Quantum Reality in the Selective Reduction of a Benzofuran System
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
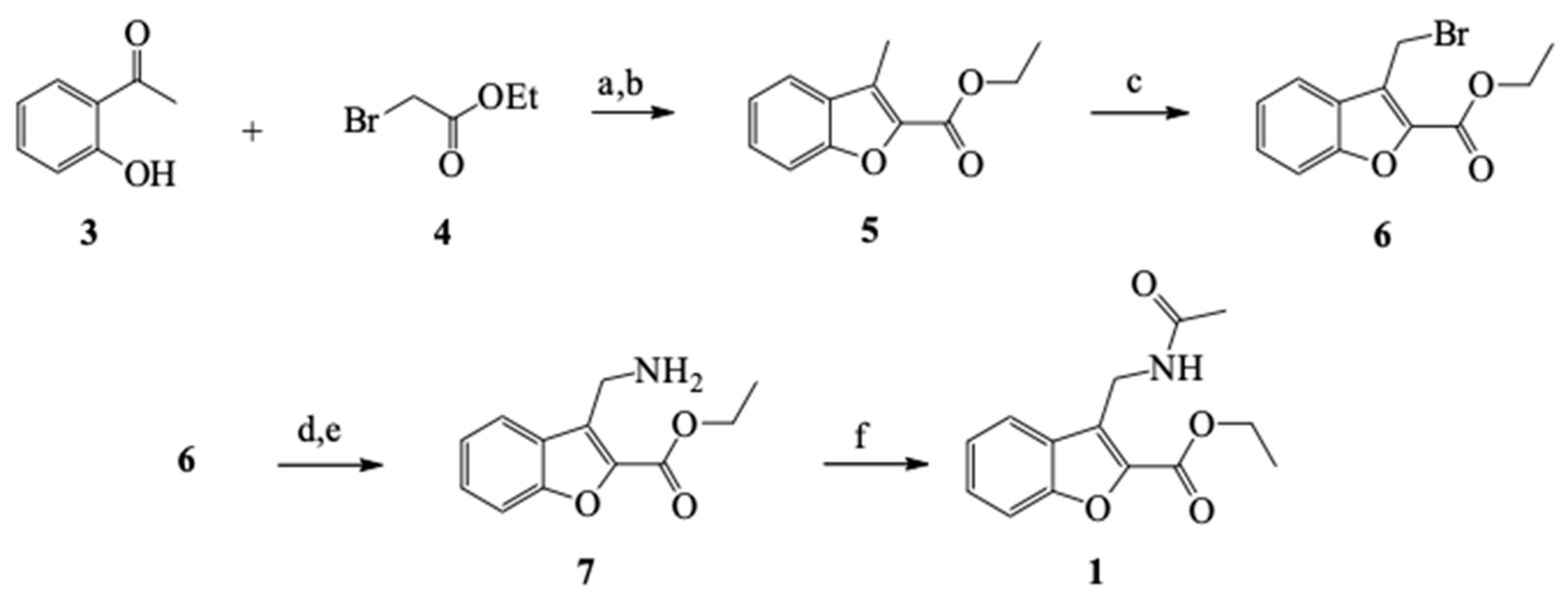
2.1.1. General Methodology for the Synthesis of 1
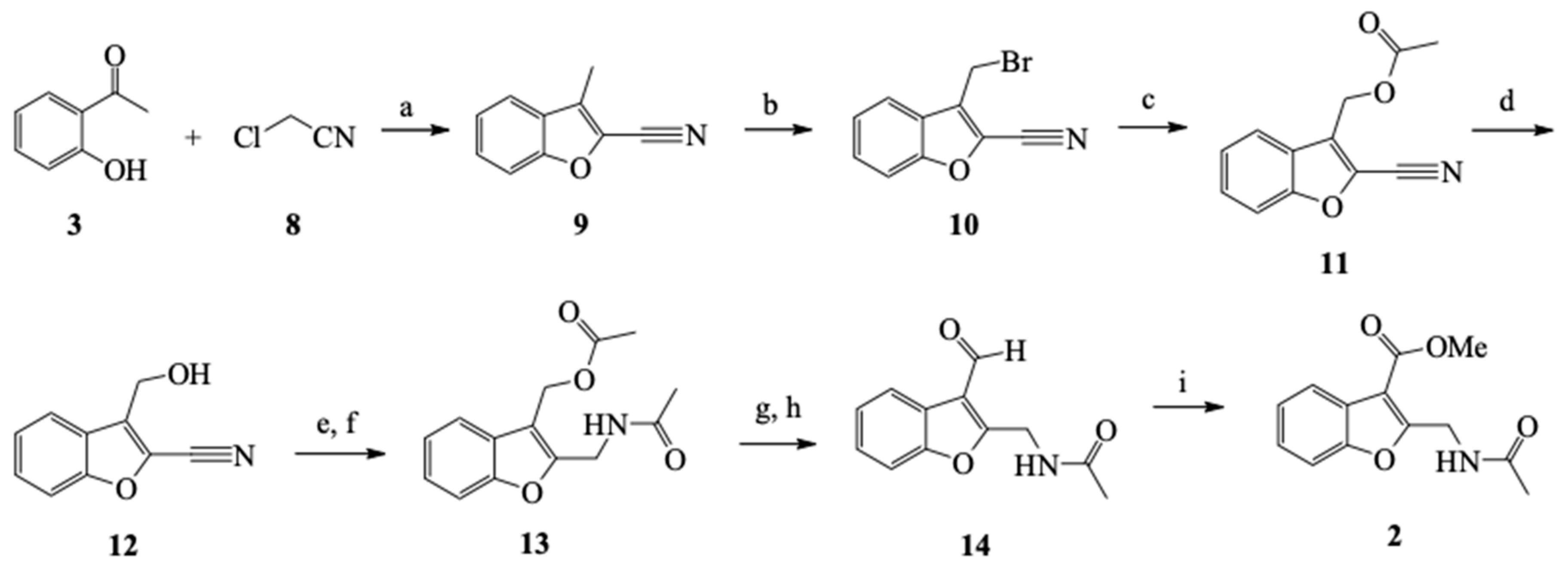
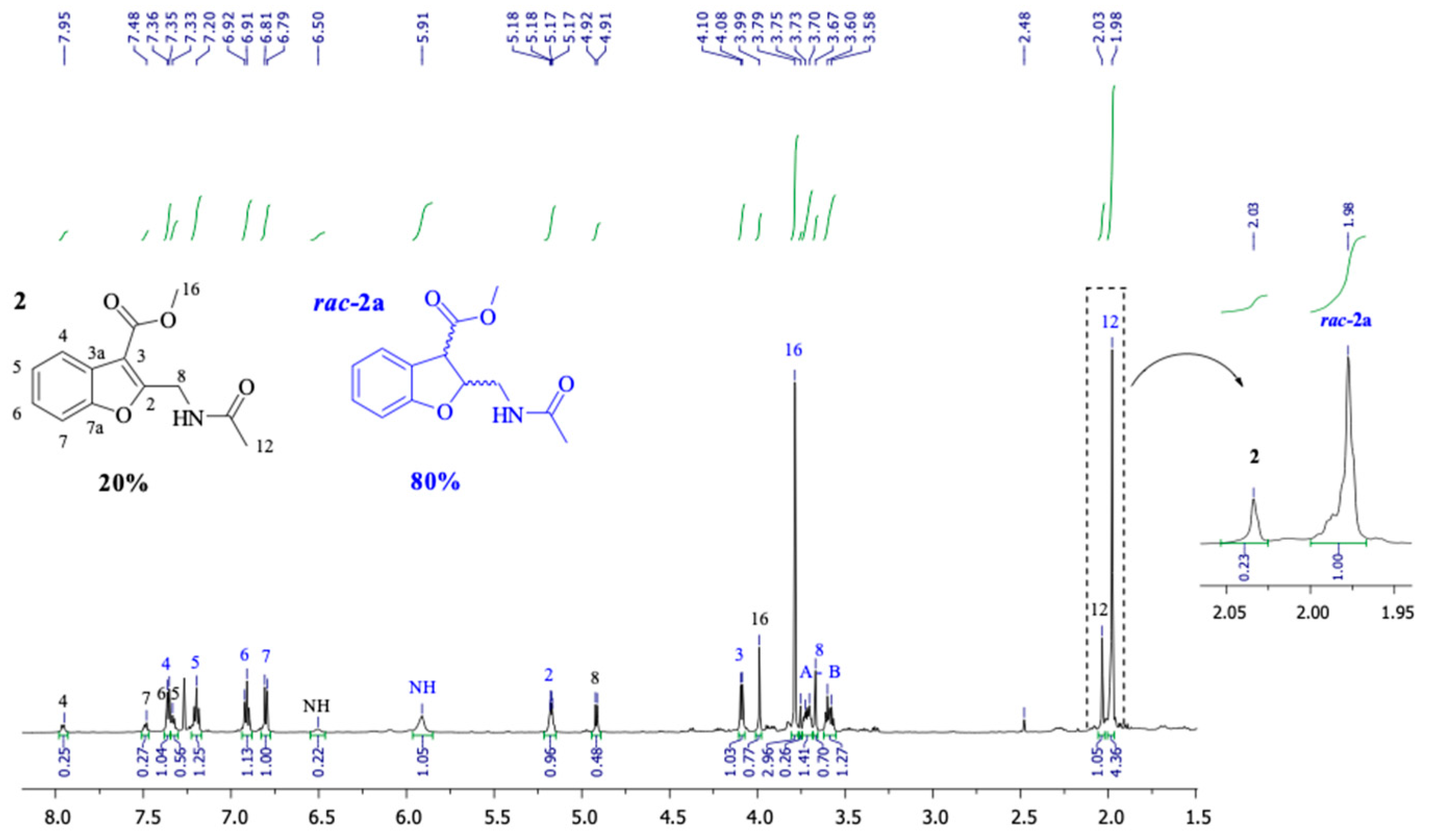
2.1.2. General Methodology for the Synthesis of 2
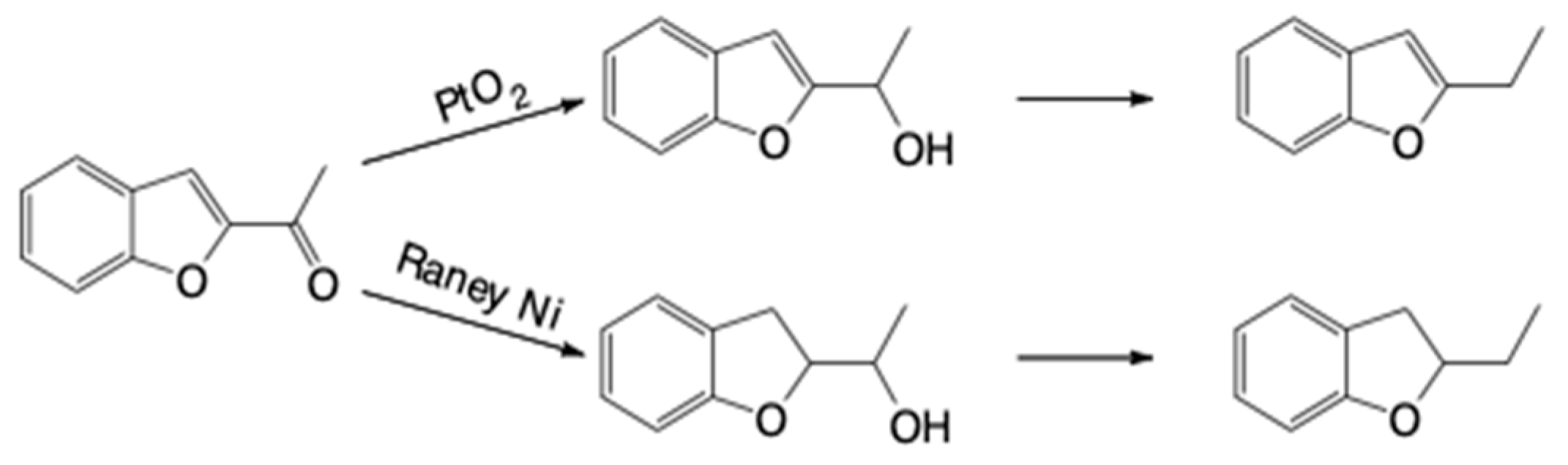
2.1.3. General Methodology for the Hydrogenation Reactions
2.2. Computational Studies (Theoretical Calculations)
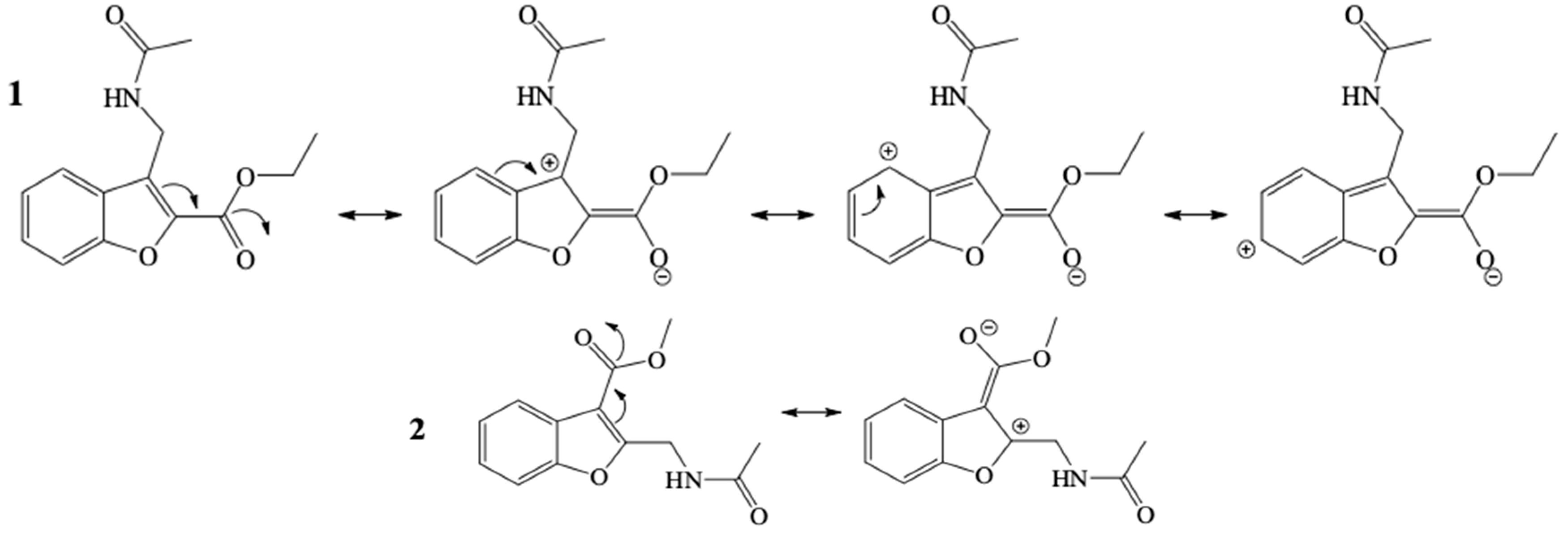
2.2.1. Conformational and Optimization Geometry
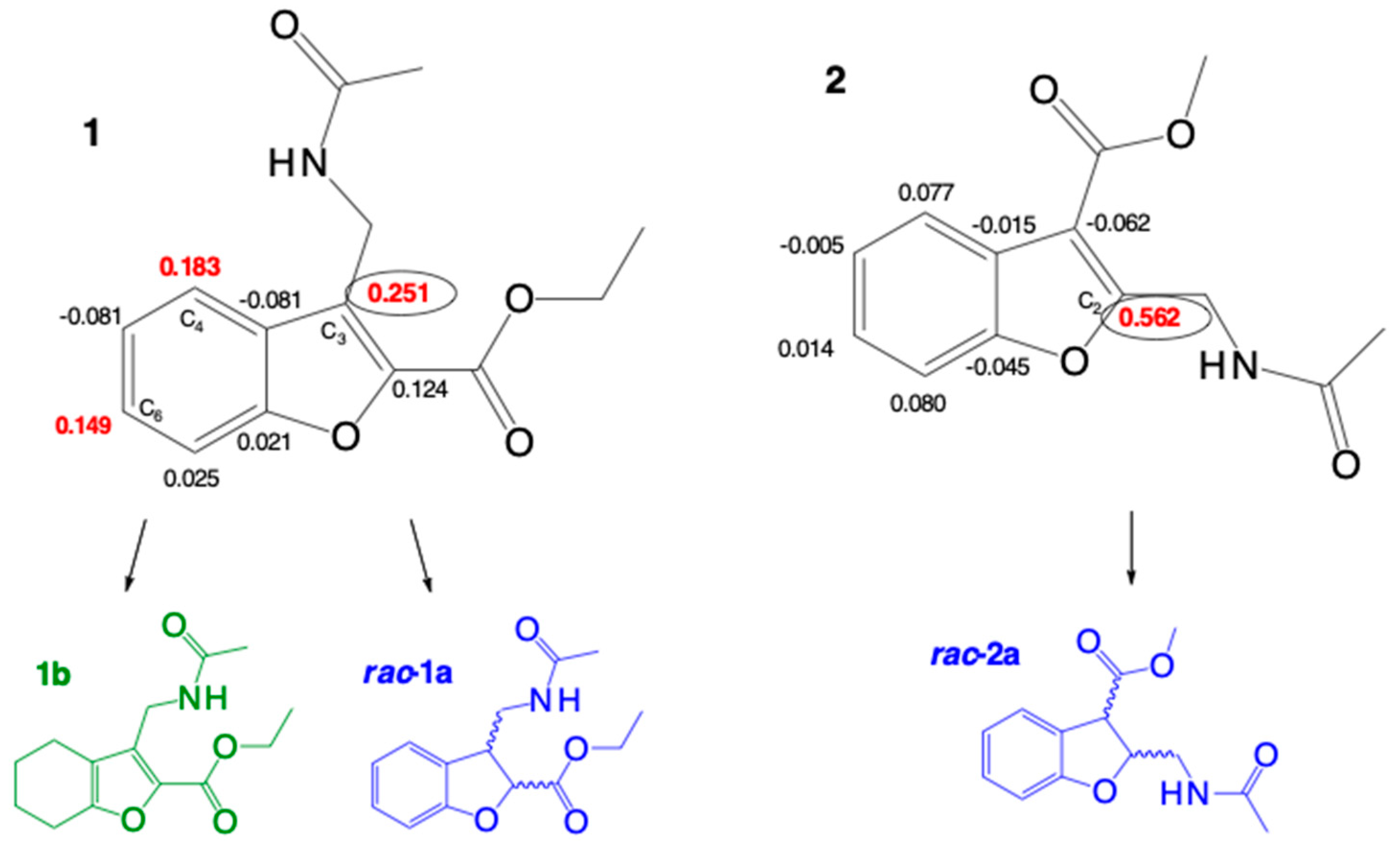
2.2.2. Indices of Global and of Local Reactivity
3. Materials and Methods
3.1. General Information
3.2. Chemistry
3.3. Theoretical Calculations
Indices of Global and Local Reactivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, P.-Y.; Wu, Y.-H.; Hsu, M.-H.; Wang, T.-P.; Wang, E.-C. Cerium ammonium nitrate-mediated the oxidative dimerization of p-alkenylphenols: a new synthesis of substituted (±)-trans-dihydrobenzofurans. Tetrahedron 2013, 69, 653–657. [Google Scholar] [CrossRef]
- Gwon, S.-H.; Kim, S.-G. One-Pot Cascade Michael-Cyclization Reactions of o-Hydroxycinnamaldehydes: Synthesis of Functionalized 2,3-Dihydrobenzofuranes. Bull. Korean Chem. Soc. 2012, 33, 2781–2784. [Google Scholar] [CrossRef]
- Baragona, F.; Lomberget, T.; Duchamp, C.; Henriques, N.; Lo Piccolo, E.; Diana, P.; Montalbano, A.; Barret, R. Synthesis of 5-substituted 2,3-dihydrobenzofurans in a one-pot oxidation/cyclization reaction. Tetrahedron 2011, 67, 8731–8739. [Google Scholar] [CrossRef]
- Karakhanov, E.A.; Viktorova, E.A. Hydrogenation and dehydrogenation reactions of benzofuran and its derivatives (review). Chem. Heterocycl. Compd. 1977, 12, 367–375. [Google Scholar] [CrossRef]
- Ortega, N.; Beiring, B.; Urban, S.; Glorius, F. Highly asymmetric synthesis of (+)-corsifuran A. Elucidation of the electronic requirements in the Ruthenium–NHC catalyzed hydrogenation of benzofurans. Tetrahedron 2012, 68, 5185–5192. [Google Scholar] [CrossRef]
- Entel, J.; Ruof, C.H.; Howard, H.C. Reactions of Benzofurans with Hydrogen 1. J. Am. Chem. Soc. 1951, 73, 4152–4158. [Google Scholar] [CrossRef]
- Shriner, R.L.; Anderson, J. Derivatives of Coumaran. VI. Reduction of 2-Acetobenzofuran and its Derivatives. J. Am. Chem. Soc. 1939, 61, 2705–2708. [Google Scholar] [CrossRef]
- Pumachagua, R.; Pecho, R.H.; Pino, R.H.; Nagles, E.O.; Hurtado, J.J. ESTUDIO TEÓRICO DE LAS PROPIEDADES ELECTRÓNICAS Y ESTRUCTURALES A TRAVÉS DE LA EVOLUCIÓN EN EL ÁNGULO DE TORSIÓN DE CHO-OH, CHS-OH y CHS-SH. Rev Soc Quím Perú. Rev Soc Quím Perú 2009, 75. [Google Scholar]
- Montes, N.; Hormaza, A. Comparación de los índices locales de reactividad Fukui de una serie de aldehídos. Rev. la Soc. Química del Perú 2008, 74, 247–251. [Google Scholar]
- Gázquez, J.L. Perspectives on the Density Functional Theory of Chemical Reactivity. J. Mex. Chem. Soc. 2008, 52, 3–10. [Google Scholar]
- Norskov, J.K.; Abild-Pedersen, F.; Studt, F.; Bligaard, T. Density functional theory in surface chemistry and catalysis. Proc. Natl. Acad. Sci. 2011, 108, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Lewars, E. Computational Chemistry—Introduction to the Theory and Applications of Molecular and Quantum Mechanics; Springer: New York, NY, USA; Boston, MA, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 2004; ISBN 9789048138623. [Google Scholar]
- Parr, R.G.; Pearson, R.G. Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Domingo, L.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the Reactivity of Captodative Ethylenes in Polar Cycloaddition Reactions. A Theoretical Study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Pratihar, S.; Roy, S. Nucleophilicity and Site Selectivity of Commonly Used Arenes and Heteroarenes. J. Org. Chem. 2010, 75, 4957–4963. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Pérez, P.; Sáez, J.A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P. The nucleophilicity N index in organic chemistry. Org. Biomol. Chem. 2011, 9, 7168. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, E.; Pérez, P.; Domingo, L.R. On the nature of Parr functions to predict the most reactive sites along organic polar reactions. Chem. Phys. Lett. 2013, 582, 141–143. [Google Scholar] [CrossRef]
- Coaviche-Yoval, A.; Luna, H.; Tovar-Miranda, R.; Soriano-Ursua, M.A.; Trujillo-Ferrara, J.G. Synthesis and biological evaluation of novel 2,3-disubstituted benzofuran analogues of GABA as neurotropic agents. Med. Chem. (Los. Angeles). 2018, 14. [Google Scholar] [CrossRef]
- Morton, J.G.M.; Kwon, L.D.; Freeman, J.D.; Njardarson, J.T. An Adler–Becker oxidation approach to vinigrol. Tetrahedron Lett. 2009, 50, 1684–1686. [Google Scholar] [CrossRef]
- Shafiee, A.; Mohamadpour, M. Synthesis of 3-formylbenzo[b]furan and 1-methyl-3,4-dihydrobenzo[b]-furo[2,3-c]pyridine. J. Heterocycl. Chem. 1978, 15, 481–483. [Google Scholar] [CrossRef]
- Schwarz, J.B.; Gibbons, S.E.; Graham, S.R.; Colbry, N.L.; Guzzo, P.R.; Le, V.-D.; Vartanian, M.G.; Kinsora, J.J.; Lotarski, S.M.; Li, Z.; et al. Novel Cyclopropyl β-Amino Acid Analogues of Pregabalin and Gabapentin That Target the α 2 -δ Protein. J. Med. Chem. 2005, 48, 3026–3035. [Google Scholar] [CrossRef]
- Satoh, T.; Suzuki, S.; Suzuki, Y.; Miyaji, Y.; Imai, Z. Reduction of organic compounds with sodium borohydride-transition metal salt systems. Tetrahedron Lett. 1969, 10, 4555–4558. [Google Scholar] [CrossRef]
- Brem, J.; Bencze, L.-C.; Liljeblad, A.; Turcu, M.C.; Paizs, C.; Irimie, F.-D.; Kanerva, L.T. Chemoenzymatic Preparation of 1-Heteroarylethanamines of Low Solubility. European J. Org. Chem. 2012, 2012, 3288–3294. [Google Scholar] [CrossRef]
- Attia, M.I.; Witt-Enderby, P.A.; Julius, J. Synthesis and pharmacological evaluation of pentacyclic 6a,7-dihydrodiindole and 2,3-dihydrodiindole derivatives as novel melatoninergic ligands. Bioorg. Med. Chem. 2008, 16, 7654–7661. [Google Scholar] [CrossRef]
- Feng, C.; Feng, D.; Luo, Y.; Loh, T.-P. Rhodium(III)-Catalyzed Olefinic C–H Alkynylation of Acrylamides Using Tosyl-Imide as Directing Group. Org. Lett. 2014, 16, 5956–5959. [Google Scholar] [CrossRef]
- Olivo, H.F.; Tovar-Miranda, R.; Barragán, E. Synthesis of (−)-Stemoamide Using a Stereoselective anti -Aldol Step. J. Org. Chem. 2006, 71, 3287–3290. [Google Scholar] [CrossRef]
- Nyangulu, J.M.; Galka, M.M.; Jadhav, A.; Gai, Y.; Graham, C.M.; Nelson, K.M.; Cutler, A.J.; Taylor, D.C.; Banowetz, G.M.; Abrams, S.R. An Affinity Probe for Isolation of Abscisic Acid-Binding Proteins. J. Am. Chem. Soc. 2005, 127, 1662–1664. [Google Scholar] [CrossRef]
- Crimmins, M.T.; O’Bryan, E.A. Enantioselective Total Synthesis of Spirofungins A and B. Org. Lett. 2010, 12, 4416–4419. [Google Scholar] [CrossRef]
- Corey, E.J.; Gilman, N.W.; Ganem, B.E. New methods for the oxidation of aldehydes to carboxylic acids and esters. J. Am. Chem. Soc. 1968, 90, 5616–5617. [Google Scholar] [CrossRef]
- Rupprecht, K.M.; Boger, J.; Hoogsteen, K.; Nachbar, R.B.; Springer, J.P. Controlling the stereochemistry of the ring junction in hexahydrodibenzofurans. J. Org. Chem. 1991, 56, 6180–6188. [Google Scholar] [CrossRef]
- Shi, G.Q.; Dropinski, J.F.; Zhang, Y.; Santini, C.; Sahoo, S.P.; Berger, J.P.; MacNaul, K.L.; Zhou, G.; Agrawal, A.; Alvaro, R.; et al. Novel 2,3-Dihydrobenzofuran-2-carboxylic Acids: Highly Potent and Subtype-Selective PPARα Agonists with Potent Hypolipidemic Activity. J. Med. Chem. 2005, 48, 5589–5599. [Google Scholar] [CrossRef] [PubMed]
- MARIS, M.; BURGI, T.; MALLAT, T.; BAIKER, A. Enantioselective hydrogenation of furancarboxylic acids: A spectroscopic and theoretical study. J. Catal. 2004, 226, 393–400. [Google Scholar] [CrossRef]
- Belmessieri, D.; de la Houpliere, A.; Calder, E.D.D.; Taylor, J.E.; Smith, A.D. Stereodivergent Organocatalytic Intramolecular Michael Addition/Lactonization for the Asymmetric Synthesis of Substituted Dihydrobenzofurans and Tetrahydrofurans. Chem. A Eur. J. 2014, 20, 9762–9769. [Google Scholar] [CrossRef]
- Antus, S.; Kurtán, T.; Juhász, L.; Kiss, L.; Hollósi, M.; Májer, Z. Chiroptical properties of 2,3-dihydrobenzo[b]furan and chromane chromophores in naturally occurring O-heterocycles. Chirality 2001, 13, 493–506. [Google Scholar] [CrossRef]
- Andrade-Jorge, E.; Godínez-Victoria, M.; Sánchez-Torres, L.E.; Fabila-Castillo, H.L.; Trujillo-Ferrara, J.G. Aryl Maleimides as Apoptosis Inducers on L5178-Y Murine Leukemia Cells (in silico, in vitro and ex vivo Study). Anticancer. Agents Med. Chem. 2016, 16, 1615–1621. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


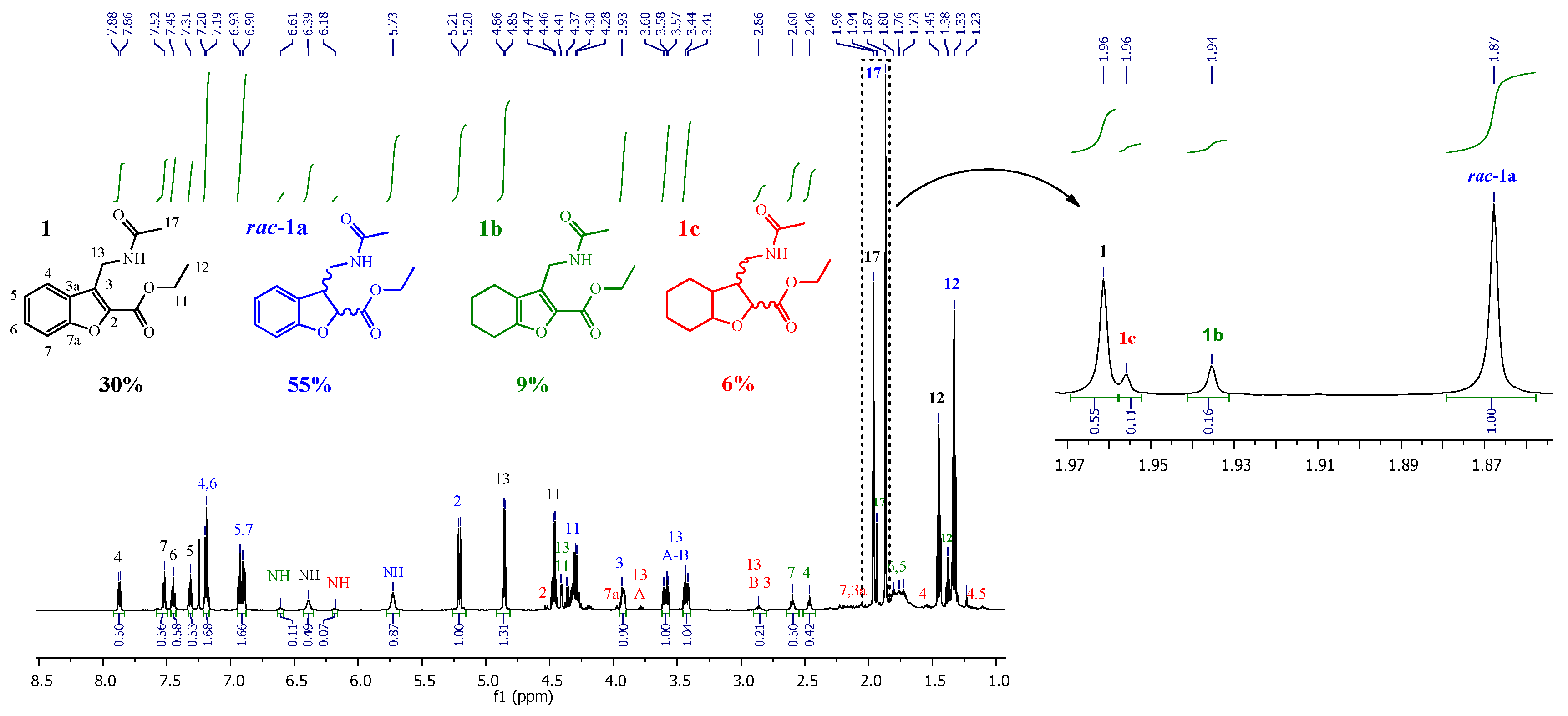
| Compound | Structure | δ NH-14 | δ CH3-17 | δ CH3-12 | |||
|---|---|---|---|---|---|---|---|
| 1 |  | 6.36 a | - | 1.96 a | 1.94 b | 1.45 a | 1.41 b |
| 1a | 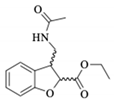 | 5.73 a | - | 1.87 a | 1.92 b | 1.34 a | 1.31 b |
| 1b | 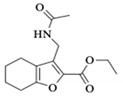 | 6.61 a | - | 1.94 a | 1.92 b | 1.38 a | 1.33 b |
| 1c | 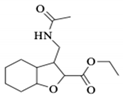 | 6.27 a | - | 1.96 a | 1.92 b | 1.34 a | 1.31 b |
| δ NH-9 | δ CH3-16 | δ CH3-12 | |||||
| 2 |  | 6.45 a | - | 3.98 a | - | 2.02 a | - |
| 2a | 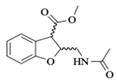 | 5.93 a | - | 3.78 a | - | 1.98 a | - |
| Entry a | Substrate (S) (mg/mmol) | Weight Ratio (S/Catal) | Concentration [mmol] | Time (h) | Yield (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | rac-1a | 1b | 1c | ||||||
| 1 | 50/0.19 | 1/0.5 | 76 | 24 | 55 | 34 | 7 | 4 | c |
| 2 | 50/0.19 | 1/1 | 76 | 24 | 23 | 54 | 10 | 13 | c |
| 3 | 50/0.19 | 1/1.5 | 76 | 24 | 13 | 42 | 14 | 32 | c |
| 4 | 50/0.19 | 1/2 | 38 | 24 | 12 | 21 | 22 | 46 | c |
| 5 | 50/0.19 | 1/0.5 | 38 | 44 | 77 | 19 | 4 | --- | c |
| 6 | 15/0.06 | 1/1 | 38 | 44 | 30 | 55 | 9 | 6 | c |
| 7 | 100/0.38 | 1/2 | 14 | 46 | 35 | 26 | 25 | --- | c |
| 8 | 100/0.38 | 1/2.4 | 82 | 48 | --- | 13 | 24 | 63 | c, d |
| 9 | 71/0.27 | 1/1 | 16 | 96 | 88 | 7 | 5 | --- | c |
| 10 | 100/0.38 | 1/4 | 14 | 24 | 7 | 47 | 31 | 15 | c, d |
| 2 | rac-2a | ||||||||
| 11 | 25/0.10 | 1/0.3 | 45 | 44 | 100 | --- | --- | --- | c |
| 12 | 15/0.06 | 1/1 | 75 | 24 | 20 | 80 | --- | --- | c |
| 13 b | 26/0.10 | 1/1 | 75 | 24 | 12 | 88 | --- | --- | c |
| Comp. | I(eV) | A(eV) | µ(eV) | η(eV) | S(1/eV) | ω(eV) | N (eV) | ω−(eV) | ω+(eV) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 8.031 | 0.136 | −4.083 | 7.895 | 0.1267 | 1.056 | 1.087 | 4.647 | 0.564 |
| 2 | 7.993 | 0.067 | −4.030 | 7.926 | 0.1261 | 1.025 | 1.125 | 4.560 | 0.530 |
| Comp. k | fk+ | fk− | sk+ | sk− | ωk a eV | Pk+ ρsra(k) | Pk− ρsrc(k) | ωk b eV | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | C2 | 0.0350 | 0.05505 | 0.0044 | 0.0070 | 0.03696 | 0.1170 | 0.2227 | 0.1236 |
| C3 | 0.0646 | 0.02911 | 0.0082 | 0.0037 | 0.06822 | 0.2372 | 0.1198 | 0.2505 | |
| C3a | 0.0059 | 0.01383 | 0.0007 | 0.0018 | 0.00623 | −0.0763 | −0.0261 | −0.0806 | |
| C4 | 0.0451 | 0.04218 | 0.0057 | 0.0053 | 0.04763 | 0.173 | 0.2393 | 0.1827 | |
| C5 | 0.0255 | 0.03098 | 0.0032 | 0.0039 | 0.02693 | −0.0768 | −0.0856 | −0.0811 | |
| C6 | 0.0462 | 0.04162 | 0.0059 | 0.0053 | 0.04879 | 0.1407 | 0.1436 | 0.1486 | |
| C7 | 0.0373 | 0.04826 | 0.0047 | 0.0061 | 0.03939 | 0.0237 | 0.1308 | 0.025 | |
| C7a | 0.0068 | 0.00060 | 0.0009 | 0.0001 | 0.00718 | 0.0199 | −0.0388 | 0.021 | |
| 2 | C2 | 0.0935 | 0.03574 | 0.0115 | 0.0044 | 0.09584 | 0.5483 | 0.1473 | 0.562 |
| C3 | 0.0270 | 0.03411 | 0.0033 | 0.0042 | 0.02768 | −0.0608 | 0.1408 | −0.0623 | |
| C3a | 0.0033 | 0.01697 | 0.0004 | 0.0021 | 0.00338 | −0.0145 | 0.0321 | −0.0149 | |
| C4 | 0.0327 | 0.03945 | 0.0040 | 0.0049 | 0.03352 | 0.0749 | 0.1899 | 0.0768 | |
| C5 | 0.0302 | 0.02737 | 0.0037 | 0.0034 | 0.03096 | −0.0045 | −0.1063 | −0.0046 | |
| C6 | 0.0366 | 0.04421 | 0.0045 | 0.0054 | 0.03752 | 0.0140 | 0.2217 | 0.0144 | |
| C7 | 0.0405 | 0.04005 | 0.0050 | 0.0049 | 0.04151 | 0.0779 | 0.0442 | 0.0798 | |
| C7a | −0.0107 | 0.01281 | −0.0013 | 0.0016 | −0.0109 | −0.0441 | 0.0156 | −0.0452 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coaviche-Yoval, A.; Andrade-Jorge, E.; Pérez-González, C.; Luna, H.; Tovar-Miranda, R.; Trujillo-Ferrara, J.G. Quantum Reality in the Selective Reduction of a Benzofuran System. Molecules 2019, 24, 2061. https://doi.org/10.3390/molecules24112061
Coaviche-Yoval A, Andrade-Jorge E, Pérez-González C, Luna H, Tovar-Miranda R, Trujillo-Ferrara JG. Quantum Reality in the Selective Reduction of a Benzofuran System. Molecules. 2019; 24(11):2061. https://doi.org/10.3390/molecules24112061
Chicago/Turabian StyleCoaviche-Yoval, Arturo, Erik Andrade-Jorge, Cuauhtémoc Pérez-González, Héctor Luna, Ricardo Tovar-Miranda, and José G. Trujillo-Ferrara. 2019. "Quantum Reality in the Selective Reduction of a Benzofuran System" Molecules 24, no. 11: 2061. https://doi.org/10.3390/molecules24112061
APA StyleCoaviche-Yoval, A., Andrade-Jorge, E., Pérez-González, C., Luna, H., Tovar-Miranda, R., & Trujillo-Ferrara, J. G. (2019). Quantum Reality in the Selective Reduction of a Benzofuran System. Molecules, 24(11), 2061. https://doi.org/10.3390/molecules24112061






