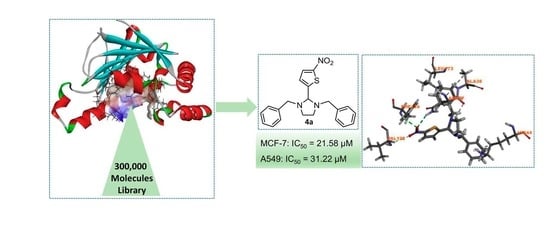
Identification and Structure-Activity Studies of 1,3-Dibenzyl-2-aryl imidazolidines as Novel Hsp90 Inhibitors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Based Virtual Screening
2.2. Biological Evaluation of Molecules Identified from Virtual Screening
2.3. Molecule Docking Analysis of Hsp90-4a Complex
2.4. Structure-Activity Relationship (SAR) Studies
2.5. Binding Affinity Assay
2.6. Western Blotting Assay
3. Materials and Methods
3.1. Virtual Screening
3.2. Evaluation of Anti-proliferative Activity In Vitro
3.3. Fluorescence Polarization (FP) Enzymatic Assay
3.4. Western Immune Blotting Assays
3.5. Chemistry
3.5.1. General Information
3.5.2. General Procedure for the Preparation of 1,3-Dibenzyl-2-aryl Imidazolidine 4c–4r
3.5.3. General Procedure for the Preparation of N,N′-Diphenyl-2-aryl Imidazolidine 6a–6d
3.5.4. General Procedure for Preparation of 1,3-Diethyl-2-aryl Imidazolidine 7a,7b
3.5.5. General Procedure for Preparation of N,N′-Dimethyl-2-aryl Imidazolidine 8a
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sherman, M.; Multhoff, G. Heat shock proteins in cancer. Ann. N. Y. Acad. Sci. 2007, 1113, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 2005, 5, 761. [Google Scholar] [CrossRef] [PubMed]
- Wandinger, S.; Richter, K.; Buchner, J. The Hsp90 chaperone machinery. J. Biol. Chem. 2008, 283, 18473–18477. [Google Scholar] [CrossRef] [PubMed]
- Pearl, L.; Prodromou, C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu. Rev. Biochem. 2006, 75, 271–294. [Google Scholar] [CrossRef] [PubMed]
- Graner, M. HSP90 and immune Modulation in cancer. Adv. Cancer Res. 2016, 129, 191–224. [Google Scholar] [PubMed]
- Wong, D.; Jay, D. Emerging roles of extracellular hsp90 in cancer. Adv. Cancer Res. 2016, 129, 141–163. [Google Scholar] [PubMed]
- Miyata, Y.; Nakamoto, H.; Neckers, L. The therapeutic target Hsp90 and cancer hallmarks. Curr. Pharm. Des. 2013, 19, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Maloney, A.; Workman, P. HSP90 as a new therapeutic target for cancer therapy: The story unfolds. Expert Opin. Biol. Ther. 2002, 2, 3–24. [Google Scholar] [CrossRef]
- Moser, C.; Lang, S.; Stoeltzing, O. Heat-shock protein 90 (Hsp90) as a molecular target for therapy of gastrointestinal cancer. Anticancer Res. 2009, 29, 2031–2042. [Google Scholar]
- Beliakoff, J.; Whitesell, L. Hsp90: An emerging target for breast cancer therapy. Anticancer Drugs 2004, 15, 651–662. [Google Scholar] [CrossRef]
- Mahalingam, D.; Swords, R.; Carew, J.; Nawrocki, S.; Bhalla, K.; Giles, F. Targeting HSP90 for cancer therapy. Br. J. Cancer 2009, 100, 1523–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özgür, A.; Tutar, Y. Heat shock protein 90 inhibition in cancer drug discovery: From chemistry to futural clinical applications. Anticancer Agents Med. Chem. 2016, 16, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Monterrey, I.; Sala, M.; Musella, S.; Campiglia, P. Heat shock protein 90 inhibitors as therapeutic agents. Recent Pat. Anticancer Drug Discov. 2012, 7, 313–336. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Tummalapalli, R.; Rotella, D. Progress in the discovery and development of heat shock protein 90 (Hsp90) inhibitors: Miniperspective. J. Med. Chem. 2014, 57, 8718–8728. [Google Scholar] [CrossRef] [PubMed]
- Claudia, C.; Andrea, P.; Celeste, C.; Paola, M.; Antonella, M.; Silvestre, B. Heat shock proteins in alzheimer’s disease: Role and targeting. Int. J. Mol. Sci. 2018, 19, 10. [Google Scholar]
- Lackie, R.; Maciejewski, A.; Ostapchenko, V.; Marques-Lopes, J. The Hsp70/Hsp90 Chaperone machinery in neurodegenerative diseases. Front Neurosci. 2017, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Ernst, J.; Neubert, T.; Liu, M.; Sperry, S.; Zuccola, H. Identification of novel HSP90α/β isoform selective inhibitors using structure-based drug design demonstration of potential utility in treating CNS disorders such as Huntington’s disease. J. Med. Chem. 2014, 57, 3382–3400. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, F.; Wang, R.; Li, F.; Wu, Y.; Kitazato, K.; Wang, Y. HSP90: A promising broad-spectrum antiviral drug target. Arch. Virol. 2017, 162, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Cowen, L.; Singh, S.; Kohler, J.; Collins, C.; Zaas, A. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc. Natl. Acad. Sci. USA 2009, 106, 2818–2823. [Google Scholar] [CrossRef] [Green Version]
- Devaney, E.; Gillan, V. Hsp90 Inhibitors in parasitic nematodes: Prospects and challenges. Curr. Top Med. Chem. 2016, 16, 2805–2811. [Google Scholar] [CrossRef]
- Wang, H.; Lu, M.; Yao, M.; Zhu, W. Effects of treatment with an Hsp90 inhibitor in tumors based on 15 phase II clinical trials. Mol. Clin. Oncol. 2016, 5, 326–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuno, A.; Lee, M.; Lee, S.; Tomita, Y.; Rekhtman, D.; Moore, B.; Trepel, J. Clinical evaluation and biomarker profiling of hsp90 inhibitors. Methods Mol. Boil. 2018, 1709, 423–441. [Google Scholar]
- Jhaveri, K.; Taldone, T.; Modi, S.; Chiosis, G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. BBA Mol. Cell Res. 2012, 1823, 742–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrikaite, V.; Matulis, D. Binding of natural and synthetic inhibitors to heat shock protein 90 and their clinical applications. Medicina (Kaunas) 2011, 47, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, D.; Xu, J.; Shi, J.; Jiang, L.; Yao, N.; Ye, W. Discovery and development of natural heat shock protein 90 inhibitors in cancer treatment. Acta Pharm. Sin. B 2012, 2, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Roe, S.; Prodromou, C.; O’Brien, R.; Ladbury, J.; Piper, P.; Pearl, L. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J. Med. Chem. 1999, 42, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Sausville, E.; Tomaszewski, J.; Ivy, P. Clinical development of 17-allylamino, 17-demethoxygeldanamycin. Curr. Cancer Drug Targets 2003, 3, 377–383. [Google Scholar] [CrossRef]
- Schulte, T.; Akinaga, S.; Soga, S.; Sullivan, W.; Stensqard, B.; Toft, D.; Neckers, L. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperone 1998, 3, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Eccles, S.; Massey, A.; Raynaud, F.; Sharp, S.; Box, G.; Valenti, V. NVP-AUY922: A novel heat shock protein 90 inhibitor active against xenograft tumor growth, angiogenesis, and metastasis. Cancer Res. 2008, 68, 2850–2859. [Google Scholar] [CrossRef] [PubMed]
- Canonici, A.; Qadir, Z.; Conlon, N.; Collins, D.; O’Brien, N. The HSP90 inhibitor NVP-AUY922 inhibits growth of HER2 positive and trastuzumab-resistant breast cancer cells. Invest. New Drugs 2018, 36, 581–589. [Google Scholar] [CrossRef]
- Shapiro, G. Hsp90 inhibitors in clinical development: STA-9090 (Ganetespib). Ann. Oncol. 2011, 22, 16–17. [Google Scholar]
- Cavenagh, J.; Oakervee, H.; Baetiong-Caguioa, P.; Davies, F.; Gharibo, M. A phase I/II study of KW-2478, an Hsp90 inhibitor, in combination with bortezomib in patients with relapsed/refractory multiple myeloma. Br. J. Cancer 2017, 117, 1295–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, K.; Cavet, J.; Johnson, P.; Morgan, G.; Williams, C. Phase I study of KW-2478, a novel Hsp90 inhibitor, in patients with B-cell malignancies. Br. J. Cancer 2016, 114, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.; Weiss, G.; Jones, S.; Tibes, R.; Bauer, T. Phase I dose-escalation studies of SNX-5422, an orally bioavailable heat shock protein 90 inhibitor, in patients with refractory solid tumours. Euro. J. Cancer 2014, 50, 2897–2904. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, R.; Matta, H.; Chaudhary, P. A purine scaffold HSP90 inhibitor BIIB021 has selective activity against KSHV-associated primary effusion lymphoma and blocks vFLIP K13-induced NF-κB. Clin. Cancer Res. 2013, 19, 5016–5026. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Hu, H. BIIB021, an Hsp90 inhibitor: A promising therapeutic strategy for blood malignancies. Oncol. Rep. 2018, 40, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Canella, A.; Welker, A.; Yoo, J.; Xu, J.; Abas, F. Efficacy of onalespib, a long-acting second-generation HSP90 inhibitor, as a single agent and in combination with temozolomide against malignant gliomas. Clin. Cancer Res. 2017, 23, 6215–6226. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 4c–4r, 6a–6d, 7a, 7b and 8a are available from the authors. |





| Entry | Product | Ar | IC50 a (μM) | |
|---|---|---|---|---|
| MCF-7 | A549 | |||
| 1 | 4a | 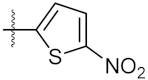 | 21.58 ± 0.57 | 31.22 ± 0.31 |
| 2 | 4b | 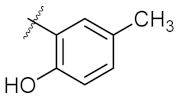 | 50.62 ± 0.26 | 88.58 ± 0.08 |
| 3 | 4c |  | 80.04 ± 0.63 | 78.18 ± 0.15 |
| 4 | 4d |  | 68.35 ± 0.44 | 56.30 ± 1.27 |
| 5 | 4e | 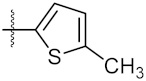 | 119.30 ± 0.42 | 112.80 ± 1.41 |
| 6 | 4f | 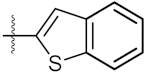 | 219.40 ± 0.57 | 135.80 ± 1.41 |
| 7 | 4g | 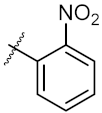 | 40.91 ± 0.05 | 40.64 ± 0.51 |
| 8 | 4h |  | 117.70 ± 0.01 | 55.60 ± 0.49 |
| 9 | 4i |  | 27.86 ± 0.01 | 59.27 ± 0.83 |
| 10 | 4j | 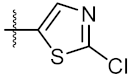 | 33.12 ± 0.23 | 35.30 ± 0.14 |
| 11 | 4k | 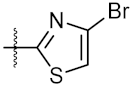 | 77.01 ± 0.01 | 498.80 ± 0.25 |
| 12 | 4l |  | 31.48 ± 0.60 | 64.66 ± 0.48 |
| 13 | 4m |  | 76.42 ± 0.31 | 70.42 ± 0.10 |
| 14 | 4n | 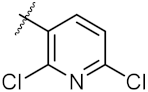 | 40.36 ± 0.51 | 52.11 ± 0.50 |
| 15 | 4o | 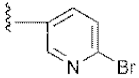 | 53.12 ± 0.77 | 56.92 ± 1.27 |
| 16 | 4p | 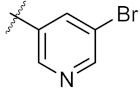 | 45.25 ± 0.63 | 72.87 ± 0.66 |
| 17 | 4q | 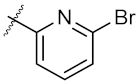 | 46.97 ± 0.04 | 71.36 ± 0.88 |
| 18 | 4r | 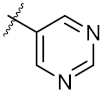 | 51.37 ± 0.54 | 80.78 ± 1.00 |
| 19 | 17-AAG | - | 7.18 ± 0.13 | 3.54 ± 0.08 |
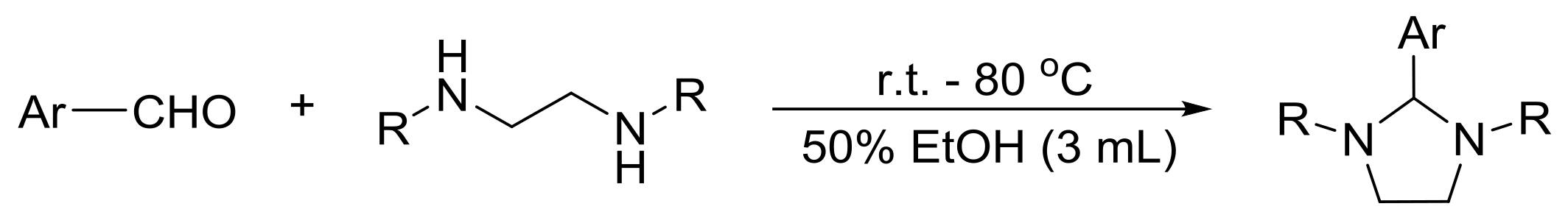
| Entry | Product | R | Ar | IC50 a (μM) | |
|---|---|---|---|---|---|
| MCF-7 | A549 | ||||
| 1 | 6a | Ph |  | 40.77 ± 0.39 | 123.40 ± 0.85 |
| 2 | 6b | Ph |  | 161.60 ± 0.57 | 172.00 ± 1.41 |
| 3 | 6c | Ph | 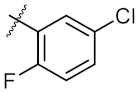 | 43.67 ± 1.03 | 46.14 ± 0.20 |
| 4 | 6d | Ph | 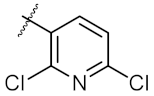 | 323.00 ± 2.28 | 248.60 ± 0.57 |
| 5 | 7a | Et | 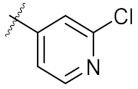 | >500 | 250.10 ± 1.27 |
| 6 | 7b | Et |  | 158.90 ± 0.14 | 164.00 ± 1.41 |
| 7 | 8a | Me | 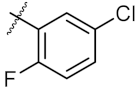 | 250.10 ± 0.27 | 148.70 ± 1.56 |
| Compounds | FP IC50 (μM) a |
|---|---|
| 17-AAG | 0.017 ± 0.003 |
| 4a | 0.012 ± 0.010 |
| 4b | 0.612 ± 0.014 |
| 4d | 0.672 ± 0.010 |
| 4i | 0.610 ± 0.014 |
| 4j | 0.310 ± 0.014 |
| 4n | 0.102 ± 0.010 |
| 6c | 1.930 ± 0.013 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, X.; Li, L.; Dai, R.; Shi, M.; Xue, H.; Liu, Y.; Wang, H. Identification and Structure-Activity Studies of 1,3-Dibenzyl-2-aryl imidazolidines as Novel Hsp90 Inhibitors. Molecules 2019, 24, 2105. https://doi.org/10.3390/molecules24112105
Liu Y, Liu X, Li L, Dai R, Shi M, Xue H, Liu Y, Wang H. Identification and Structure-Activity Studies of 1,3-Dibenzyl-2-aryl imidazolidines as Novel Hsp90 Inhibitors. Molecules. 2019; 24(11):2105. https://doi.org/10.3390/molecules24112105
Chicago/Turabian StyleLiu, Yajun, Xiaoxia Liu, Lihong Li, Rui Dai, Meiyun Shi, Hongyu Xue, Yong Liu, and Hecheng Wang. 2019. "Identification and Structure-Activity Studies of 1,3-Dibenzyl-2-aryl imidazolidines as Novel Hsp90 Inhibitors" Molecules 24, no. 11: 2105. https://doi.org/10.3390/molecules24112105
APA StyleLiu, Y., Liu, X., Li, L., Dai, R., Shi, M., Xue, H., Liu, Y., & Wang, H. (2019). Identification and Structure-Activity Studies of 1,3-Dibenzyl-2-aryl imidazolidines as Novel Hsp90 Inhibitors. Molecules, 24(11), 2105. https://doi.org/10.3390/molecules24112105