Abstract
A series of oxime Cyclohexyl (E)-4-(hydroxyimino)-4-phenylbutanoates and their ethers were designed, synthesized, and evaluated for anti-hepatitis B virus (HBV) activities with HepG 2.2.15 cell line in vitro. Most of these compounds possessed anti-HBV activities, and among them, compound 4B-2 showed significant inhibiting effects on the secretion of HBsAg (IC50 = 63.85 ± 6.26 μM, SI = 13.41) and HBeAg (IC50 = 49.39 ± 4.17 μM, SI = 17.34) comparing to lamivudine (3TC) in HBsAg (IC50 = 234.2 ± 17.17 μM, SI = 2.2) and HBeAg (IC50 = 249.9 ± 21.51 μM, SI = 2.07). Docking study of these compounds binding to a protein residue (PDB ID: 3OX8) from HLA-A2 that with the immunodominant HBcAg18–27 epitope (HLA-A2.1- restricted CTL epitope) active site was carried out by using molecular operation environment (MOE) software. Docking results showed that behaviors of these compounds binding to the active site in HLA-A protein residue partly coincided with their behaviors in vitro anti-HBV active screening.
1. Introduction
Hepatitis B virus (HBV) remains a serious health problem for leading risks of liver related diseases for many people in the world. An estimation of 257 million people is chronically infected with HBV in the world, and among them, about 15–40% develop liver cirrhosis, hepatic failure, and hepatocellular carcinoma. More than 887,000 people die every year for complications of HBV [1,2]. Therapies of hepatitis B infection currently include methods of direct-acting antivirals (just as with nucleoside analogs) and host-targeting antivirals (just as with interferon) [3]. The nucleoside analogs in treatment of anti-HBV play a role of Troy horse in synthesis of HBV DNA and suppressed replication of HBV, but they are not effective to eliminate virus completely from patients [4,5,6]. Meanwhile, therapies of HBV with nucleoside analogs in the long term cause side effects and resistance [7,8,9]. Therefore, it is still a tremendous challenge to develop new anti-HBV agents for further improvement of anti-HBV therapy. Many non-nucleoside analog compounds with anti-HBV activities have been found and invented from synthesized compounds [10,11] and natural products [12,13,14]. Researches showed that HLA-A2 with the immunodominant HBcAg18–27 epitope (HLA-A2.1- restricted CTL epitope) binding peptides of vaccine or of HBcAg initiated specific respond of T cell and resolved cute HBV infection [15,16,17,18,19]. Other peptides specifically bound to the HLA-A2 residue activate CTL responds to prevent infection and eliminate HBV [20,21]. Therefore, a proposal can be implied that other non-peptide compounds specifically interact with protein residue in HLA-A2.1 restricted CTL epitope may initiate CTL respond to prevent infection of HBV. A protein residue from HLA-A2.1-restricted CTL epitope (PDB ID: 3OX8) interacted intensively with anti-HBV active compounds in docking investigations, was used as a virtual target to design anti-HBV new compounds in our previous works. Series of oxime-contained compounds with C6-C3 and C6-C4 skeleton were designed, synthesized, and assayed for anti-HBV activities. Results showed these compounds possessed significant anti-HBV activities [22,23,24,25]. On the basis of these results, we designed and synthesized a series of another part of novel oximes and their derivatives with C6-C4 skeletons, and screened for anti-HBV activities in our present works. All these compounds were evaluated theoretically with a mimic target HLA-A protein (PDB ID 3OX8) by molecular docking using molecular operating environment (MOE) method. Results showed these compounds with significant anti-HBV activities in vitro partly coincided with molecular docking.
2. Results and Discussion
2.1. Chemistry
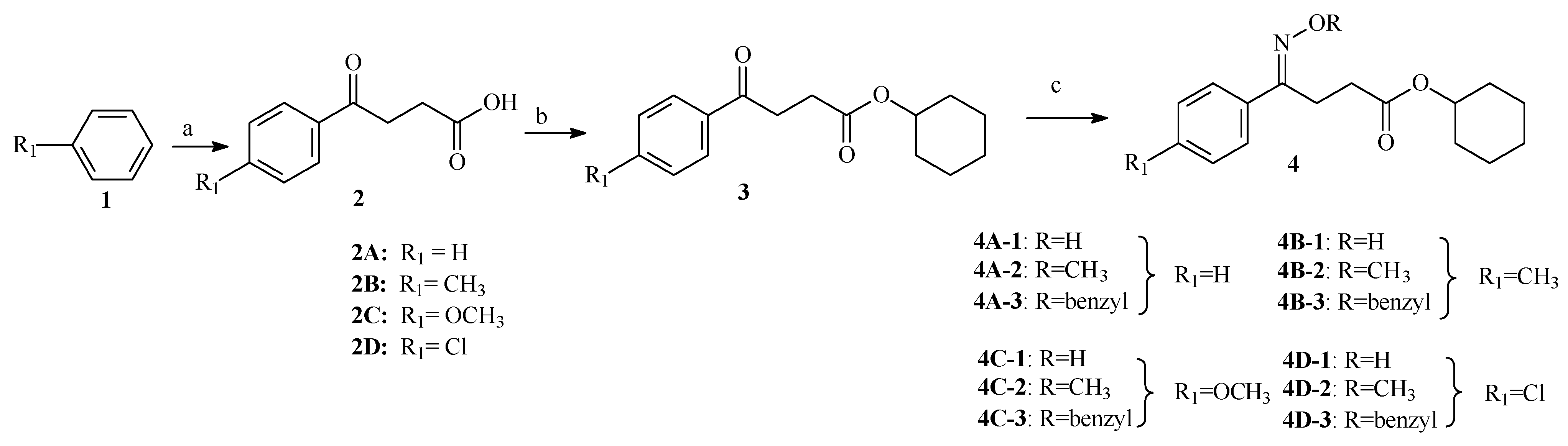
A series of oxime and oxime ether derivatives of (E)-4-(alkyl-imino)-4-(4-substitute-phenyl) cyclohexylbutanoate were designed for anti-HBV agents according molecular docking conclusions. General synthesis of these compounds was depicted in Scheme 1. Compounds 2A–2D were prepared by Friedel-Crafts reaction of succinic anhydride with substituted benzene in yields of 95–99%. Compounds 3A–3D were prepared by reaction of compounds 2A–2D with cyclohexanol catalyzed with p-CH3C6H4SO3H (TsOH) in yields of 81-89%. Oxime ether derivatives (4A-1–4A-3, 4B-1–4B-3, 4C-1–4C-3, 4D-1–4D-3) were obtained by reaction of substitute-hydroxylamine hydrochloride (hydroxylamine hydrochloride methoxylamine hydrochloride and benzylhydroxylamine hydrochloride) with compounds 3A–3D in yields of 75–88%. The structures of the newly synthesized compounds were characterized by 1H NMR, 13C NMR and MS. 1H NMR spectra of the derivatives showed a singlet at about 8.69–9.23 ppm corresponding to N-O-H proton in oxime compounds 4A-1, 4C-1 and 4D-1 (Compound 4B-1 was deuterated in CH3OD). The signal at 6.92–7.64 ppm of multiplets in the region attributed to aromatic hydrogens of the phenyl ring, especially 6.92–7.63 and 7.37–7.69 ppm of two doubles in the region attributed to the phenyl ring of R1. 13C NMR spectrum for the derivatives showed signals at 155.68–158.24, 171.90–172.35 corresponding to C=N and C=O, respectively.
Scheme 1.
Synthesis of Oximes and their Ethers.
2.2. Anti-HBV Activities In Vitro
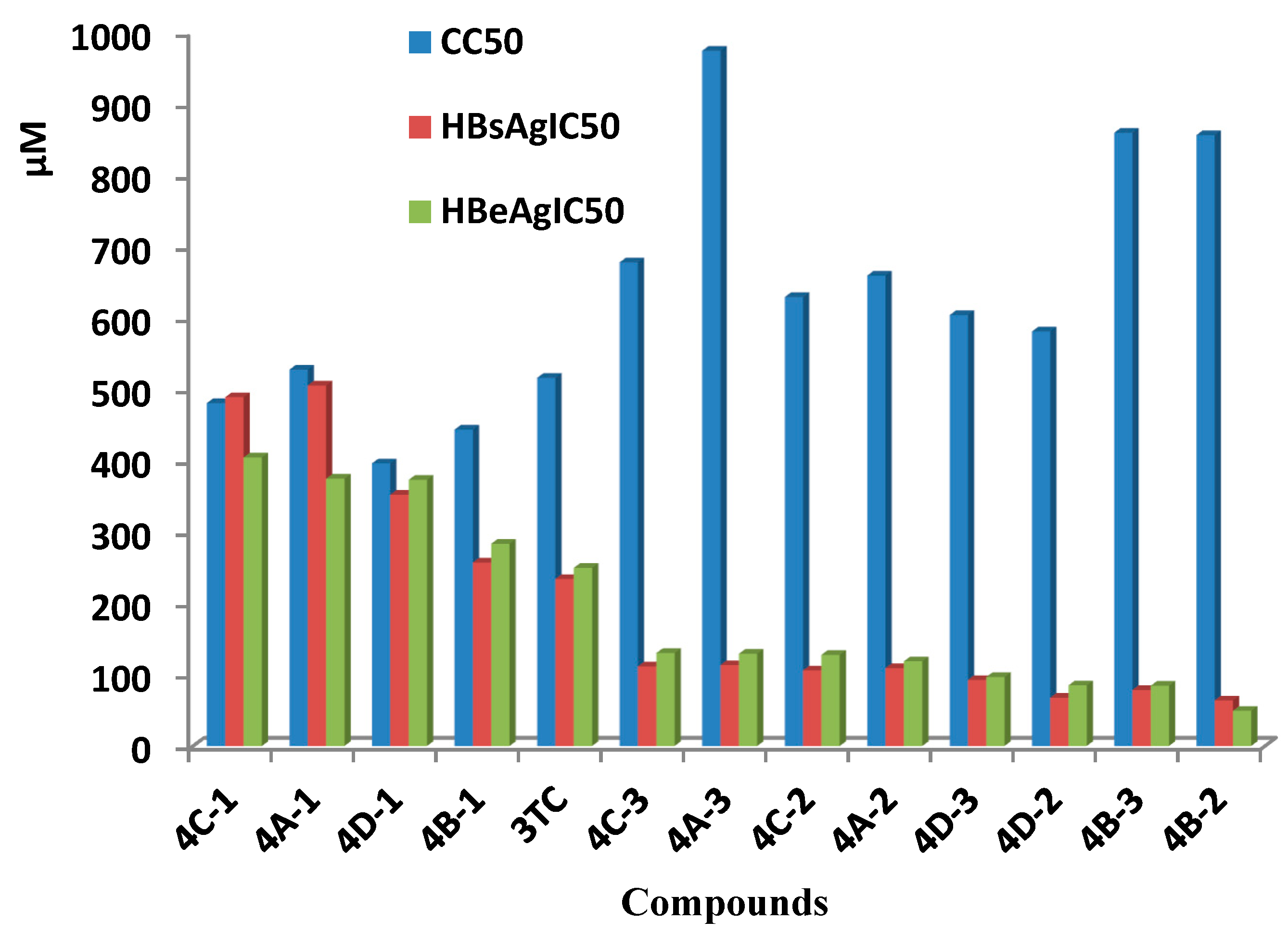
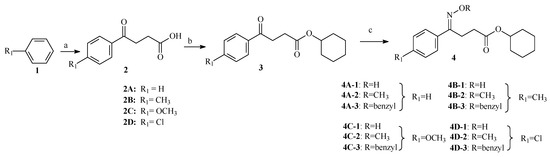
Inhibition of secreting HBsAg and HBeAg of these synthesized compounds were assayed in HepG 2.2.15 cells with lamivudine (3TC) as a positive control. Results of cytotoxicity and anti-HBV activities were showed in Table 1 and Figure 1. Many compounds showed more effective in inhabiting secretion of HBsAg and HBeAg than that of lamivudine, and among them compound 4B-2 showed inhibition of HBsAg (IC50 = 63.85 μM, SI = 13.41) and HBeAg (IC50 = 49.39 μM, SI = 17.34), with low toxicity (CC50 = 856.2 μM), and compound 4B-3 in inhibiting secretion of HBsAg (IC50 = 78.46 μM,) and HBeAg (IC50 = 84.57 μM) and low toxicity (CC50 = 859.5 μM) with SI values (SIHBsAg = 12.90, SIHBeAg = 10.89). When R=H, all oximes in the four groups decreased inhibitions of HBsAg in 505.7, 257.4, 489.3, and 352.4μM, respectively, HBeAg in 375.0, 283.7, 404.7, and 373.4 μM, respectively, and elevated cytotoxicity than their relative ethers.

Table 1.
In Vitro Anti-HBV Activity and Cytotoxicity of the Target Compounds.
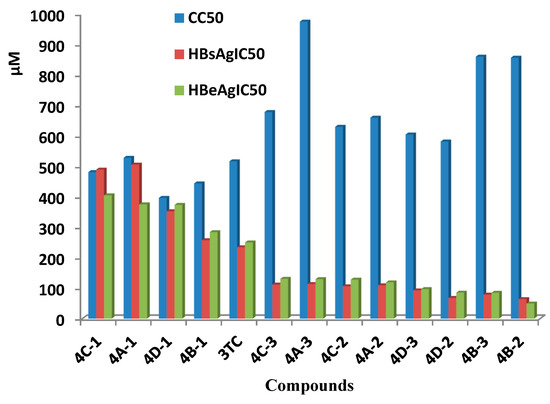
Figure 1.
Anti-HBV activities and toxicity of compounds in vitro (concentration of compounds in μM).
2.3. Molecular Docking Study
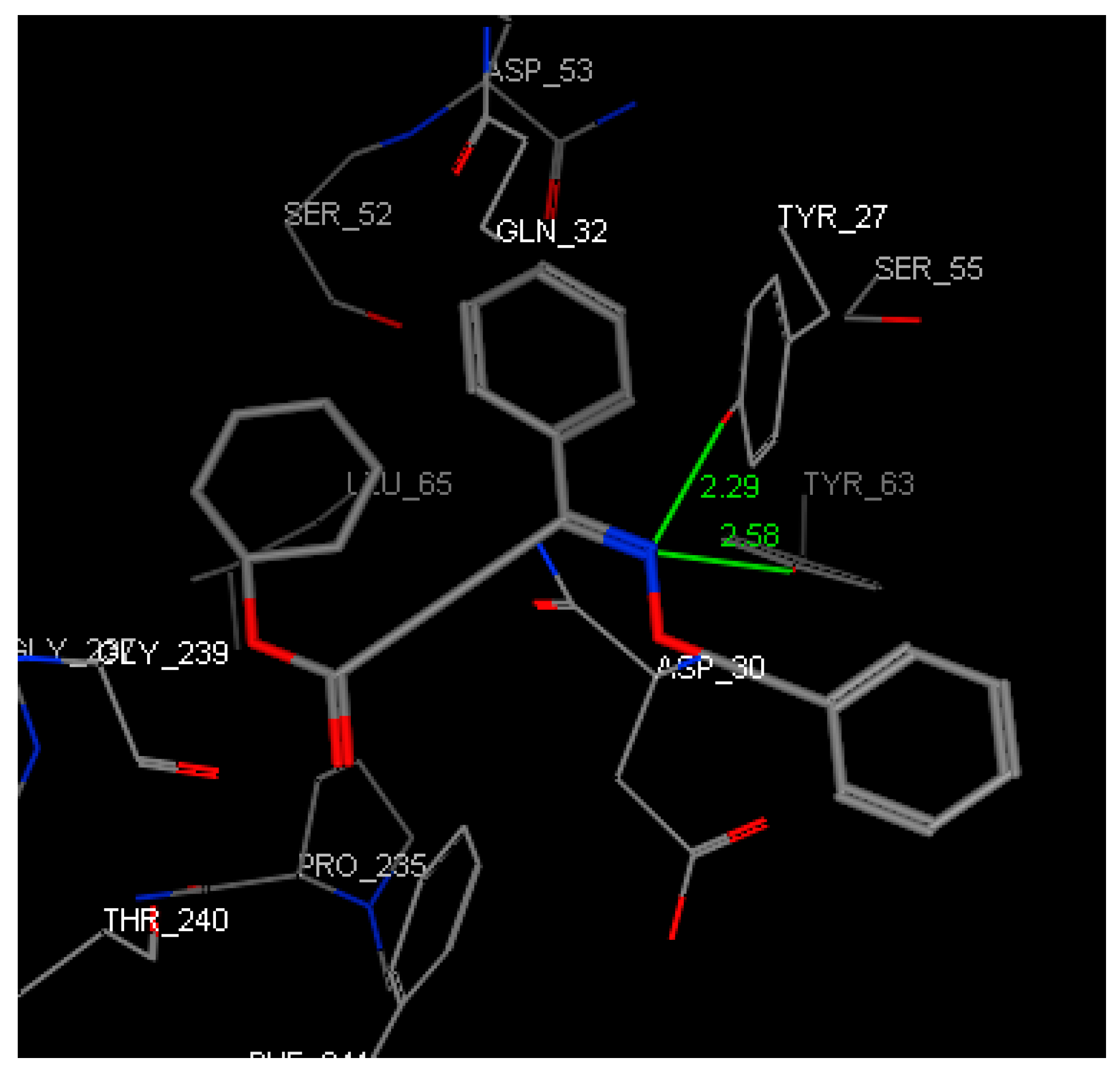
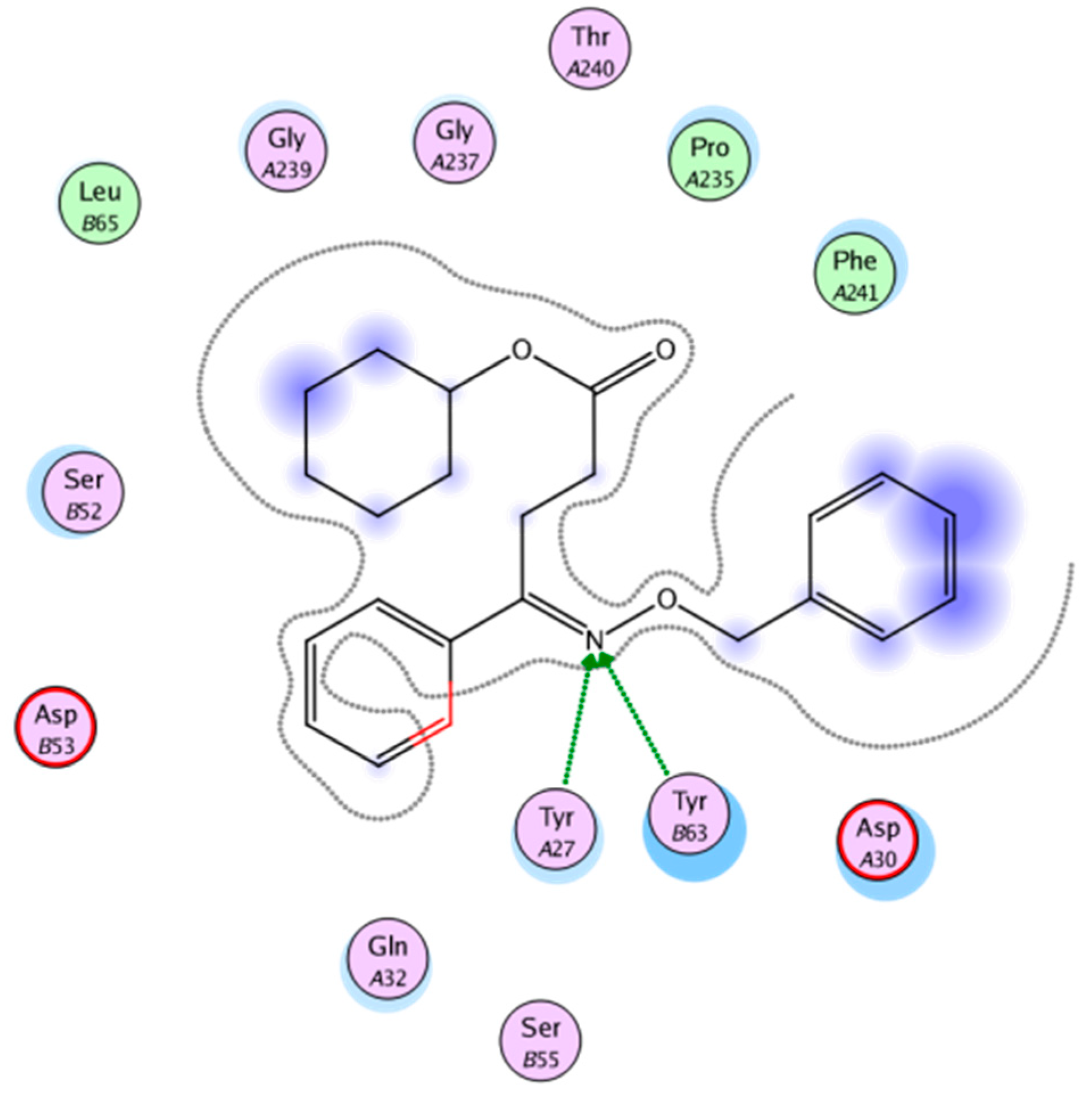
The docking software MOE 2008.10 was used for molecular docking of oxime derivatives and a protein residue in HLA-A2.1-restricted CTL epitope (PDB ID: 3OX8) was selected for docking study. Molecular docking results clearly revealed interactions between ligands and protein in the active site. Affinity scoring function ΔG of the protein-ligand complexes and other docking results were shown in Table 2. Significant interactions with protein residue in docking were compound 4A-3 and 4D-3 for −22.8858 kcal/mol and −21.2040 kcal/mol, respectively. Two hydrogen bonds of lengths 2.29 and 2.58 Å for N of O-N in the oxime group with Tyr27 and Tyr63, respectively, was found for compound 4A-3 (Figure 2 and Figure 3).

Table 2.
Docking score and bond interaction of synthesized compounds.
Figure 2.
Three-dimensional (3D) representation showing the binding mode of compound 4A-3 into the binding site of HLA-A2. The hydrogen bond formed is colored in green.
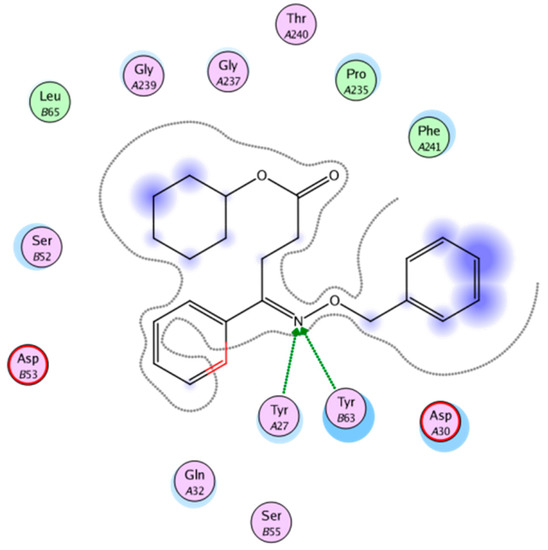
Figure 3.
Two-dimensional (2D) representation showing the docking pose interactions of compound 4A-3 in the HLA-A2 active site.
2.4. Structure-Activity Relationship (SAR)
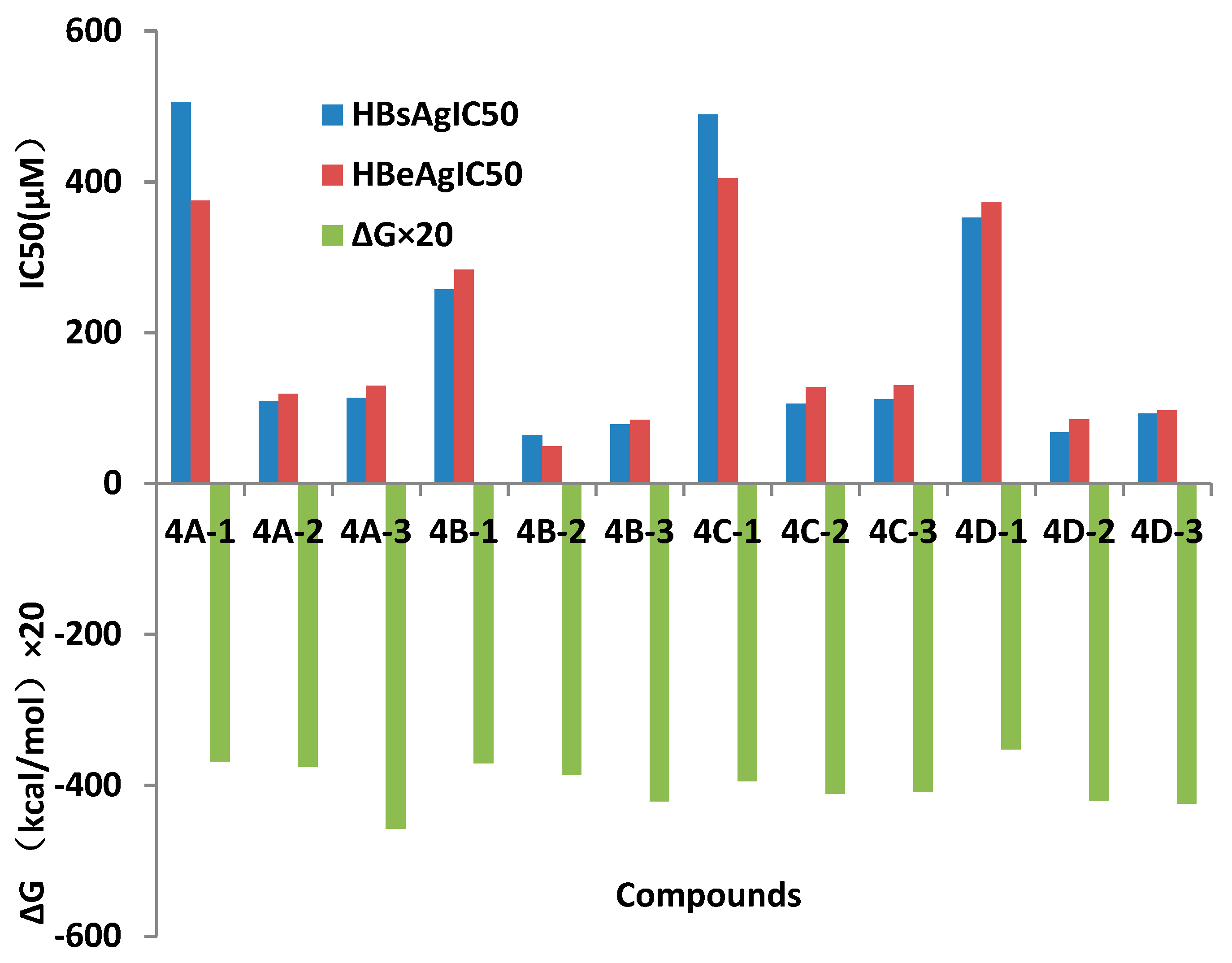
Oximes (4A-1, 4B-1, 4C-1 and 4D-1) which R=H showed weaker anti-HBsAg and anti-HBeAg activities, weaker affinities to the selected target protein in docking than those compounds which R=Me and benzyl. When these oximes were etherified, anti-HBV activities of their ethers increased, meanwhile cytotoxicity of these ethers decreased by comparing to their relative oximes. Methyl ethers 4A-2, 4B-2, 4C-2 and 4D-2 showed more significant anti-HBV active in their relative groups (Figure 4). Results also revealed that substitute groups in phenyl (groups 4B, 4C and 4D) increased anti-HBV activities by comparing to relative compounds in 4A group. Docking results showed that N or O atoms in ON fragment and O atom in C=O groups in the oxime derivatives interacted with ammonic acid residues by hydrogen bond, and their docking affinity partly coincided with their anti-HBV activities in vitro. Similar structure-activity relationships were found in our previous works [23].
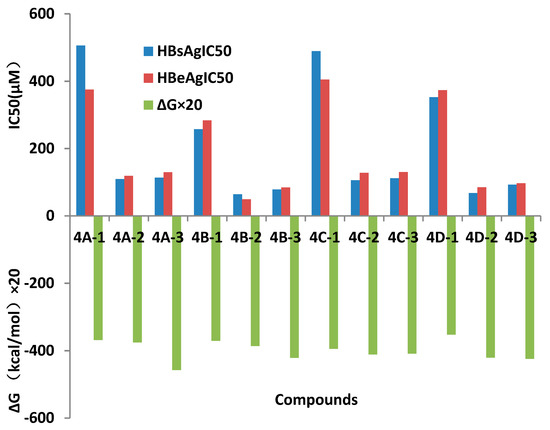
Figure 4.
Affinity energy and anti-HBV activities of compounds in vitro (concentration of compounds in μM).
3. Materials and Methods
Melting points were measured on an uncorrected WRX-4 electrothermal melting point apparatus (Shanghai, China). 1H NMR and 13C NMR spectra were recorded on Bruker AVANCE III HD600 (1H/13C, 600MHz/150MHz) spectrometer (Bruker, Bremerhaven, Germany) using TMS as an internal standard. The mass spectra were recorded on a Finnigan LCQ Deca XP MAX mass spectrometer (Thermo Fisher, San Jose, CA, USA) equipped with an ESI source and an ion trap analyzer in the positive ion mode/in the negative ion. Silica gel GF-254 was used in thin-layer chromatography, and silica gel H used in flash column chromatography (Qingdao Haiyang Chemical, Qingdao, China). All solvents and reagents were analytical grade. The purity of target compounds was assessed on the basis of analytical HPLC (Thermo Fisher Ultimate 3000), and the results were > 95%.
3.1. Chemistry
3.1.1. Preparation of 4- oxo-4-(substituted phenyl) Butanoic Acids (2) and Their Esters (3)
Preparation of 4- oxo-4-(substituted phenyl) butanoic acids (2). These Compounds were synthesized according to reports [26,27]. Succinic anhydride (1 equiv, 25 mmol) in 30 mL CH2Cl2 reacted with substituted benzenes (1, 25 mmol) under stirring for 0.5 h in ice-water bath, then anhydrous aluminum chloride (37.5 mmol) was added to the CH2Cl2 solution (Scheme 1). The reaction was then kept on room temperature for 6 h. After the reaction was stopped, icy aqueous hydrochloric acid solution (0.1 mol/L, 10 mL) was drop to resultant solution under stirring for 10 min. Organic phase solution was evaporated to give crude product. The crude product was further purified by crystallization repeatedly with 70% ethanol solution to afford 4-oxo-4-phenylbutanoic acid (2A) and4-oxo-4-(p-tolyl)butanoic acid (2B) [26], 4-(4-Methoxyphenyl)-4-oxobutanoic acid (2C) and 4-(4-Chlorophenyl)-4-oxobutanoic acid (2D) [27].
Reagents and conditions: (a) Succinic anhydride, AlCl3, CH2Cl2, 0.5h, 0 °C, then 6h rt; (b) Cyclohexanol, TsOH, CH2Cl2, 4h, reflux; (c) Hydroxylamine hydrdochloride (methoxylamine hydrochloride, benzylhydroamine hydrochloride), pyridine, CH2Cl2,12h, reflux.
Preparation of Cyclohexyl 4-(4-substituted phenyl) -4-oxobutanoates (3) Compound 2 (2A, 2B, 2C, 2D, 15 mmol) reacted with cyclohexanol (30 mmol) in CH2Cl2 (20 mL) with p-methylbenzenesulfonic acid (TsOH) (2g, 11.6 mmol) under refluxing for 4 h (Scheme 1). After stopping, the resultant solution was washed with icy aqueous sodium bicarbonate solution (3%, 15 mL) for three times, then the organic solution was dried with anhydrous sodium sulfate evaporated to give crude product. This crude product was purified by flash column chromatography eluting with an eluent of ethyl acetate/petroleum ether (1:7 v/v) to afford 3A, 3B, 3C, and 3D, respectively.
Cyclohexyl 4-oxo-4-phenylbutanoate (3A) Colorless liquid, yield 91.6%. 1H NMR (CDCl3) δ 7.98 (d, J = 7.3 Hz, 2H, H-2, 6), 7.55 (t, J = 7.4 Hz, 1H, H-4), 7.45 (t, J = 7.8 Hz, 2H, H-3, 5), 4.81–4.75 (m, 1H, H-14), 3.29 (t, J = 6.7 Hz, 2H, H-8), 2.74 (t, J = 6.6 Hz, 2H, H-9), 1.84 (dd, J = 12.6, 4.2 Hz, 2H, H-15), 1.70 (dd, J = 8.9, 4.3 Hz, 2H, H-19), 1.52 (dd, J = 8.9, 3.9 Hz, 1H, H-17), 1.45–1.32 (m, 4H, H-16,18), 1.24 (dd, J = 20.5, 7.5 Hz, 1H, H-17). 13C NMR (CDCl3) δ 198.23 (C-7), 172.29 (C-10), 136.66 (C-1), 133.14 (C-4), 128.59 (C-2, 6), 128.01 (C-3, 5), 72.90 (C-14), 33.44 (C-8), 31.57 (C-15, 19), 28.69 (C-9), 25.37 (C-17), 23.71 (C-16, 18) [28].
Cyclohexyl 4-oxo-4-(p-tolyl) butanoate (3B). Colorless crystal, yield 90.8%. m.p. 40.8–41.6 °C. 1H NMR (MeOD) δ 7.90 (d, J = 8.2 Hz, 2H, H-2, 6), 7.31 (d, J = 8.0 Hz, 2H, H-3, 5), 4.76–4.72 (m, 1H, H-15), 3.32–3.28 (m, 2H, H-8), 2.70–2.68 (m, 2H, H-9), 2.41 (s, 3H, H-14, -phCH3), 1.82 (dd, J = 11.8, 5.7 Hz, 2H, H-16), 1.71 (dd, J = 9.7, 3.3 Hz, 2H, H-20), 1.53 (dd, J = 11.0, 8.0 Hz, 1H, H-18), 1.45–1.33 (m, 4H, H-17,19), 1.29 (dd, J = 12.8, 9.8 Hz, 1H, H-18). 13C NMR (CDCl3) δ 197.88 (C-7), 172.41 (C-10), 143.92 (C-1), 134.22 (C-4), 129.27 (C-2, 6), 128.15 (C-3,5), 72.89 (C-15), 33.34 (C-8), 31.59 (C-16, 20), 28.76 (C-9), 25.39 (C-18), 23.73 (C-17, 19), 21.64 (C-14). ESIMS: m/z 275.1646 [M + H]+, 297.1461 [M + Na]+, calculated for C17H22O3 (274.36).
Cyclohexyl 4-(4-methoxyphenyl)-4-oxobutanoate (3C). Colorless crystal, yield 90.2%. m.p. 43.7–44.6 °C. 1H NMR (MeOD) δ 7.99 (d, J = 9.0 Hz, 2H, H-2, 6), 7.01 (d, J = 9.0 Hz, 2H, H-3, 5), 4.77–4.70 (m, 1H, H-16), 3.88 (s, 3H, H-15, OCH3), 3.30–3.26 (m, 2H, H-8), 2.70–2.67 (m, 2H, H-9), 1.82 (dd, J = 12.0, 5.7 Hz, 2H, H-17), 1.72 (dd, J = 8.0, 4.9 Hz, 2H, H-21), 1.56–1.50 (m, 1H, H-19), 1.44 (dd, J = 14.4, 8.0 Hz, 2H, H-18), 1.38 (dd, J = 9.9, 6.7 Hz, 2H, H-20), 1.32–1.26 (m, 1H, H-19). 13C NMR (151 MHz, MeOD) δ 197.60 (C-7), 172.77 (C-10), 163.91 (C-4), 130.04 (C-2, 6), 129.55 (C-1), 113.49 (C-3, 5), 72.64 (C-16), 54.68 (C-15), 32.61 (C-8), 31.14 (C-17, 21), 28.29 (C-9), 25.09 (C-19), 23.25 (C-18, 20). ESIMS: m/z 291.1593 [M + H]+, 313.1413 [M + Na]+, calculated for C17H22O4 (290.36).
Cyclohexyl 4-(4-chlorophenyl)-4-oxobutanoate (3D). Colorless crystal, yield 89.5%. m.p. 67.5–68.7 °C. 1H NMR (CDCl3) δ 7.92 (d, J = 8.7 Hz, 2H, H-2, 6), 7.44 (d, J = 8.6 Hz, 2H, H-3, 5), 4.80-4.75 (m, 1H, H-14), 3.26 (t, J = 6.6 Hz, 2H, H-8), 2.74 (t, J = 6.6 Hz, 2H, H-9), 1.84 (dd, J = 11.4, 5.4 Hz, 2H, H-19), 1.71 (dd, J = 8.8, 4.4 Hz, 2H, H-15), 1.55-1.51 (m, 1H, H-17), 1.44–1.32 (m, 4H, H-16,18), 1.26 (t, J = 11.6 Hz, 1H, H-17). 13C NMR (CDCl3) δ 197.04 (C-7), 172.16 (C-10), 139.58 (C-4), 134.99 (C-1), 129.45 (C-2, 6), 128.91 (C-3, 5), 73.01 (C-14), 33.40 (C-8), 31.57 (C-15, 19), 28.61 (C-9), 25.36 (C-17), 23.72 (C-16, c18). ESIMS: m/z 295.1102 [M + H]+, 317.0922 [M + Na]+, calculated for C16H19ClO3 (294.78).
3.1.2. Preparation of Oximes and Their Ethers
Compound 3 (3A, 3B, 3C, and 3D, 3.84mmol) and substituted hydroxylamine hydrochloride (7.7 mmol) in CH2Cl2 (20 mL) with pyridine (2 mL) was refluxed for 12 h (Scheme 1). After stopping, the resultant solution was evaporated to give a residue under vacuum. This residue was dissolved in ethyl acetate (20 mL), then washed with saturated sodium chloride solution (20 mL × 3). The organic phase was dried with anhydrous sodium sulfate, and evaporated to give a crude product. This crude product was purified by flash column chromatography with an eluent of ethyl acetate/petroleum ether (1:5 to 1:13 v/v) to afford ethers.
Cyclohexyl (E)-4-hydroxyimino-4-phenylbutanoate (4A-1). Colorless crystal, yield 88.1%. m.p. 100.8–102.0 °C. 1H NMR (CDCl3) δ 9.23 (s, 1H, H-21, COOH), 7.63 (d, J = 3.7 Hz, 2H, H-2, 6), 7.44–7.39 (m, 3H, H-3, 4, 5), 4.81–4.74 (m, 1H, H-14), 3.18-3.13 (m, 2H, H-8), 2.65-2.60 (m, 2H, H-9), 1.83 (dd, J = 8.7, 3.8 Hz, 2H, H-15), 1.72 (dd, J = 9.8, 4.0 Hz, 2H, H-19), 1.57–1.52 (m, 1H, H-17), 1.38 (dd, J = 22.6, 12.5 Hz, 4H, H-16, 18), 1.26 (dd, J = 16.3, 6.4 Hz, 1H, H-17). 13C NMR (CDCl3) δ 172.15 (C-10), 158.24 (C-7), 135.21 (C-1), 129.42 (C-4), 128.67 (C-2, 6), 126.36 (C-3, 5), 73.04 (C-14), 31.56 (C-15, 19), 31.00 (C-9), 25.36 (C-8), 23.73 (C-17), 22.03 (C-16, 18). ESIMS: m/z 276.1601 [M + H]+, 298.1420 [M + Na]+, calculated for C16H21NO3 (275.35) [29].
Cyclohexyl (E)-4-methoxyimino-4-phenylbutanoate (4A-2). Colorless liquid, yield 81.5%. 1H NMR (CDCl3) δ 7.64 (d, J = 1.9 Hz, 2H, H-2, 6), 7.40–7.34 (m, 3H, H-3, 4, 5), 4.78–4.74 (m, 1H, H-14), 4.00 (s, 3H, H-21), 3.08–3.04 (m, 2H, H-8), 2.57–2.53 (m, 2H, H-9), 1.83 (dd, J = 8.9, 3.7 Hz, 2H, H-15), 1.72 (dd, J = 9.7, 3.9 Hz, 2H, H-19), 1.54 (dd, J = 10.1, 2.5 Hz, 1H, H-17), 1.38 (dd, J = 22.0, 12.3 Hz, 4H, H-16,18), 1.27 (dd, J = 13.6, 5.8 Hz, 1H, H-17). 13C NMR (CDCl3) δ 172.03 (C-10), 156.98 (C-7), 135.27 (C-4), 129.19 (C-1), 128.53 (C-2, 6), 126.33 (C-3, 5), 72.90 (C-14), 62.01 (C-21), 31.57 (C-15, 19), 31.20 (C-9), 25.38 (C-8), 23.72 (C-17), 22.38 (C-16, 18). ESIMS: m/z 290.1748 [M + H]+, 312.1567 [M + Na]+, calculated for C17H23NO3 (289.38).
Cyclohexyl (E)-4-benzyloxyimino-4-phenylbutanoate (4A-3). Pale yellow liquid, yield 79.5%. 1H NMR CDCl3) δ 7.69 (d, J = 3.3 Hz, 2H, H-2, 6), 7.46 (d, J = 7.1 Hz, 2H, H-3, 5), 7.43–7.38 (m, 5H, H-23,24,25,26,27), 7.35 (t, J = 7.3 Hz, 1H, H-4), 5.29 (s, 2H, H-21), 4.78 (dt, J = 8.9, 4.6 Hz, 1H, H-14), 3.16–3.12 (m, 2H, H-8), 2.62–2.58 (m, 2H, H-9), 1.84 (dd, J = 9.7, 3.6 Hz, 2H, H-15), 1.75–1.72 (m, 2H, H-19), 1.57 (dd, J = 7.9, 4.9 Hz, 1H, H-17), 1.44–1.35 (m, 4H, H-16, 18), 1.30 (dd, J = 16.6, 6.5 Hz, 1H, H-17). 13C NMR (CDCl3) δ 172.09 (C-10), 157.36 (C-7), 138.01 (C-22), 135.29 (C-1), 129.27 (C-4), 128.55 (C-24, 26), 128.41 (C-2, 6), 128.11 (C-3, 5), 127.81 (C-25), 126.42 (C-23, 27), 76.35 (C-21), 72.94 (C-14), 31.59 (C-19), 31.25 (C-9), 25.41 (C-8), 23.75 (C-17), 22.57 (C-16, 18). ESIMS: m/z 366.2070 [M + H]+, 388.1886 [M + Na]+, calculated for C23H27NO3 (365.47).
Cyclohexyl (E)-4-hydroxyimino-4-(4-methylphenyl)butanoate (4B-1). Colorless crystal, yield 88.2%. m.p. 80.1–80.7 °C. 1H NMR (MeOD) δ 7.51 (d, J = 8.2 Hz, 2H, H-2, 6), 7.20 (d, J = 8.0 Hz, 2H, H-3, 5), 4.70 (dt, J = 8.7, 4.5 Hz, 1H, H-15), 3.08-3.05 (m, 2H, H-8), 2.54 (t, J = 7.9 Hz, 2H, H-9), 2.36 (s, 3H, H-14), 1.80 (d, J = 9.3 Hz, 2H, H-16), 1.75–1.71 (m, 2H, H-20), 1.57–1.54 (m, 1H, H-18), 1.39 (dd, J = 17.8, 8.7 Hz, 4H, H-17, 19), 1.32 (d, J = 11.5 Hz, 1H, H-18). 13C NMR (CDCl3) δ 172.15 (C-10), 158.23 (C-7), 139.48 (C-1), 132.32 (C-4), 129.35 (C-2, 6), 126.24 (C-3, 5), 72.96 (C-15), 31.57 (C-16, 20), 31.06 (C-9), 25.37 (C-8), 23.73 (C-18), 21.89 (C-17, 19), 21.27 (C-14). ESIMS: m/z 290.1754 [M + H]+, 312.1573 [M + Na]+, calculated for C17H23NO3 (289.38).
Cyclohexyl (E)-4-methoxyimino-4-(4-methoxyphenyl)butanoate (4B-2). Colorless liquid, yield 81.7%. 1H NMR (CDCl3) δ 7.55 (d, J = 8.2 Hz, 2H, H-2, 6), 7.19 (d, J = 8.0 Hz, 2H, H-3, 5), 4.80–4.73 (m, 1H, H-15), 3.99 (s, 3H, H-22), 3.06-3.03 (m, 2H, H-8), 2.56–2.53 (m, 2H, H-9), 2.38 (s, 3H, H-14), 1.83 (dd, J = 8.8, 3.8 Hz, 2H, H-20), 1.73 (dd, J = 9.0, 4.7 Hz, 2H, H-16), 1.55 (dd, J = 9.6, 6.5 Hz, 1H, H-18), 1.39 (dd, J = 22.4, 12.4 Hz, 4H, H-17, 19), 1.29–1.24 (m, 1H, H-18). 13C NMR (CDCl3) δ 172.11 (C-10), 156.95 (C-7), 139.22 (C-4), 132.40 (C-1), 129.24 (C-2, 6), 126.22 (C-3, 5), 72.87 (C-15), 61.93 (C-22), 31.59 (C-16, 20), 31.27 (C-9), 25.39 (C-8), 23.73 (C-18), 22.34 (C-17, 19), 21.25 (C-14). ESIMS: m/z 326.1726 [M+Na]+, calculated for C18H25NO3 (303.40).
Cyclohexyl (E)-4-benzyloxyimino-4-(4-methylphenyl)butanoate (4B-3). Pale yellow liquid, yield 79.3%. 1H NMR (CDCl3) δ 7.37–7.29 (m, 7H, H-2, 6, 24, 25, 26, 27, 28), 7.22 (d, J = 7.9 Hz, 2H, H-3, 5), 5.09 (s, 2H, H-22), 4.74 (dt, J = 8.9, 4.6 Hz, 1H, H-15), 2.87–2.83 (m, 2H, H-8), 2.57–2.54 (m, 2H, H-9), 2.38 (s, 3H, H-14), 1.84-1.78 (m, 2H, H-16), 1.72 (dd, J = 9.4, 3.6 Hz, 2H, H-20), 1.55 (dd, J = 6.9, 3.7 Hz, 1H, H-18), 1.41–1.33 (m, 4H, H-17, 19), 1.27 (t, J = 7.0 Hz, 1H, H-18). 13C NMR (CDCl3) δ 172.16 (C-10), 155.68 (C-7), 138.81 (C-4), 138.41 (C-23), 130.80 (C-1), 128.86 (C-3, 5), 128.22 (C-25, 27), 127.89 (C-26), 127.82 (C-24, 28), 127.47 (C-2, 6), 75.89 (C-22), 72.64 (C-15), 31.61 (C-16, 20), 31.45 (C-9), 30.75 (C-8), 25.40 (C-18), 23.76 (C-17, 19), 21.37 (C-14). ESIMS: m/z 380.2223 [M + H]+, 402.2037 [M + Na]+, calculated for C24H29NO3 (379.50).
Cyclohexyl (E)-4-hydroxyimino-4-(4-methoxyphenyl)butanoate (4C-1). Colorless crystal, yield 89.0%. m.p. 67.7–68.5 °C. 1H NMR (CDCl3) δ 8.69 (s, 1H, H-23), 7.59 (d, J = 8.9 Hz, 2H, H-2, 6), 6.93 (d, J = 8.9 Hz, 2H, H-3, 5), 4.79-4.75 (m, 1H, H-16), 3.85 (s, 3H, H-15), 3.13- 3.10 (m, 2H, H-8), 2.62-2.59 (m, 2H, H-9), 1.83 (dd, J = 8.8, 4.0 Hz, 2H, H-17), 1.72 (dd, J = 8.7, 3.7 Hz, 2H, H-21), 1.54 (dd, J = 5.7, 3.2 Hz, 1H, H-19), 1.38 (dd, J = 23.1, 11.8 Hz, 4H, H-18, 20), 1.26 (dd, J = 13.0, 3.1 Hz, 1H, H-19). 13C NMR (CDCl3) δ 172.27 (C-10), 160.61 (C-4), 157.68 (C-7), 127.71 (C-2, 6), 127.63 (C-1), 114.05 (C-3, 5), 73.00 (C-16), 55.33 (C-15), 31.57 (C-17, 21), 31.08 (C-9), 25.37 (C-8), 23.73 (C-19), 21.95 (C-18, 20). ESIMS: m/z 306.1701 [M + H]+, 328.1521 [M + Na]+, calculated for C17H23NO4 (305.37).
Cyclohexyl (E)-4-methoxyimino-4-(4-methoxyphenyl)butanoate (4C-2). Colorless liquid, yield 80.7%. 1H NMR (MeOD) δ 7.59 (d, J = 8.9 Hz, 2H, H-2, 6), 6.94 (d, J = 8.9 Hz, 2H, H-3, 5), 4.72–4.64 (m, 1H, H-16), 3.95 (s, 3H, H-23), 3.83 (s, 3H, H-15), 3.03–2.97 (m, 2H, H-8), 2.52-2.46 (m, 2H, H-9), 1.82-1.76 (m, 2H, H-17), 1.74-1.67 (m, 2H, H-21), 1.55 (dd, J = 10.7, 4.6 Hz, 1H, H-19), 1.42-1.33 (m, 4H, H-18,20), 1.29 (dd, J = 9.6, 3.0 Hz, 1H, H-19). 13C NMR (MeOD) δ 172.28 (C-10), 160.78 (C-4), 156.67 (C-7), 127.48 (C-2, 6), 127.35 (C-1), 113.53 (C-3, 5), 72.89 (C-16), 60.84 (C-23), 54.42 (C-15), 31.16 (C-17, 21), 30.99 (C-9), 25.07 (C-8), 23.34 (C-19), 21.81 (C-18, 20). ESIMS: m/z 320.1845 [M + H]+, 342.1661 [M + Na]+, calculated for C18H25NO4 (319.40).
Cyclohexyl (E)-4-benzyloxyimino-4-(4-methoxyphenyl)butanoate (4C-3). Pale yellow liquid, yield 76.9%. 1H NMR (MeOD) δ 7.58 (d, J = 8.9 Hz, 2H, H-2, 6), 7.41 (d, J = 7.1 Hz, 2H, H-25, 29), 7.36 (t, J = 7.5 Hz, 2H, H-26, 28), 7.30 (t, J = 7.3 Hz, 1H, H-27), 6.92 (d, J = 8.9 Hz, 2H, H-3, 5), 5.19 (s, 2H, H-23), 4.66 (dt, J = 8.7, 4.5 Hz, 1H, H-16), 3.81 (s, 3H, H-15), 3.06-3.03 (m, 2H, H-8), 2.52-2.49 (m, 2H, H-9), 1.75 (dd, J = 13.6, 6.0 Hz, 2H, H-17), 1.68 (dd, J = 9.2, 4.9 Hz, 2H, H-21), 1.55–1.50 (m, 1H, H-19), 1.39–1.32 (m, 4H, H-18,20), 1.30–1.24 (m, 1H, H-19). 13C NMR (MeOD) δ 172.35 (C-10), 160.82 (C-4), 157.11 (C-7), 138.08 (C-24), 127.99 (C-26, 28), 127.79 (C-2, 6), 127.52 (C-27), 127.43 (C-25, 29), 127.38 (C-1), 113.50 (C-3, 5), 75.75 (C-23), 72.87 (C-16), 54.39 (C-15), 31.09 (C-17, 21), 30.99 (C-9), 25.04 (C-8), 23.28 (C-19), 22.02 (C-18, 20). ESIMS: m/z 396.2179 [M + H]+, 418.1994 [M +Na]+, calculated for C24H29NO4 (395.50).
Cyclohexyl (E)-4-hydroxyimino-4-(4-chlorophenyl)butanimidate (4D-1). Colorless crystal, yield 86.3%. m.p. 92.9-93.6 °C. 1H NMR (CDCl3) δ 9.12 (s, 1H, H-21), 7.57 (d, J = 8.6 Hz, 2H, H-2, 6), 7.37 (d, J = 8.6 Hz, 2H, H-3, 5), 4.76 (dt, J = 8.9, 4.6 Hz, 1H, H-14), 3.12-3.09 (m, 2H, H-8), 2.62–2.59 (m, 2H, H-9), 1.84–1.80 (m, 2H, H-15), 1.71 (dd, J = 9.5, 3.7 Hz, 2H, H-19), 1.54 (dd, J = 9.7, 3.2 Hz, 1H, H-17), 1.37 (dd, J = 20.0, 11.6 Hz, 4H, H-16, 18), 1.28–1.23 (m, 1H, H-17). 13C NMR (CDCl3) δ 172.06 (C-10), 157.39 (C-7), 135.48 (C-4), 133.65 (C-1), 128.87 (C-3, 5), 127.68 (C-2, 6), 73.19 (C-14), 31.56 (C-15,19), 30.91 (C-9), 25.34 (C-8), 23.73 (C-17), 21.89 (C-16, 18). ESIMS: m/z 332.1024 [M + Na]+, calculated for C16H20ClNO3 (309.79).
Cyclohexyl (E)-4 -methoxyimino-4-(4-chlorophenyl)butanoate (4D-2). Colorless liquid, yield 75.5%. 1H NMR (CDCl3) δ 7.59 (d, J = 8.6 Hz, 2H, H-2, 6), 7.34 (d, J = 8.6 Hz, 2H, H-3, 5), 4.75 (dt, J = 9.0, 4.6 Hz, 1H, H-14), 3.99 (s, 3H, H-21), 3.03-3.00 (m, 2H, H-8), 2.53 (dd, J = 8.6, 7.5 Hz, 2H, H-9), 1.83-1.79 (m, 2H, H-19), 1.71 (dd, J = 9.3, 3.5 Hz, 2H, H-15), 1.56-1.51 (m, 1H, H-17), 1.37 (dd, J = 18.8, 10.5 Hz, 4H, H-16,18), 1.28-1.22 (m, 1H, H-17). 13C NMR (CDCl3) δ 171.90 (C-10), 155.87 (C-7), 135.19 (C-4), 133.71 (C-1), 128.70 (C-2, 6), 127.61 (C-3, 5), 72.99 (C-14), 62.13 (C-21), 31.57 (C-15, 19), 31.10 (C-9), 25.35 (C-8), 23.72 (C-17), 22.16 (C-16, 18). ESIMS: m/z 324.1370 [M + H]+, 346.1187 [M + Na]+, calculated for C17H22ClNO3 (323.82).
Cyclohexyl (E)-4-benzyloxyimino-4-(4-chlorophenyl)butanoate (4D-3). Pale yellow liquid, yield 70.8%. 1H NMR (CDCl3) δ 7.60 (d, J = 8.4 Hz, 2H, H-2, 6), 7.42 (ddd, J = 19.1, 15.0, 6.3 Hz, 10H, H-25, 26, 27, 28, 29, 17, 18, 19, 20, 21), 7.22 (d, J = 7.8 Hz, 2H, H-3, 5), 5.29 (s, 2H, H-15), 5.14 (s, 2H, H-23), 3.19-3.16 (m, 2H, H-8), 2.71–2.65 (m, 2H, H-9), 2.41 (s, 3H, H-14). 13C NMR (CDCl3) δ 172.52 (C-10), 157.05 (C-7), 139.37(C-4), 138.04 (C-24), 135.91 (C-16), 132.32 (C-4),129.30 (C-2, 6), 129.29 (C-26, 28), 128.60 (C-18, 20), 128.42 (C-27), 128.27 (C-19), 128.17 (C-25, 29), 127.81 (C-17, 21), 126.30 (C2, 6), 76.33 (C-23), 66.47 (C-15), 30.95(C-9), 22.45 (C-8), 21.32 (C-14). ESIMS: m/z 388.1918 [M + H]+, 410.1739 [M + Na]+, calculated for C25H25NO3 (387.48).
3.1.3. Evaluation of Compounds for Biological Activity in vitro
Cytotoxicity of Compounds In Vitro
These synthesized compounds were evaluated for anti-HBV activity with referring to reported method [25,30]. The HepG2.2.15 cell, a human hepatoblastoma cell line stably transfected with cloned HBV DNA, was obtained from Chinese Academy of Medical Sciences (Beijing, China), and was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum and 1.5 g/L of sodium bicarbonate, 10 mL/L of streptomycin, 10 mL/L of penicillin, and 200 mg/L of G418 at 37 °C in a 5% CO2 atmosphere with 95–98% humidity. Then, HepG2.2.15 cell was plated at a density of 1 × 105 cells/mL in 96-well plates and incubated at 37 °C for 24 h and then treated with variant concentration of compounds in 300, 150, 75, and 35.5 μM/mL; every three days, in a nine day period, C (OD) values were recorded at 450 nm. After nine days, the percent of cell death was measured using the method of MTT assay after drug treatment. Cytotoxicity of these compounds was expressed as the concentration of compound required to kill 50% (CC50) of the HepG 2.2.15 cells.
Method for the HBsAg and HBeAg Inhibition Assays. The levels of HBV surface antigen (HBsAg) and HBV e antigen (HBeAg) in the supernatant of the HepG2.2.15 cells were determined by the enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocol (Shanghai Kehua Biotech Co., Ltd., Shanghai, China). The synthesized compounds were expressed as the concentration that achieved 50% inhibition (IC50) to the secretion of HBsAg and HBeAg. The selectivity index (SI) was determined as the ratio of CC50 to IC50 which a major pharmaceutical parameter of estimates possible future clinical application.
3.2. Statistical Analysis
SPSS 20.0 and MICROSOFT EXCEL 2010 were used for the statistical analysis. All data were expressed as the mean ± standard error of mean (S.E.M.). The differences among the groups were analyzed by one-way analysis of variance (ANOVA) with a Tukey post hoc test when comparing multiple groups. P values lower than 0.05 were regarded as statistically significant.
4. Conclusions
A series of cyclohexyl (E)-4-(alkyl imino)-4-(4-substituted-phenyl) butanoates possessed obvious and significant anti-HBV activities with SIHBsAg values from 0.89 to 13.41 and SIHBeAg values from 1.06 to 17.34 compared to control lamivudine in SIHBsAg values 2.20 and SIHBeAg values 2.07. Results also revealed that oximes were weaker in inhibiting HBV, and more cytotoxic than their relative ethers; meanwhile, methyl ethers were the most active in inhibiting HBV among compounds in every group. Compound 4B-2 among these compounds showed the best inhibition of HBsAg and HBeAg secretion.
Author Contributions
Conceptualization, W.W. and X.-C.; Methodology, M.Z.; Software, C.L.; Validation, X.C. and M.Z.; Formal Analysis, P.L.; Investigation, X.C. and Z.W.; Data Curation, J.T.; Writing-Original Draft Preparation, X.C.; Writing-Review & Editing, M.Z.; Visualization, X.C. and M.Z.; Supervision, W.W.; Project Administration, W.W.; Funding Acquisition, W.W.
Funding
This work was financially supported by the National natural science foundation of China (No. 81760635).
Acknowledgments
We are thankful to Jun Xu from the School of Chemical & Chemistry Engineering, Sun Yat Sen University, for providing generously help in MOE docking.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- World Health Organization (WHO). Hepatitis B, Fact Sheet; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Lok, A.S. Chronic hepatitis B. N. Engl. J. Med. 2002, 346, 1682–1683. [Google Scholar] [CrossRef] [PubMed]
- Block, T.M.; Rawat, S.; Brosgart, C.L. Chronic hepatitis B: A wave of new therapies on the horizon. Antivir. Res. 2015, 121, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, S.J.; Lok, A.S. Effectiveness of hepatitis B treatment in clinical practice. Gastroenterology 2012, 142, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Clercq, D.E.; Ferir, G.; Kaptein, S.; Neyts, J. Antiviral treatment of chronic hepatitis B virus (HBV) infections. Viruses 2010, 2, 1279–1305. [Google Scholar] [CrossRef] [PubMed]
- Pradere, U.; Ethel, C.; Steven, J.C.; Franck, A.; Raymond, F.S. Synthesis of nucleoside phosphate and phosphonate prodrugs. Chem. Rev. 2014, 114, 9154–9218. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.K.; Cheung, A.M.; O’Rourke, K.; Naylor, C.D.; Detsky, A.S.; Heathcote, J. Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B: A meta-analysis. Ann. Intern. Med. 1993, 4, 312–323. [Google Scholar] [CrossRef]
- Zarski, J.P.; Causse, X.; Cohard, M.; Cougnard, J.; Trepo, C. A randomized, controlled trial of interferon alfa-2b alone and after prednisone withdrawal for the treatment of chronic hepatitis B. N. Engl. J. Med. 1990, 5, 295–301. [Google Scholar]
- Dusheiko, G.; Bertoletti, A. Resistance to lamivudine therapy: Is there more than meets the eye. Gut 2011, 1, 4–6. [Google Scholar] [CrossRef][Green Version]
- Gao, L.M.; Han, Y.X.; Wang, Y.P.; Li, Y.H.; Shan, Y.Q.; Li, X.; Peng, Z.G.; Bi, C.W.; Zhang, T.; Du, N.N.; et al. Design and Synthesis of Oxymatrine Analogues Overcoming Drug Resistance in Hepatitis B Virus through Targeting Host Heat Stress Cognate 7. J. Med. Chem. 2011, 54, 869–876. [Google Scholar] [CrossRef]
- Huang, T.-J.; Chuang, H.; Liang, Y.-C.; Lin, H.-H.; Horng, J.-C.; Kuo, Y.-C.; Chen, W.; Tsai, F.-Y.; Yen, S.-C.; Chou, S.-C.; et al. Design, Synthesis, and Bioevaluation of Paeonol Derivatives as Potential Anti-HBV Agents. Eur. J. Med. Chem. 2015, 90, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.-J.; Yu, S.; Wang, Y.-F.; Wang, D.; Zhu, H.-T.; Cheng, R.-R.; Yang, C.-R.; Xu, M.; Zhang, Y.-J. Anti-Hepatitis B Virus Norbisabolane Sesquiterpenoids from Phyllanthus acidus and the Establishment of Their Absolute Configurations Using Theoretical Calculations. J. Org. Chem. 2014, 79, 5432–5447. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wei, W.X.; Li, Y.B.; Lin, X.; Shi, K.C.; Cao, X.; Zhou, M. In vitro and in vivo anti-hepatitis B virus activities of the lignan nirtetralin B isolated from Phyllanthus niruri L. J. Ethnopharmacol. 2014, 157, 62–68. [Google Scholar] [CrossRef]
- Yan, M.H.; Cheng, P.; Jiang, Z.Y.; Chen, J.J. Periglaucines A-D, Anti-HBV and –HIV-1 Alkaloids from Pericampylus glaucus. J. Nat. Prod. 2008, 71, 760–763. [Google Scholar] [CrossRef] [PubMed]
- de Niet, A.; Stelma, F.; Jansen, L.; Sinnige, M.J.; Remmerswaal, E.B.M.; Takkenberg, R.B.; Kootstra, N.A.; Reesink, H.W.; van Lier, R.A.W.; van Leeuwen, E.M.M. Restoration of T cell function in chronic hepatitis B patients upon treatment with interferon based combination therapy. J. Hepatol. 2015, 3, 539–546. [Google Scholar]
- Liu, J.X.; Chen, K.Y.; Ren, E.C. Structural insights into the binding of hepatitis B virus core peptide to HLA-A2 alleles: Towards designing better vaccines. Eur. J. Immunol. 2011, 41, 2097–2106. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, Y.; Yu, Y.; Tan, Q.; Huang, X. Identification of HLA-A*0201-restricted CD8+ T-cell epitope C64–72 from hepatitis B virus core protein. Int. Immunopharmacol. 2012, 13, 141–147. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Zhao, B.; Deng, M.-M.; Liu, J.; Li, X.; Hou, J.-W.; Gui, M.-M.; Zhang, S.-J.; Li, X.-D.; et al. A new unconventional HLA-A2-restricted epitope from HBV core protein elicits antiviral cytotoxic T lymphocytes. Protein Cell 2014, 4, 317–327. [Google Scholar] [CrossRef]
- Xu, W.; Chu, Y.W.; Zhang, R.H.; Xu, H.B.; Wang, Y.; Xiong, S.D. Endoplasmic reticulum targeting sequence enhances HBV-specific cytotoxic T lymphocytes induced by a CTL epitope-based DNA vaccine. Virology 2005, 334, 255–263. [Google Scholar] [CrossRef]
- Yu, D.B.; Liu, H.; Shi, S.; Dong, L.W.; Wang, H.G.; Wu, N.T.; Gao, H.; Cheng, Z.J.; Zheng, Q.; Cai, J.J.; et al. A novel dendritic-cell-targeting DNA vaccine for hepatitis B induces T cell and humoral immune responses and potentiates the antivirus activity in HBV transgenic mic. Immunol. Lett. 2015, 2, 293–299. [Google Scholar] [CrossRef]
- Li, J.Q.; Ge, J.; Ren, S.L.; Zhou, T.; Sun, Y.; Sun, H.L.; Gu, Y.; Huang, H.Y.; Xu, Z.X.; Chen, X.X.; et al. Hepatitis B surface antigen (HBsAg) and core antigen (HBcAg) combine CpG oligodeoxynucletides as a novel therapeutic vaccine for chronic hepatitis B infection. Vaccine 2015, 35, 4247–4254. [Google Scholar] [CrossRef]
- Liu, S.; Wei, W.X.; Li, Y.B.; Liu, X.; Cao, X.J.; Lei, K.C.; Zhou, M. Design, synthesis, biological evaluation and molecular docking studies of phenylpropanoid derivatives as potent anti-hepatitis B virus agents. Eur. J. Med. Chem. 2015, 95, 473–482. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.B.; Wei, W.X.; Wei, J.C. Design, Synthesis, Molecular Docking Studies and Anti-HBV Activity of Phenylpropanoid Derivatives. Chem.-Biol. Interact. 2016, 251, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Y.B.; Wei, W.X.; Wang, K.W.; Wang, L.S.; Wang, J.Y. Synthesis and biological evaluation of phenylpropanoid derivatives. Med. Chem. Res. 2016, 6, 1074–1086. [Google Scholar]
- Tan, J.; Zhou, M.; Cui, X.H.; Wei, Z.C.; Wei, W.X. Discovery of Oxime Ethers as Hepatitis B Virus (HBV) Inhibitors by Docking, Screening and In Vitro Investigation. Molecules 2018, 23, 637. [Google Scholar] [CrossRef]
- Ikawa, T.; Sajiki, H.; Hirota, K. Highly chemoselective hydrogenation method using novel finely dispersed palladium catalyst on silk-fibroin: Its preparation and activity. Tetrahedron 2005, 8, 2217–2231. [Google Scholar] [CrossRef]
- Ali, Y.; Alam, M.S.; Hamid, H.; Husain, A.; Dhulap, A.; Bano, S.; Kharbanda, C.; Nazreen, S.; Haider, S. Design and synthesis of butenolide-based amide derivatives as anti-inflammatory agents. Med. Chem. Res. 2015, 11, 3775–3784. [Google Scholar] [CrossRef]
- Iftime, G.; Kazmaier, P.M.; Norsten, T.B. Inkless Reimageable Printing Paper and Method. U.S. Patent 7582398, 1 September 2009. [Google Scholar]
- Wei, W.X.; Zhou, M.; Cui, X.H.; Tan, J. Oxime and Oxime Ether compounds with Anti-Hepatitis B virus activity. China Patent CN201610718025.4, 24 August 2016. [Google Scholar]
- Sells, M.A.; Chen, M.-L.; Acs, G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. PNAS 1987, 4, 1005–1009. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).