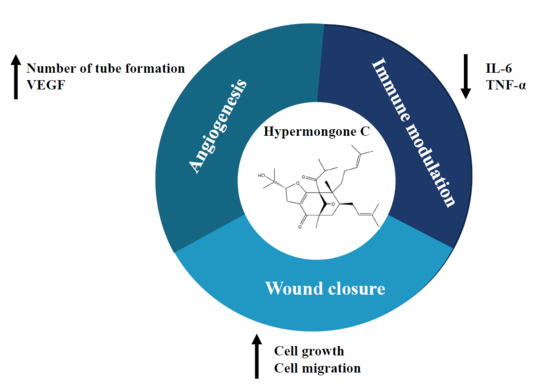
Hypermongone C Accelerates Wound Healing through the Modulation of Inflammatory Factors and Promotion of Fibroblast Migration
Abstract
:1. Introduction
2. Results
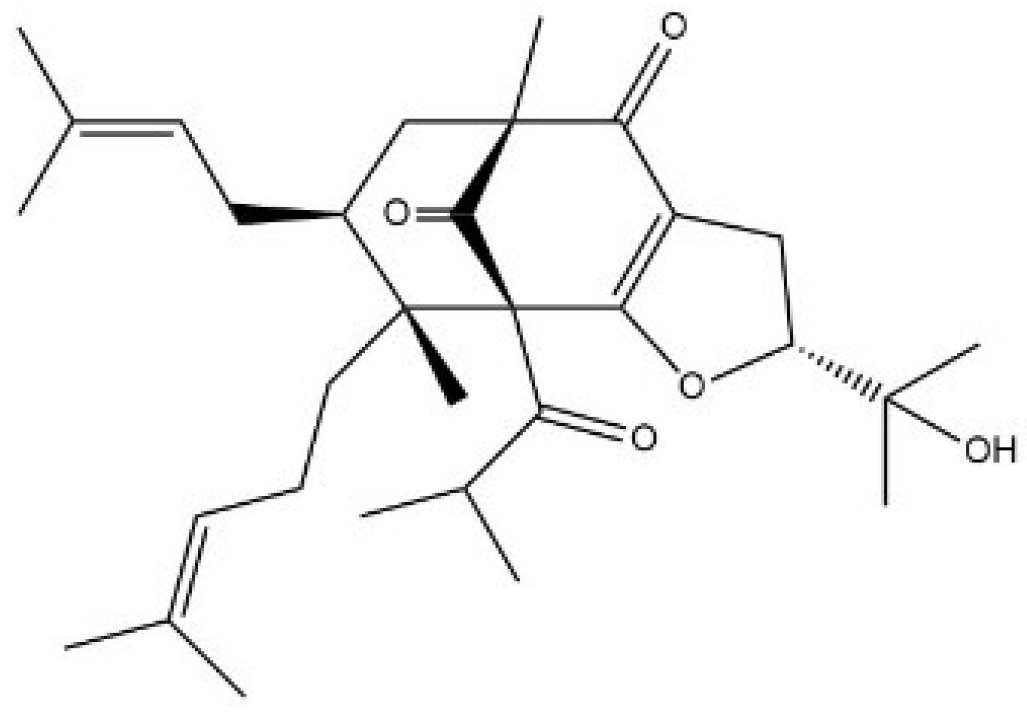
2.1. Hypermongone C Identification
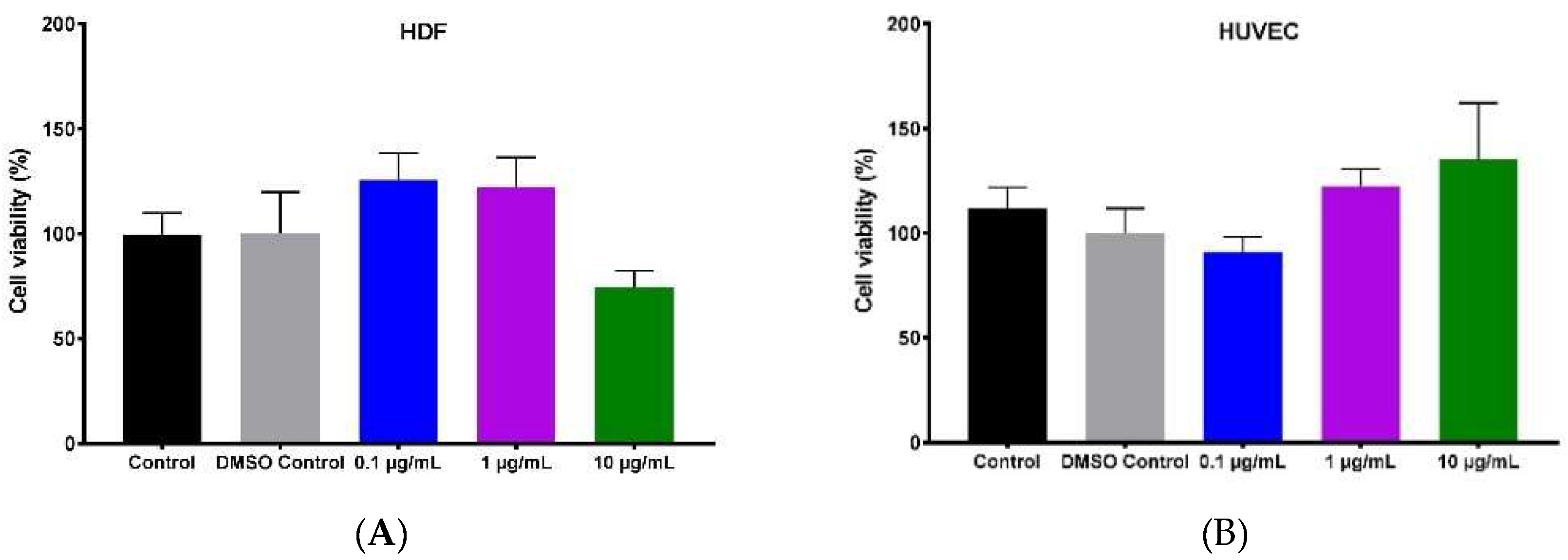
2.2. Effect of Hypermongone C on Cell Proliferation and Viability
2.3. Hypermongone C-Induced Cell Migration
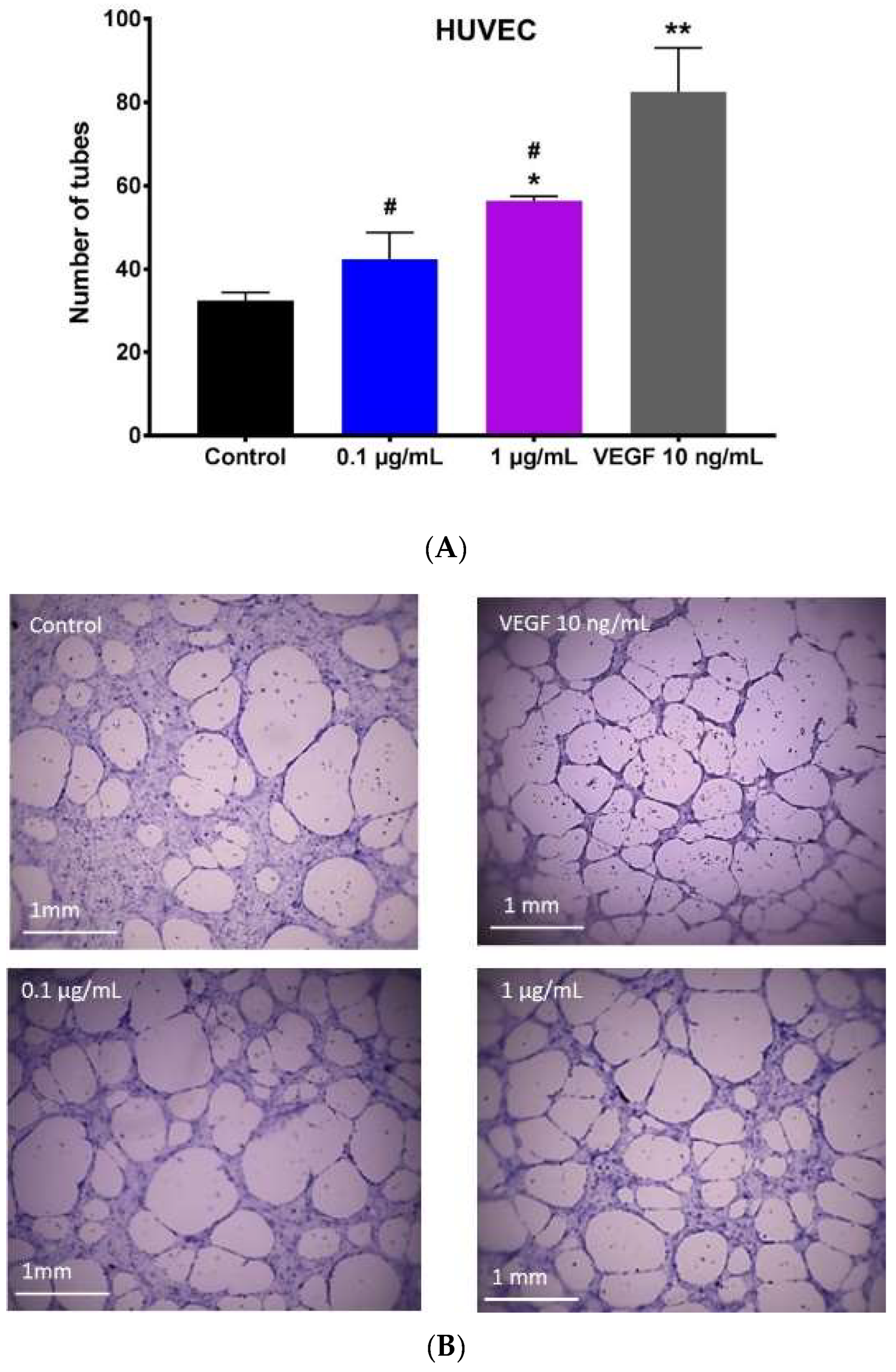
2.4. Effect of Hypermongone C on Angiogenesis
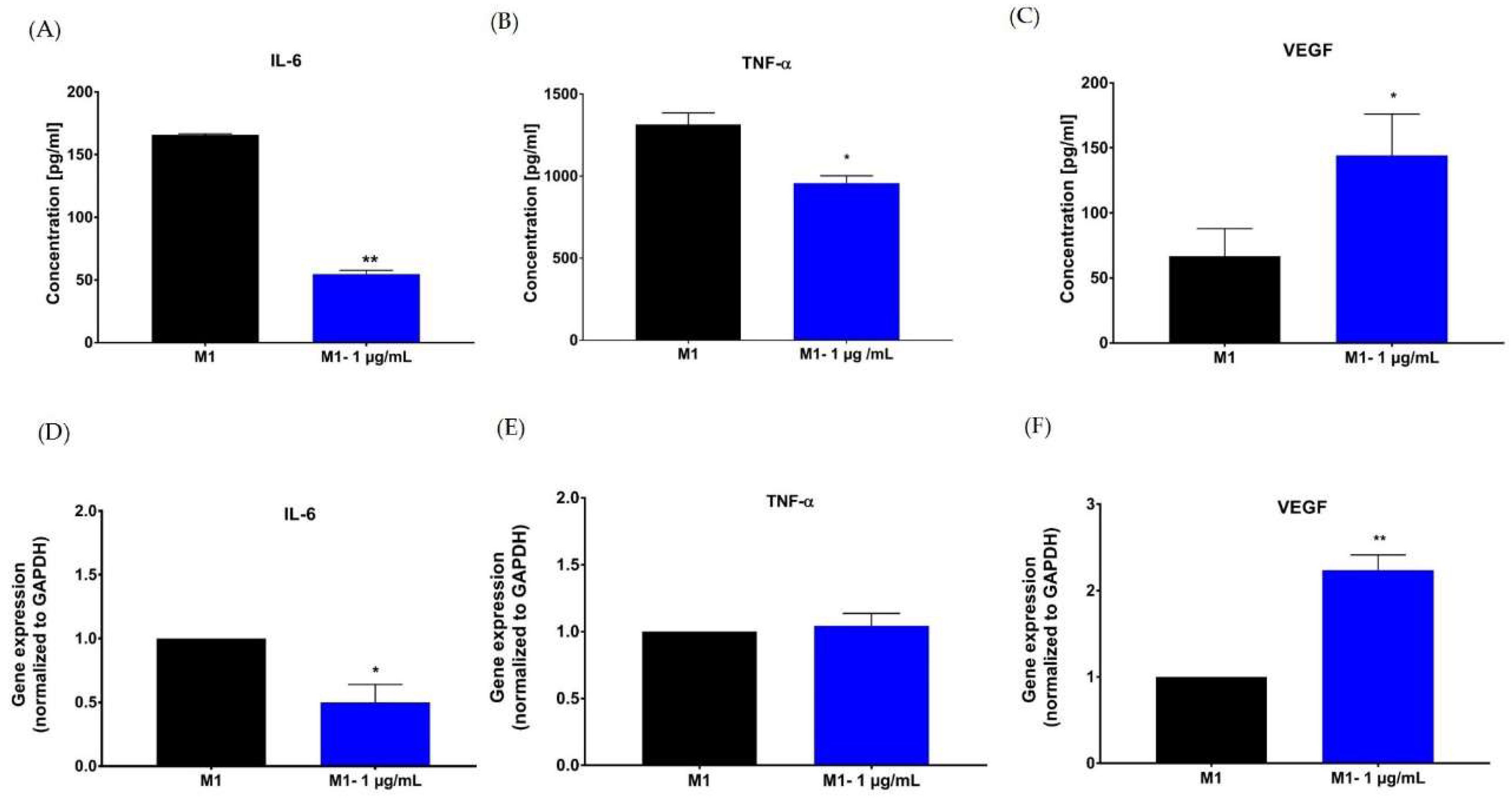
2.5. Effect of Hypermongone C on TNF-α, IL-6, and VEGF Production
3. Discussion
4. Materials and Methods
4.1. Hypermongone C Source and Identification
4.2. Cell Culture and Reagents
4.3. Cytotoxicity Assay (MTS)
4.4. LIVE/DEAD® Assay
4.5. In Vitro Migration Assay (Wound Healing Assay)
4.6. Capillary Tube Formation
4.7. Macrophage Polarization
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holt, D.J.; Chamberlain, L.M.; Grainger, D.W. Cell-cell signaling in co-cultures of macrophages and fibroblasts. Biomaterials 2010, 31, 9382–9394. [Google Scholar] [CrossRef]
- Ferrer, R.A.; Saalbach, A.; Gruenwedel, M.; Lohmann, N.; Forstreuter, I.; Saupe, S.; Wandel, E.; Simon, J.C.; Franz, S. Dermal fibroblasts promote alternative macrophage activation improving impaired wound healing. J. Investig. Dermatol. 2017, 137, 941–950. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Eming, S.A.; Hammerschmidt, M.; Krieg, T.; Roers, A. Interrelation of immunity and tissue repair or regeneration. Semin. Cell Dev. Biol. 2009, 20, 517–527. [Google Scholar] [CrossRef]
- Delavary, B.M.; Van der Veer, W.M.; Van Egmond, M.; Niessen, F.B.; Beelen, R.H. Macrophages in skin injury and repair. Immunobiology 2011, 216, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Ploeger, D.T.; Hosper, NA.; Schipper, M.; Koerts, J.A.; De rond, S.; Bank, R.A. Cell plasticity in wound healing: Paracrine factors of M1/M2 polarized macrophages influence the phenotypical state of dermal fibroblasts. Cell Commun. Signal. 2013, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Minutti, C.M.; Knipper, J.A.; Allen, J.E.; Zaiss, D.M. Tissue-specific contribution of macrophages to wound healing. Semin. Cell Dev. Biol. 2017, 61, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Hussain, Z.; Thu, H.E.; Ng, S.-F.; Khan, S.; Katas, H. Nanoencapsulation, an efficient and promising approach to maximize wound healing efficacy of curcumin: A review of new trends and state-of-the-art. Colloids Surf. B Biointerfaces 2017, 150, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.d.O.; Costa, T.F.; Andrade, Z.d.A.; Medrado, A.R.A.P. Wound healing-A literature review. Annis Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22. [Google Scholar] [CrossRef]
- Mescher, A.L. Macrophages and fibroblasts during inflammation and tissue repair in models of organ regeneration. Regeneration 2017, 4, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Maquart, F.X.; Monboisse, J.C. Extracellular matrix and wound healing. Pathol. Biol. 2014, 62, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Jackson, C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Jetten, N.; Verbruggen, S.; Gijbels, M.J.; Post, M.J.; De Winther, M.P.; Donners, M.M. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 2014, 17, 109–118. [Google Scholar] [CrossRef]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef]
- Li, W.W.; Tsakaynnis, D.; Li, V.W. Angiogenesis: A control point for normal and delayed wound healing. Contemp. Surg. 2003, 1, 5–11. [Google Scholar]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [Green Version]
- Corliss, B.A.; Azimi, M.S.; Munson, J.M.; Peirce, S.M.; Murfee, W.L. Macrophages: An Inflammatory Link between Angiogenesis and Lymphangiogenesis. Microcirculation 2016, 23, 95–121. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Farzaei, M.H.; Rahimi, R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: An integrative review. Arch. Dermatol. Res. 2014, 306, 601–617. [Google Scholar] [CrossRef]
- Sivamani, R.K.; Ma, B.R.; Wehrli, L.N.; Maverakis, E. Phytochemicals and Naturally Derived Substances for Wound Healing. Adv. Wound Care 2012, 1, 213–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.W.; Grossman, R.B.; Xu, G. Research Progress of Polycyclic Polyprenylated Acylphloroglucinols. Chem. Rev. 2018, 118, 3508–3558. [Google Scholar] [CrossRef] [PubMed]
- Cakir, A.; Mavi, A.; Yildirim, A.; Duru, M.A.; Harmander, M.; Kazaz, C. Isolation and characterization of antioxidant phenolic compounds from the aerial parts of Hypericum hyssopifolium L. by activity-guided fractionation. J. Ethnopharmacol. 2003, 87, 73–83. [Google Scholar] [CrossRef]
- Crockett, S.L.; Wenzig, E.-M.; Kunert, O.; Bauer, R. Anti-inflammatory phloroglucinol derivatives from Hypericum empetrifolium. Phytochem. Lett. 2008, 1, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Bridi, H.; Meirelles, G.C.; von Poser, G.L. Structural diversity and biological activities of phloroglucinol derivatives from Hypericum species. Phytochemistry 2018, 155, 203–232. [Google Scholar] [CrossRef]
- Gao, W.; Hou, W.-Z.; Zhao, J.; Xu, F.; Li, L.; Xu, F.; Sun, H.; Xing, J.-G.; Peng, Y.; Wang, X.-L. Polycyclic polyprenylated acylphloroglucinol congeners from Hypericum scabrum. J. Nat. Prod. 2016, 79, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Verotta, L. Are acylphloroglucinols lead structures for the treatment of degenerative diseases. Phytochem. Rev. 2002, 1, 389–407. [Google Scholar] [CrossRef]
- Prisacaru, A.I.; Andriţoiu, C.; Andriescu, C.; Hăvârneanu, E.; Popa, M.; Motoc, A.; Sava, A. Evaluation of the wound-healing effect of a novel Hypericum perforatum ointment in skin injury. Rom. J. Morphol. Embryol. 2013, 54, 1053–1059. [Google Scholar]
- Franco, P.; Potenza, I.; Moretto, F.; Segantin, M.; Grosso, M.; Lombardo, A.; Taricco, D.; Vallario, P.; Filippi, A.R.; Rampino, M. Hypericum perforatum and neem oil for the management of acute skin toxicity in head and neck cancer patients undergoing radiation or chemo-radiation: A single-arm prospective observational study. Radiat. Oncol. 2014, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-J.; Zhu, M.D.; Wang, X.B.; Yang, M.H.; Luo, J.; Kong, L.Y. Hypermongones A–J, rare methylated polycyclic polyprenylated acylphloroglucinols from the flowers of Hypericum monogynum. J. Nat. Prod. 2015, 78, 1093–1100. [Google Scholar] [CrossRef]
- Lordani, T.V.A.; de Lara, C.E.; Ferreira, F.B.P.; de Souza Terron Monich, M.; Mesquita da Silva, C.; Lordani, F.; Regina, C.; Giacomini Bueno, F.; Vieira Teixeira, J.J.; Lonardoni, M.V.C. Therapeutic effects of medicinal plants on cutaneous wound healing in humans: A systematic review. Mediat. Inflamm. 2018. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, S.E.; Ebrahimi, S.; Salehi, P.; Moridi Farimani, M.; Hamburger, M.; Jabbarzadeh, E. Wound Healing Potential of Chlorogenic Acid and Myricetin-3-O-β-Rhamnoside Isolated from Parrotia persica. Molecules 2017, 22, 1501. [Google Scholar] [CrossRef]
- Shah, A.; Amini-Nik, S. The role of phytochemicals in the inflammatory phase of wound healing. Int. J. Mol. Sci. 2017, 18, 1068. [Google Scholar] [CrossRef] [PubMed]
- Yanez, M.; Blanchette, J.; Jabbarzadeh, E. Modulation of Inflammatory Response to Implanted Biomaterials Using Natural Compounds. Curr. Pharm. Des. 2017, 23, 6347–6357. [Google Scholar] [CrossRef]
- Taylor, W.F.; Jabbarzadeh, E. The use of natural products to target cancer stem cells. Am. J. Cancer Res. 2017, 7, 1588. [Google Scholar]
- Suntar, I.P.; Akkol, E.K.; Yilmazer, D.; Baykal, T.; Kirmizibekmez, H.; Alper, M.; Yesilada, E. Investigations on the in vivo wound healing potential of Hypericum perforatum L. J. Ethnopharmacol. 2010, 127, 468–477. [Google Scholar]
- Tonks, A.J.; Cooper, R.; Jones, K.; Blair, S.; Parton, J.; Tonks, A. Honey stimulates inflammatory cytokine production from monocytes. Cytokine 2003, 21, 242–247. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murry, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Hwang, S.J.; Beckenkamp, A.; Ccana-Ccapatinta, G.V.; de Loreto Bordignon, S.A.; Buffon, A.; von Poser, G. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Witzel, I.D.; Langosch, K.M.; Wirtz, R.M.; Roth, C.; Ihnen, M.; Mahner, S.; Eulenburg, C.z.; Janicke, F.; Muller, V. Comparison of microarray-based RNA expression with ELISA-based protein determination of HER2, uPA and PAI-1 in tumour tissue of patients with breast cancer and relation to outcome. J. Cancer Res. Clin. Oncol. 2010, 136, 1709–1718. [Google Scholar] [CrossRef]
- Amsen, D.; de Visser, K.E.; Town, T. Approaches to determine expression of inflammatory cytokines. Methods Mol. Biol. 2009, 511, 107–142. [Google Scholar] [PubMed]
- Bridi, H.; Beckenkamp, A.; Ccana-Ccapatinta, G.V.; de Loreto Bordignon, S.A.; Buffon, A.; von Poser, G.L. Characterization of Phloroglucinol-enriched Fractions of Brazilian Hypericum Species and Evaluation of Their Effect on Human Keratinocytes Proliferation. Phytother. Res. 2017, 31, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Lucas, T.; Waisman, A.; Ranjan, R.; Roes, J.; Krieg, T.; Müller, W.; Roers, A.; Eming, S.A. Differential roles of macrophages in diverse phases of skin repair. J. Immunol. 2010, 184, 3964–3977. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Krieg, T. Molecular mechanisms of VEGF-A action during tissue repair. J. Investig. Dermatol. Symp. Proc. 2006, 11, 79–86. [Google Scholar] [CrossRef] [PubMed]
- DeCicco-Skinner, K.L.; Henry, G.H.; Cataisson, C.; Tabib, T.; Gwilliam, J.C.; Watson, N.J.; Bulwinkle, E.M.; Falkenburg, L.; O’Neil, R.C.; Morin, A.; Wiest, J.S. Endothelial cell tube formation assay for the in vitro study of angiogenesis. J. Vis. Exp. 2014, e51312. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moghadam, S.E.; Moridi Farimani, M.; Soroury, S.; Ebrahimi, S.N.; Jabbarzadeh, E. Hypermongone C Accelerates Wound Healing through the Modulation of Inflammatory Factors and Promotion of Fibroblast Migration. Molecules 2019, 24, 2022. https://doi.org/10.3390/molecules24102022
Moghadam SE, Moridi Farimani M, Soroury S, Ebrahimi SN, Jabbarzadeh E. Hypermongone C Accelerates Wound Healing through the Modulation of Inflammatory Factors and Promotion of Fibroblast Migration. Molecules. 2019; 24(10):2022. https://doi.org/10.3390/molecules24102022
Chicago/Turabian StyleMoghadam, Sara E., Mahdi Moridi Farimani, Sara Soroury, Samad N. Ebrahimi, and Ehsan Jabbarzadeh. 2019. "Hypermongone C Accelerates Wound Healing through the Modulation of Inflammatory Factors and Promotion of Fibroblast Migration" Molecules 24, no. 10: 2022. https://doi.org/10.3390/molecules24102022
APA StyleMoghadam, S. E., Moridi Farimani, M., Soroury, S., Ebrahimi, S. N., & Jabbarzadeh, E. (2019). Hypermongone C Accelerates Wound Healing through the Modulation of Inflammatory Factors and Promotion of Fibroblast Migration. Molecules, 24(10), 2022. https://doi.org/10.3390/molecules24102022