Electrochemical Reduction of CO2 to Formate on Easily Prepared Carbon-Supported Bi Nanoparticles
Abstract
:1. Introduction
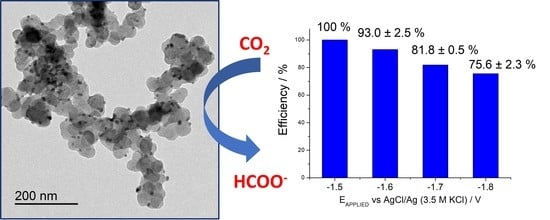
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Synthesis of Bismuth Electrocatalysts
3.3. Preparation of the Catalytic Ink and Cathode
3.4. Physicochemical Characterisation
3.5. Electrochemical Characterisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The U.S. Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Con. 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the Valorization of Exhaust Carbon: From CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Liu, Y.; Zhang, J. Electrochemical Reduction of Carbon Dioxide: Fundamentals and Technologies, 1st ed.; Qiao, J., Liu, Y., Zhang, J., Eds.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Wu, J.J.; Zhou, X.D. Catalytic conversion of CO2 to value added fuels: Current status, challenges, and future directions. Chin. J. Catal. 2016, 37, 999–1015. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.J.; Gong, J.L. Nanostructured Materials for Heterogeneous Electrocatalytic CO2 Reduction and their Related Reaction Mechanisms. Angew. Chem. 2017, 56, 11326–11353. [Google Scholar] [CrossRef]
- Arán-Ais, R.M.; Gao, D.; Roldan Cuenya, B. Structure- and Electrolyte-Sensitivity in CO2 Electroreduction. Acc. Chem. Res. 2018, 51, 2906–2917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, Y.; Ma, L.; Zhu, G.; Wang, Y.; Xue, X.; Chen, R.; Yang, S.; Jin, Z. Progress and Perspective of Electrocatalytic CO2 Reduction for Renewable Carbonaceous Fuels and Chemicals. Adv. Sci. 2018, 5. [Google Scholar] [CrossRef]
- Wanfeng, Y.; Kamran, D.; Chen, J.; Chuan, Z. Design of Electrocatalysts and Electrochemical Cells for Carbon Dioxide Reduction Reactions. Adv. Mater. Technol. 2018, 1700377. [Google Scholar] [CrossRef]
- Feng, D.M.; Sun, Y.; Liu, Z.Q.; Zhu, Y.P.; Ma, T.Y. Designing nanostructured metal-based CO2 reduction electrocatalysts. J. Nanosci. Nanotechnol. 2019, 19, 3079–3096. [Google Scholar] [CrossRef]
- Kortlever, R.; Shen, J.; Schouten, K.J.P.; Calle-Vallejo, F.; Koper, M.T.M. Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide. J. Phys. Chem. Lett. 2015, 6, 4073–4082. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, K.P.; Hatsukade, T.; Cave, E.R.; Abram, D.N.; Kibsgaard, J.; Jaramillo, T.F. Electrocatalytic Conversion of Carbon Dioxide to Methane and Methanol on Transition Metal Surfaces. J. Am. Chem. Soc. 2014, 136, 14107–14113. [Google Scholar] [CrossRef]
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic process of co selectivity in electrochemical reduction of CO2 at metal-electrodes in aqueous-media. Electrochim. Acta 1994, 39, 1833–1839. [Google Scholar] [CrossRef]
- Lim, R.J.; Xie, M.; Sk, M.A.; Lee, J.-M.; Fisher, A.; Wang, X.; Lim, K.H. A review on the electrochemical reduction of CO2 in fuel cells, metal electrodes and molecular catalysts. Catal. Today 2014, 233, 169–180. [Google Scholar] [CrossRef]
- Lu, Q.; Jiao, F. Electrochemical CO2 reduction: Electrocatalyst, reaction mechanism, and process engineering. Nano Energy 2016, 29, 439–456. [Google Scholar] [CrossRef]
- Hoshi, N.; Sato, E.; Hori, Y. Electrochemical reduction of carbon dioxide on kinked stepped surfaces of platinum inside the stereographic triangle. J. Electroanal. Chem. 2003, 540, 105–110. [Google Scholar] [CrossRef]
- Hori, Y.; Takahashi, I.; Koga, O.; Hoshi, N. Electrochemical reduction of carbon dioxide at various series of copper single crystal electrodes. J. Mol. Catal. Chem. 2003, 199, 39–47. [Google Scholar] [CrossRef]
- Tang, W.; Peterson, A.A.; Varela, A.S.; Jovanov, Z.P.; Bech, L.; Durand, W.J.; Dahl, S.; Norskov, J.K.; Chorkendorff, I. The importance of surface morphology in controlling the selectivity of polycrystalline copper for CO2 electroreduction. Phys. Chem. Chem. Phys. 2012, 14, 76–81. [Google Scholar] [CrossRef]
- Chen, C.S.; Handoko, A.D.; Wan, J.H.; Ma, L.; Ren, D.; Yeo, B.S. Stable and selective electrochemical reduction of carbon dioxide to ethylene on copper mesocrystals. Catal. Sci. Technol. 2015, 5, 161–168. [Google Scholar] [CrossRef]
- Zhu, S.Q.; Shao, M.H. Surface structure and composition effects on electrochemical reduction of carbon dioxide. J. Solid State Electrochem. 2016, 20, 861–873. [Google Scholar] [CrossRef]
- Luo, W.J.; Nie, X.W.; Janik, M.J.; Asthagiri, A. Facet Dependence of CO2 Reduction Paths on Cu Electrodes. ACS Catal. 2016, 6, 219–229. [Google Scholar] [CrossRef]
- Liu, S.; Tao, H.; Zeng, L.; Liu, Q.; Xu, Z.; Liu, Q.; Luo, J.-L. Shape-Dependent Electrocatalytic Reduction of CO2 to CO on Triangular Silver Nanoplates. J. Am. Chem. Soc. 2017, 139, 2160–2163. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Song, J.T.; Ryoo, H.; Kim, J.G.; Chung, S.Y.; Oh, J. Morphology-controlled Au nanostructures for efficient and selective electrochemical CO2 reduction. J. Mater. Chem. A 2018, 6, 5119–5128. [Google Scholar] [CrossRef]
- Back, S.; Yeom, M.S.; Jung, Y. Understanding the Effects of Au Morphology on CO2 Electrocatalysis. J. Phys. Chem. C 2018, 122, 4274–4280. [Google Scholar] [CrossRef]
- Gao, D.F.; Zhou, H.; Cai, F.; Wang, J.G.; Wang, G.X.; Bao, X.H. Pd-Containing Nanostructures for Electrochemical CO2 Reduction Reaction. ACS Catal. 2018, 8, 1510–1519. [Google Scholar] [CrossRef]
- Strasser, P.; Gliech, M.; Kuehl, S.; Moeller, T. Electrochemical processes on solid shaped nanoparticles with defined facets. Chem. Soc. Rev. 2018, 47, 715–735. [Google Scholar] [CrossRef]
- Kim, D.; Resasco, J.; Yu, Y.; Asiri, A.M.; Yang, P.D. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold-copper bimetallic nanoparticles. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef]
- Monzo, J.; Malewski, Y.; Kortlever, R.; Vidal-Iglesias, F.J.; Solla-Gullon, J.; Koper, M.T.M.; Rodriguez, P. Enhanced electrocatalytic activity of Au@Cu core@shell nanoparticles towards CO2 reduction. J. Mater. Chem. A 2015, 3, 23690–23698. [Google Scholar] [CrossRef]
- Zhao, X.; Luo, B.; Long, R.; Wang, C.; Xiong, Y. Composition-dependent activity of Cu-Pt alloy nanocubes for electrocatalytic CO2 reduction. J. Mater. Chem. A 2015, 3, 4134–4138. [Google Scholar] [CrossRef]
- Zhang, F.-Y.; Sheng, T.; Tian, N.; Liu, L.; Xiao, C.; Lu, B.-A.; Xu, B.-B.; Zhou, Z.-Y.; Sun, S.-G. Cu overlayers on tetrahexahedral Pd nanocrystals with high-index facets for CO2 electroreduction to alcohols. Chem. Commun. 2017, 53, 8085–8088. [Google Scholar] [CrossRef]
- Kim, D.; Xie, C.; Becknell, N.; Yu, Y.; Karamad, M.; Chan, K.; Crumlin, E.J.; Nørskov, J.K.; Yang, P. Electrochemical Activation of CO2 through Atomic Ordering Transformations of AuCu Nanoparticles. J. Am. Chem. Soc. 2017, 139, 8329–8336. [Google Scholar] [CrossRef]
- Shan, C.; Martin, E.T.; Peters, D.G.; Zaleski, J.M. Site-Selective Growth of AgPd Nanodendrite-Modified Au Nanoprisms: High Electrocatalytic Performance for CO2 Reduction. Chem. Mater. 2017, 29, 6030–6043. [Google Scholar] [CrossRef]
- Mistry, H.; Reske, R.; Zeng, Z.H.; Zhao, Z.J.; Greeley, J.; Strasser, P.; Cuenya, B.R. Exceptional Size-Dependent Activity Enhancement in the Electroreduction of CO2 over Au Nanoparticles. J. Am. Chem. Soc. 2014, 136, 16473–16476. [Google Scholar] [CrossRef]
- Zhu, W.L.; Michalsky, R.; Metin, O.; Lv, H.F.; Guo, S.J.; Wright, C.J.; Sun, X.L.; Peterson, A.A.; Sun, S.H. Monodisperse Au Nanoparticles for Selective Electrocatalytic Reduction of CO2 to CO. J. Am. Chem. Soc. 2013, 135, 16833–16836. [Google Scholar] [CrossRef]
- Gao, D.; Zhou, H.; Wang, J.; Miao, S.; Yang, F.; Wang, G.; Wang, J.; Bao, X. Size-Dependent Electrocatalytic Reduction of CO2 over Pd Nanoparticles. J. Am. Chem. Soc. 2015, 137, 4288–4291. [Google Scholar] [CrossRef]
- Loiudice, A.; Lobaccaro, P.; Kamali, E.A.; Thao, T.; Huang, B.H.; Ager, J.W.; Buonsanti, R. Tailoring Copper Nanocrystals towards C-2 Products in Electrochemical CO2 Reduction. Angew. Chem. 2016, 55, 5789–5792. [Google Scholar] [CrossRef]
- Duan, X.C.; Xu, J.T.; Wei, Z.X.; Ma, J.M.; Guo, S.J.; Wang, S.Y.; Liu, H.K.; Dou, S.X. Metal-Free Carbon Materials for CO2 Electrochemical Reduction. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Guanyu, L.; Thanh, T.P.; Hongjun, C.; Antonio, T. A Review of Metal- and Metal-Oxide-Based Heterogeneous Catalysts for Electroreduction of Carbon Dioxide. Adv. Sustainable Syst. 2018. [Google Scholar] [CrossRef]
- Schouten, K.J.P.; Gallent, E.P.; Koper, M.T.M. The influence of pH on the reduction of CO and CO2 to hydrocarbons on copper electrodes. J. Electroanal. Chem. 2014, 716, 53–57. [Google Scholar] [CrossRef]
- Varela, A.S.; Kroschel, M.; Reier, T.; Strasser, P. Controlling the selectivity of CO2 electroreduction on copper: The effect of the electrolyte concentration and the importance of the local pH. Catal. Today 2016, 260, 8–13. [Google Scholar] [CrossRef]
- Verma, S.; Lu, X.; Ma, S.C.; Masel, R.I.; Kenis, P.J.A. The effect of electrolyte composition on the electroreduction of CO2 to CO on Ag based gas diffusion electrodes. Phys. Chem. Chem. Phys. 2016, 18, 7075–7084. [Google Scholar] [CrossRef]
- Varela, A.S.; Ju, W.; Reier, T.; Strasser, P. Tuning the Catalytic Activity and Selectivity of Cu for CO2 Electroreduction in the Presence of Halides. ACS Catal. 2016, 6, 2136–2144. [Google Scholar] [CrossRef]
- Medina-Ramos, J.; Lee, S.S.; Fister, T.T.; Hubaud, A.A.; Sacci, R.L.; Mullins, D.R.; DiMeglio, J.L.; Pupillo, R.C.; Velardo, S.M.; Lutterman, D.A.; et al. Structural Dynamics and Evolution of Bismuth Electrodes during Electrochemical Reduction of CO2 in Imidazolium-Based Ionic Liquid Solutions. ACS Catal. 2017, 7, 7285–7295. [Google Scholar] [CrossRef]
- Rumayor, M.; Dominguez-Ramos, A.; Irabien, A. Formic Acid manufacture: Carbon dioxide utilization alternatives. Appl. Sci. 2018, 8, 914. [Google Scholar] [CrossRef]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef]
- Pérez-Fortes, M.; Schöneberger, J.C.; Boulamanti, A.; Harrison, G.; Tzimas, E. Formic acid synthesis using CO2 as raw material: Techno-economic and environmental evaluation and market potential. Int. J. Hydrog. Energy 2016, 41, 16444–16462. [Google Scholar] [CrossRef]
- Rees, N.V.; Compton, R.G. Sustainable energy: A review of formic acid electrochemical fuel cells. J. Solid State Electrochem. 2011, 15, 2095–2100. [Google Scholar] [CrossRef]
- Ong, B.C.; Kamarudin, S.K.; Basri, S. Direct liquid fuel cells: A review. Int. J. Hydrog. Energy 2017, 42, 10142–10157. [Google Scholar] [CrossRef]
- Jeong, K.J.; Miesse, C.A.; Choi, J.H.; Lee, J.; Han, J.; Yoon, S.P.; Nam, S.W.; Lim, T.H.; Lee, T.G. Fuel crossover in direct formic acid fuel cells. J. Power Sources 2007, 168, 119–125. [Google Scholar] [CrossRef]
- Czaun, M.; Kothandaraman, J.; Goeppert, A.; Yang, B.; Greenberg, S.; May, R.B.; Olah, G.A.; Prakash, G.K.S. Iridium-Catalyzed Continuous Hydrogen Generation from Formic Acid and Its Subsequent Utilization in a Fuel Cell: Toward a Carbon Neutral Chemical Energy Storage. ACS Catal. 2016, 6, 7475–7484. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, S.; Kumar, A. Hydrogen energy future with formic acid: a renewable chemical hydrogen storage system. Catal. Sci. Technol. 2016, 6, 12–40. [Google Scholar] [CrossRef]
- Noda, H.; Ikeda, S.; Oda, Y.; Imai, K.; Maeda, M.; Ito, K. Electrochemical reduction of carbon-dioxide at various metal-electrodes in aqueous potassium hydrogen carbonate solution. Bull. Chem. Soc. Jpn 1990, 63, 2459–2462. [Google Scholar] [CrossRef]
- Chen, Z.P.; Wang, N.L.; Yao, S.Y.; Liu, L.C. The flaky Cd film on Cu plate substrate: An active and efficient electrode for electrochemical reduction of CO2 to formate. J. CO2 Util. 2017, 22, 191–196. [Google Scholar] [CrossRef]
- Detweiler, Z.M.; White, J.L.; Bernasek, S.L.; Bocarsly, A.B. Anodized Indium Metal Electrodes for Enhanced Carbon Dioxide Reduction in Aqueous Electrolyte. Langmuir 2014, 30, 7593–7600. [Google Scholar] [CrossRef]
- White, J.L.; Bocarsly, A.B. Enhanced Carbon Dioxide Reduction Activity on Indium-Based Nanoparticles. J. Electrochem. Soc. 2016, 163, H410–H416. [Google Scholar] [CrossRef]
- Del Castillo, A.; Alvarez-Guerra, M.; Solla-Gullon, J.; Saez, A.; Montiel, V.; Irabien, A. Electrocatalytic reduction of CO2 to formate using particulate Sn electrodes: Effect of metal loading and particle size. Appl. Energy 2015, 157, 165–173. [Google Scholar] [CrossRef]
- Medina-Ramos, J.; Pupillo, R.C.; Keane, T.P.; DiMeglio, J.L.; Rosenthal, J. Efficient Conversion of CO2 to CO Using Tin and Other Inexpensive and Easily Prepared Post-Transition Metal Catalysts. J. Am. Chem. Soc. 2015, 137, 5021–5027. [Google Scholar] [CrossRef]
- Zhang, R.; Lv, W.X.; Lei, L.X. Role of the oxide layer on Sn electrode in electrochemical reduction of CO2 to formate. Appl. Surf. Sci. 2015, 356, 24–29. [Google Scholar] [CrossRef]
- Wu, J.J.; Risalvato, F.G.; Ma, S.G.; Zhou, X.D. Electrochemical reduction of carbon dioxide III. The role of oxide layer thickness on the performance of Sn electrode in a full electrochemical cell. J. Mater. Chem. A 2014, 2, 1647–1651. [Google Scholar] [CrossRef]
- Vanýsek, P. Handbook of Chemistry and Physics, 93rd ed.; Haynes, W.M., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 5–80. [Google Scholar]
- Greeley, J.; Jaramillo, T.F.; Bonde, J.; Chorkendorff, I.; Nørskov, J.K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef]
- Zu, M.Y.; Zhang, L.; Wang, C.; Zheng, L.R.; Yang, H.G. Copper-modulated bismuth nanocrystals alter the formate formation pathway to achieve highly selective CO2 electroreduction. J. Mater. Chem. A 2018, 6, 16804–16809. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Y.; Quan, F.J.; Huang, J.J.; Jia, F.L.; Zhang, L.Z. Selective electro-reduction of CO2 to formate on nanostructured Bi from reduction of BiOCl nanosheets. Electrochem. Commun. 2014, 46, 63–66. [Google Scholar] [CrossRef]
- Zhong, H.X.; Qiu, Y.L.; Zhang, T.T.; Li, X.F.; Zhang, H.M.; Chen, X.B. Bismuth nanodendrites as a high performance electrocatalyst for selective conversion of CO2 to formate. J. Mater. Chem. A 2016, 4, 13746–13753. [Google Scholar] [CrossRef]
- Bei, J.J.; Zhang, R.; Chen, Z.D.; Lv, W.X.; Wang, W. Efficient Reduction of CO2 to Formate Using in Situ Prepared Nano-Sized Bi Electrocatalyst. Int. J. Electrochem. Sci. 2017, 12, 2365–2375. [Google Scholar] [CrossRef]
- Lv, W.X.; Zhou, J.; Bei, J.J.; Zhang, R.; Wang, L.; Xu, Q.; Wang, W. Electrodeposition of nano-sized bismuth on copper foil as electrocatalyst for reduction of CO2 to formate. Appl. Surf. Sci. 2017, 393, 191–196. [Google Scholar] [CrossRef]
- Lv, W.X.; Bei, J.J.; Zhang, R.; Wang, W.J.; Kong, F.Y.; Wang, L.; Wang, W. Bi2O2CO3 Nanosheets as Electrocatalysts for Selective Reduction of CO2 to Formate at Low Overpotential. ACS Omega 2017, 2, 2561–2567. [Google Scholar] [CrossRef]
- Kim, S.; Dong, W.J.; Gim, S.; Sohn, W.; Park, J.Y.; Yoo, C.J.; Jang, H.W.; Lee, J.L. Shape-controlled bismuth nanoflakes as highly selective catalysts for electrochemical carbon dioxide reduction to formate. Nano Energy 2017, 39, 44–52. [Google Scholar] [CrossRef]
- Sun, X.F.; Zhu, Q.G.; Kang, X.C.; Liu, H.Z.; Qian, Q.L.; Zhang, Z.F.; Han, B.X. Molybdenum-Bismuth Bimetallic Chalcogenide Nanosheets for Highly Efficient Electrocatalytic Reduction of Carbon Dioxide to Methanol. Angew. Chem. Int. Ed. 2016, 55, 6771–6775. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, T.; Liu, Y.Y.; Qiao, J.L. Enhancing CO2 electrolysis to formate on facilely synthesized Bi catalysts at low overpotential. Appl. Catal. B-Environ. 2017, 218, 46–50. [Google Scholar] [CrossRef]
- Bertin, E.; Garbarino, S.; Roy, C.; Kazemi, S.; Guay, D. Selective electroreduction of CO2 to formate on Bi and oxide-derived Bi films. J. CO2 Util. 2017, 19, 276–283. [Google Scholar] [CrossRef]
- García de Arquer, F.P.; Bushuyev, O.S.; De Luna, P.; Dinh, C.-T.; Seifitokaldani, A.; Saidaminov, M.I.; Tan, C.-S.; Quan, L.N.; Proppe, A.; Kibria, M.G.; et al. 2D Metal Oxyhalide-Derived Catalysts for Efficient CO2 Electroreduction. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Qiu, Y.; Du, J.; Dai, C.; Dong, W.; Taoz, C. Bismuth nano-flowers as a highly selective catalyst for electrochemical reduction of CO2 to formate. J. Electrochem. Soc. 2018, 165, H594–H600. [Google Scholar] [CrossRef]
- Miao, C.-C.; Yuan, G.-Q. Morphology-Controlled Bi2O3 Nanoparticles as Catalysts for Selective Electrochemical Reduction of CO2 to Formate. ChemElectroChem 2018, 5, 3741–3747. [Google Scholar] [CrossRef]
- Koh, J.H.; Won, D.H.; Eom, T.; Kim, N.K.; Jung, K.D.; Kim, H.; Hwang, Y.J.; Min, B.K. Facile CO2 Electro-Reduction to Formate via Oxygen Bidentate Intermediate Stabilized by High-Index Planes of Bi Dendrite Catalyst. ACS Catal. 2017, 7, 5071–5077. [Google Scholar] [CrossRef]
- Lee, C.W.; Hong, J.S.; Yang, K.D.; Jin, K.; Lee, J.H.; Ahn, H.-Y.; Seo, H.; Sung, N.-E.; Nam, K.T. Selective Electrochemical Production of Formate from Carbon Dioxide with Bismuth-Based Catalysts in an Aqueous Electrolyte. ACS Catal. 2018, 8, 931–937. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, X.; Zhang, Q.; Cai, Y.; Liu, Y.; Qiao, J. Polyethylene glycol induced reconstructing Bi nanoparticle size for stabilized CO2 electroreduction to formate. J. Catal. 2018, 365, 63–70. [Google Scholar] [CrossRef]
- Qiu, Y.; Du, J.; Dong, W.; Dai, C.; Tao, C. Selective conversion of CO2 to formate on a size tunable nano-Bi electrocatalyst. J. CO2 Util. 2017, 20, 328–335. [Google Scholar] [CrossRef]
- Han, N.; Wang, Y.; Yang, H.; Deng, J.; Wu, J.; Li, Y.; Li, Y. Ultrathin bismuth nanosheets from in situ topotactic transformation for selective electrocatalytic CO2 reduction to formate. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- Su, P.; Xu, W.; Qiu, Y.; Zhang, T.; Li, X.; Zhang, H. Ultrathin Bismuth Nanosheets as a Highly Efficient CO2 Reduction Electrocatalyst. ChemSusChem. 2018, 11, 848–853. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F.; Zhang, X.; Williams, T.; Easton, C.D.; Bond, A.M.; Zhang, J. Electrochemical reduction of CO2 on defect-rich Bi derived from Bi2S3 with enhanced formate selectivity. J. Mater. Chem. A 2018, 6, 4714–4720. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X Ray Photoelectron Spectroscopy: A Reference Book of Standard for Identification and Interpretation of Xps Data, 1st ed.; Physical Electronics Division, Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1995. [Google Scholar]
- Del Castillo, A.; Alvarez-Guerra, M.; Solla-Gullon, J.; Saez, A.; Montiel, V.; Irabien, A. Sn nanoparticles on gas diffusion electrodes: Synthesis, characterization and use for continuous CO2 electroreduction to formate. J. CO2 Util. 2017, 18, 222–228. [Google Scholar] [CrossRef]
Sample Availability: Samples of the Bi and Bi/C are available from the authors. |






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila-Bolívar, B.; García-Cruz, L.; Montiel, V.; Solla-Gullón, J. Electrochemical Reduction of CO2 to Formate on Easily Prepared Carbon-Supported Bi Nanoparticles. Molecules 2019, 24, 2032. https://doi.org/10.3390/molecules24112032
Ávila-Bolívar B, García-Cruz L, Montiel V, Solla-Gullón J. Electrochemical Reduction of CO2 to Formate on Easily Prepared Carbon-Supported Bi Nanoparticles. Molecules. 2019; 24(11):2032. https://doi.org/10.3390/molecules24112032
Chicago/Turabian StyleÁvila-Bolívar, Beatriz, Leticia García-Cruz, Vicente Montiel, and José Solla-Gullón. 2019. "Electrochemical Reduction of CO2 to Formate on Easily Prepared Carbon-Supported Bi Nanoparticles" Molecules 24, no. 11: 2032. https://doi.org/10.3390/molecules24112032
APA StyleÁvila-Bolívar, B., García-Cruz, L., Montiel, V., & Solla-Gullón, J. (2019). Electrochemical Reduction of CO2 to Formate on Easily Prepared Carbon-Supported Bi Nanoparticles. Molecules, 24(11), 2032. https://doi.org/10.3390/molecules24112032