaceae | Proscillardin A324 |
| Senecio retrorsus | Asteraceae | Retrorsine343 |
| Senecio serratuloides | Asteraceae | Platyphylline317, senecionine359 |
| Smodingium argutum | Anacardiaceae | 3,8,11-Heptadecadienylcatechol15 |
| Solanum pseudocapsicum | Solanaceae | Solanocapsine367 |
| Spirostachys africana | Euphorbiaceae | Stachenol372, stachenone373 |
| Strophanthus speciosus | Apocynaceae | Christyoside107 |
| Synadenium grantii | Euphorbiaceae | 4-Deoxy-13-O-phenylacetyl-12-O-tigloylphorbol21 |
| Synaptolepis kirkii | Thymelaeaceae | Synaptolepis factor K1381, synaptolepis factor K7382 |
| Tetradenia riparia | Lamiaceae | Ibozol228, 8-(14)-15-isopimaradiene-7,18-diol26 |
| Thesium minkwitzianum | Santalaceae | Thesinine389 |
| Thesium hystrix | Santalaceae | Quercetin*331 |
| Thevetia peruviana | Apocynaceae | Thevetin A390, thevetin B391 |
| Tylecodon wallichii | Crassulaceae | Cotyledoside121 |
| Typha capensis | Typhaceae | Catechin*100, typhaphtalide398, typharin399, thyphasterol400 |
| Urginea maritima | Asparagaceae | Scillaren A357, scillarenin358 |
| Urginea sanguinea | Asparagaceae | Scillaren A357 |
| Valeriana capensis | Valerianaceae | Valerenic acid405 |
| Vinca minor | Apocynaceae | Eburnamonine*160, vincamine*417 |
| Xerophyta retinervis | Velloziaceae | Amentoflavone*49 |
| Zanthoxylum capense | Rutaceae | Sanguinarine*354 |
| Target Enzyme | Total Number of Compounds | Largest Cluster | Cluster Centroid | Maximum Common Substructure |
|---|---|---|---|---|
| 11HSDB1 | 208 | 40 | 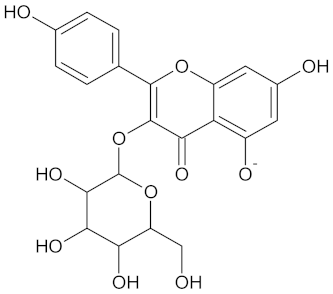 Kaempferol-3-glucoside | 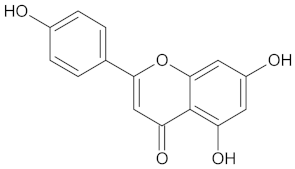 |
| AKR1B1 | 135 | 71 | 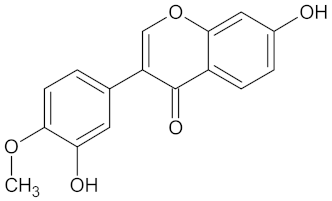 Calycosin | 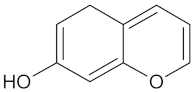 |
| AMY2A | 129 | 38 | 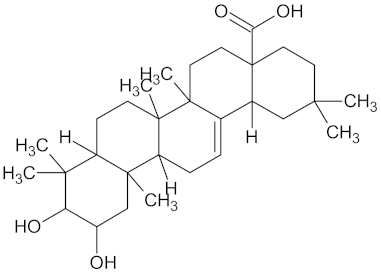 Maslinic acid | 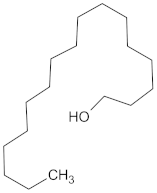 |
| DPP4 | 149 | 23 |  Balanitin-6 | 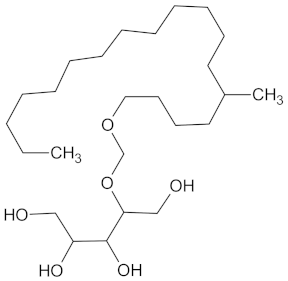 |
| FFAR1 | 37 | 26 | 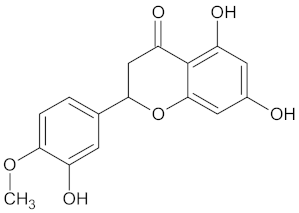 Hesperitin |  |
| GCK | 77 | 33 | 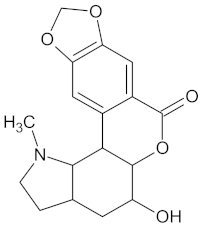 Clivonine | 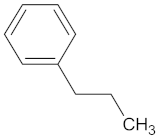 |
| MGAM | 18 | 12 |  Kolaflavanone | 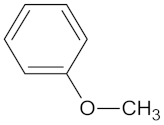 |
| PPARD | 190 | 57 | 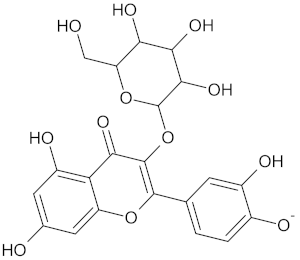 Hyperin | 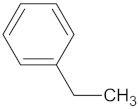 |
| PPARG | 124 | 89 | 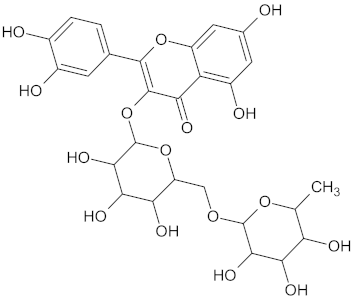 Rutin |  |
| RBP4 | 85 | 48 | 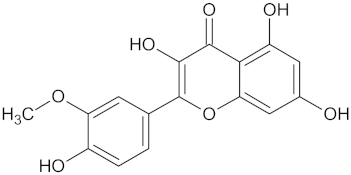 Isorhamnetin | 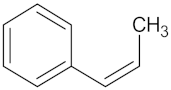 |
| ADMET Property | Unknown Compounds | Known Compounds | Diabetes Drugs |
|---|---|---|---|
| Lipinski violations (1–4) | 136/305 (45%) | 75/125 (60%) | 12/48 (25%) |
| Veber violations (1–2) | 89/305 (29%) | 42/125 (36%) | 9/48 (19%) |
| Aqueous solubility QPlogS | 34/305 (11%) | 33/125 (26%) | 6/48 (13%) |
| Caco-2 cell permeability (<25 nm/s) | 66/305 (22%) | 40/125 (32%) | 3/48 (6%) |
| Binding to human serum albumin | 37/305 (12%) | 23/125 (18%) | 6/48 (13%) |
| Human oral absorption (<25%) | 55/305 (18%) | 32/125 (26%) | 3/48 (6%) |
| Rat oral LD50 (1–50 mg/kg) | 53/305 (17%) | 4/125 (3%) | 1/48 (2%) |
| Hepatotoxicity | 4/305 (1%) | 4/125 (3%) | 8/48 (17%) |
| Carcinogenicity | 70/305 (23%) | 31/125 (25%) | 6/48 (13%) |
| Immunotoxicity | 233/305 (76%) | 89/125 (71%) | 16/48 (33%) |
| Mutagenicity | 49/305 (16%) | 17/125 (14%) | 1/48 (2%) |
| Cytotoxicity | 58/305 (19%) | 11/125 (9%) | 1/48 (2%) |
| Blockage of hERG K+ channels | 132/305 (43%) | 58/125 (46%) | 20/48 (42%) |
| Compound | Structure | Predicted Targets (Docking Score in kcal/mol) | Potential Anti-Diabetic Effect | Plant |
|---|---|---|---|---|
| 2-Hydroxygenistein | 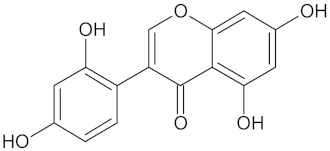 | AKR1B1 (−9.1) | Regulation of glucose metabolism | Cajanus cajan |
| Apigenin | 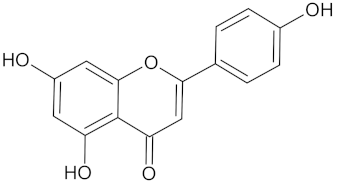 | AKR1B1 (−9.1), HSD11B1 (−9.0), RBP4 (−9.9), and RXRA (−9.1) | Regulation of insulin secretion, glucose metabolism, and lipid metabolism | Cajanus cajan |
| Autumnarinol | 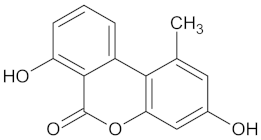 | RBP4 (−9.0) | Regulation of insulin secretion | Eucomis autumnalis |
| Catechin | 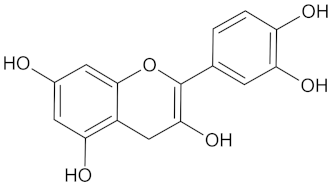 | AKR1B1 (−9.0), HSD11B1 (−9.5), and RBP4 (−9.3) | Regulation of insulin secretion and glucose metabolism | Adansonia digitate, Combretum micranthum, Prunus africana, Sclerocarya birrea, Pelargonium sidoides, and Typha capensis |
| Crotofoline A | 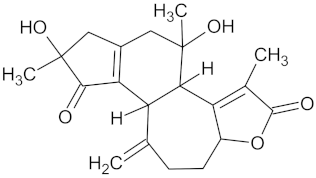 | AMY2A (−9.2), HSD11B1 (−9.9), and PPARD (−9.3) | Regulation of insulin secretion, glucose metabolism, and lipid metabolism | Croton gratissimus |
| Cyanidin | 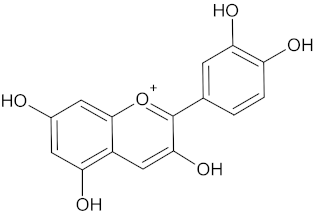 | AKR1B1 (−9.1), HSD11B1 (−9.5), and RBP4 (−9.2) | Regulation of insulin secretion and glucose metabolism | Rhoicissus tridentate |
| Desacetylformonoakuammiline | 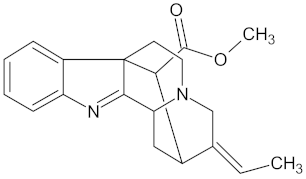 | HSD11B1 (−9.1), PPARD (−9.0) | Regulation of insulin secretion and lipid metabolism | Rauvolfia vomitoria |
| Eburnamonine | 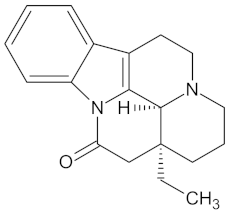 | AKR1B1 (-9.4), HSD11B1 (−9.2), PPARD (−9.3), and RBP4 (−9.4) | Regulation of insulin secretion, glucose metabolism, and lipid metabolism | Vinca minor |
| Ent-16-kauran-19-oic acid | 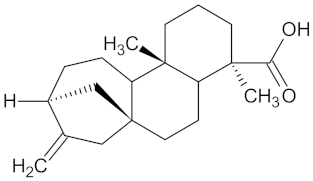 | HSD11B1 (−9.4) and PPARD (−9.4) | Regulation of insulin secretion and lipid metabolism | Aster bakeranus |
| Epicatechin | 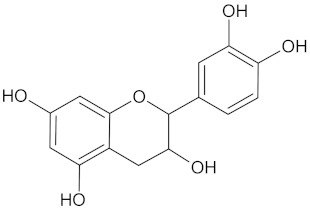 | AKR1B1 (−9.2) and RBP4 (−9.3) | Regulation of insulin secretion and glucose metabolism | Acacia karroo, Harungana madagascariensis, and Prunus Africana |
| Ergine |  | HSD11B1 (−9.5) and RBP4 (−9.4) | Regulation of insulin secretion | Ipomoea purpurea |
| Eriodictyol | 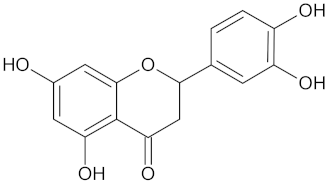 | HSD11B1 (−9.2) and RBP4 (−9.5) | Regulation of insulin secretion | Cyclopia intermedia |
| Erythraline |  | AKR1B1 (−9.0), GCK (−9.8), and RBP4 (−9.0) | Regulation of insulin secretion and glucose metabolism | Erythrina caffra and Erythrina lysistemon |
| Furanoeudesma-1,3-diene | 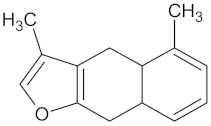 | RBP4 (−9.0) | Regulation of insulin secretion | Commiphora myrrha |
| Hautriwaic acid | 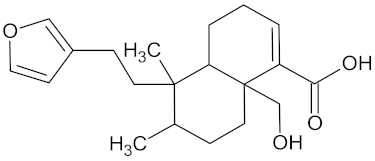 | AKR1B1 (−9.3) | Regulation of glucose metabolism | Dodonaea angustifolia |
| Henningsiine |  | AMY2A (−9.1), HSD11B1 (−9.6), PPARD (−10.0), and PPARG (−9.0) | Regulation of insulin secretion, glucose metabolism, and lipid metabolism | Strychnos henningsii |
| Ibozol | 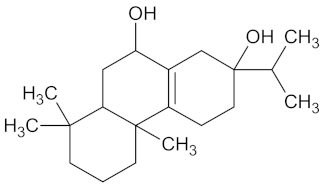 | GCK (−9.7) | Regulation of glucose metabolism | Tetradenia riparia |
| Integerrimine | 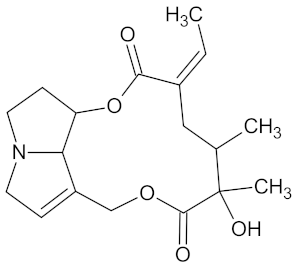 | HSD11B1 (−9.1) and PPARD (−9.3) | Regulation of insulin secretion and lipid metabolism | Lotononis laxa |
| Lapachol | 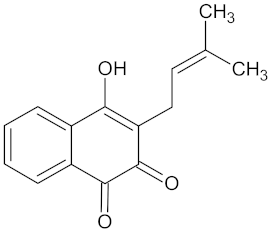 | AKR1B1 (−9.2) | Regulation of glucose metabolism | Kigelia africana |
| Nauclefidine | 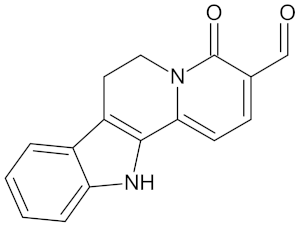 | AKR1B1 (−10.1), HSD11B1 (−9.0), and RBP4 (−10.0) | Regulation of insulin secretion and glucose metabolism | Nauclea latifolia |
| N-methylflindersine | 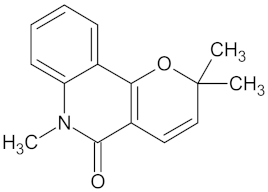 | AKR1B1 (−9.2) and RBP4 (−9.5) | Regulation of insulin secretion and glucose metabolism | Toddalia asiatica |
| Platyphylline | 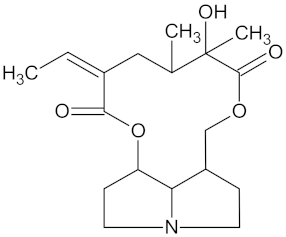 | HSD11B1 (−9.4) and PPARD (−9.3) | Regulation of insulin secretion and lipid metabolism | Senecio serratuloides |
| Rhinocerotinoic acid |  | HSD11B1 (−9.2) and RBP4 (−9.9) | Regulation of insulin secretion | Elytropappus rhinocerotis |
| Senecionine | 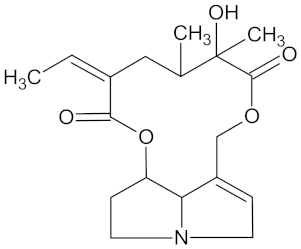 | HSD11B1 (−10.3) and PPARD (−9.4) | Regulation of insulin secretion and lipid metabolism | Senecio serratuloides |
| Valerenic acid |  | AKR1B1 (−9.0) | Regulation of glucose metabolism | Valeriana capensis |
| Vinburnine | 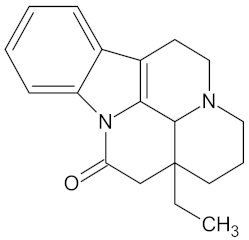 | AKR1B1 (−9.6), HSD11B1 (−9.1), PPARD (−9.3), PPARG (−9.4), RBP4 (−10.7), and RXRA (−9.3) | Regulation of insulin secretion, glucose metabolism and lipid metabolism | Voacanga africana |
| Voaphylline | 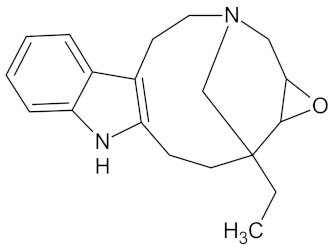 | AMY2A (−9.0), DPP4 (−9.6), GCK (−9.1), HSD11B1 (−9.3), PPARD (−9.1), PPARG (−9.8), and RBP4 (−9.2) | Regulation of insulin secretion, glucose metabolism, and lipid metabolism | Voacanga africana |
| Withasomnine | 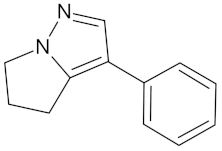 | FFAR1 (−9.1) | Regulation of insulin secretion | Voacanga africana |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.S.P.; den Haan, H.; Peña-García, J.; Moreno, M.M.; Pérez-Sánchez, H.; Apostolides, Z. Exploring African Medicinal Plants for Potential Anti-Diabetic Compounds with the DIA-DB Inverse Virtual Screening Web Server. Molecules 2019, 24, 2002. https://doi.org/10.3390/molecules24102002
Pereira ASP, den Haan H, Peña-García J, Moreno MM, Pérez-Sánchez H, Apostolides Z. Exploring African Medicinal Plants for Potential Anti-Diabetic Compounds with the DIA-DB Inverse Virtual Screening Web Server. Molecules. 2019; 24(10):2002. https://doi.org/10.3390/molecules24102002
Chicago/Turabian StylePereira, Andreia S.P., Helena den Haan, Jorge Peña-García, Marién M. Moreno, Horacio Pérez-Sánchez, and Zeno Apostolides. 2019. "Exploring African Medicinal Plants for Potential Anti-Diabetic Compounds with the DIA-DB Inverse Virtual Screening Web Server" Molecules 24, no. 10: 2002. https://doi.org/10.3390/molecules24102002
APA StylePereira, A. S. P., den Haan, H., Peña-García, J., Moreno, M. M., Pérez-Sánchez, H., & Apostolides, Z. (2019). Exploring African Medicinal Plants for Potential Anti-Diabetic Compounds with the DIA-DB Inverse Virtual Screening Web Server. Molecules, 24(10), 2002. https://doi.org/10.3390/molecules24102002