Comparative Analysis of Pentacyclic Triterpenic Acid Compositions in Oleogum Resins of Different Boswellia Species and Their In Vitro Cytotoxicity against Treatment-Resistant Human Breast Cancer Cells
Abstract
:1. Introduction
2. Results
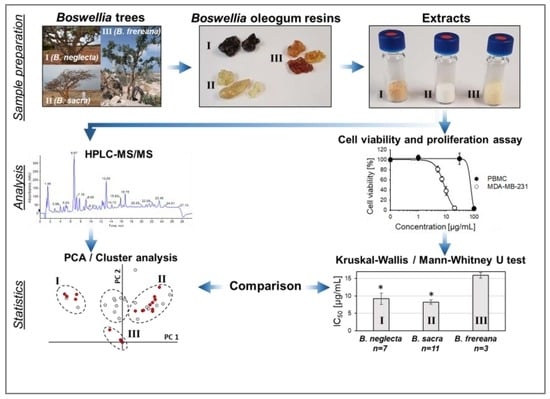
2.1. Extraction of Boswellia Oleogum Resins
2.2. Analysis of Pentacyclic Triterpenic Acids in Boswellia Extracts
2.3. Differences in Pentacyclic Triterpenic Acid Composition of Various Boswellia Oleogum Resins
2.4. Classification of Frankincense Sampleas by the Boswellia Index (Bosi)
2.5. PTA Composition of Boswellia occulta Oleogum Resin
2.6. Cytotoxicity of Frankincense Extracts towards Triple Negative Human Breast Cancer Cells
2.7. Cytotoxicity of Different Extraction Fractions Obtained from Oleogum Resins of the Species Boswellia sacra
3. Materials and Methods
3.1. Plant Material
3.2. Materials
3.3. Extraction Procedure
3.4. HPLC-MS/MS Analysis
3.5. Validation of the HPLC-MS/MS Method
3.6. Analysis of Cancer Cell Cytotoxicity
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Compliance with Ethical Standards
References
- Huber, G. Weihrauch; Ansata: Munich, Germany, 2018. [Google Scholar]
- Martinetz, D.; Lohs, K.; Janzen, J. Weihrauch und Myrrhe: Kulturgeschichte und wirtschaftliche Bedeutung, Botanik, Chemie, Medizin; Wissenschaftliche Verlangsgesellschaft mbH Stuttgart: Suttgart, Germany, 1988. [Google Scholar]
- Moussaieff, A.; Mechoulam, R. Boswellia resin: From religious ceremonies to medical uses; a review of in-vitro, in-vivo and clinical trials. J. Pharm. Pharmacol. 2009, 61, 1281–1293. [Google Scholar] [CrossRef]
- Schrott, E. Weihrauch; Mosaik: Munich, Germany, 1998. [Google Scholar]
- Ernst, E. Frankincense: Systematic review. BMJ 2008, 337, a2813. [Google Scholar] [CrossRef]
- Roy, N.K.; Deka, A.; Bordoloi, D.; Mishra, S.; Kumar, A.P.; Sethi, G.; Kunnumakkara, A.B. The potential role of boswellic acids in cancer prevention and treatment. Cancer Lett. 2016, 377, 74–86. [Google Scholar] [CrossRef]
- Belsner, K.; Büchele, B.; Werz, U.; Simmet, T. Structural analysis of 3-α-acetyl-20(29)-lupene-24-oic acid, a novel pentacyclic triterpene isolated from the gum resin of Boswellia serrata, by NMR spectroscopy. Magn. Reson. Chem. 2003, 41, 629–632. [Google Scholar] [CrossRef]
- Belsner, K.; Büchele, B.; Werz, U.; Syrovets, T.; Simmet, T. Structural analysis of pentacyclic triterpenes from the gum resin of Boswellia serrata by NMR spectroscopy. Magn. Reson. Chem. 2003, 41, 115–122. [Google Scholar] [CrossRef]
- Büchele, B.; Simmet, T. Analysis of 12 different pentacyclic triterpenic acids from frankincense in human plasma by high-performance liquid chromatography and photodiode array detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003, 795, 355–362. [Google Scholar] [CrossRef]
- Büchele, B.; Zugmaier, W.; Simmet, T. Analysis of pentacyclic triterpenic acids from frankincense gum resins and related phytopharmaceuticals by high-performance liquid chromatography. Identification of lupeolic acid, a novel pentacyclic triterpene. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003, 791, 21–30. [Google Scholar] [CrossRef]
- Frank, A.; Unger, M. Analysis of frankincense from various Boswellia species with inhibitory activity on human drug metabolising cytochrome P450 enzymes using liquid chromatography mass spectrometry after automated on-line extraction. J. Chromatogr. A 2006, 1112, 255–262. [Google Scholar] [CrossRef]
- Mathe, C.; Culioli, G.; Archier, P.; Vieillescazese, C. High-performance liquid chromatographic analysis of triterpenoids in commercial frankincense. Chromatographia 2004, 60, 493–499. [Google Scholar] [CrossRef]
- Paul, M.; Brüning, G.; Bergmann, J.; Jauch, J. A thin-layer chromatography method for the identification of three different olibanum resins (Boswellia serrata, Boswellia papyrifera and Boswellia carterii, respectively, Boswellia sacra). Phytochem. Anal. 2012, 23, 184–189. [Google Scholar] [CrossRef]
- Woolley, C.L.; Suhail, M.M.; Smith, B.L.; Boren, K.E.; Taylor, L.C.; Schreuder, M.F.; Chai, J.K.; Casabianca, H.; Haq, S.; Lin, H.K.; et al. Chemical differentiation of Boswellia sacra and Boswellia carterii essential oils by gas chromatography and chiral gas chromatography-mass spectrometry. J. Chromatogr. A 2012, 1261, 158–163. [Google Scholar] [CrossRef]
- Paul, M.; Brüning, G.; Weihrather, J.; Jauch, J. Qualitative and quantitative analysis of 17 different types of tetra- and pentacyclic triterpenic acids in Boswellia papyrifera by a semi-automatic homomodal 2D HPLC method. Chromatographia 2011, 74, 29. [Google Scholar] [CrossRef]
- Thulin, M.; DeCarlo, A.; Johnson, S.P. Boswellia occulta (Burseraceae), a new species of frankincense tree from Somalia (Somaliland). Phytotaxa 2019, 394, 219–224. [Google Scholar] [CrossRef]
- Ammon, H.P.T.; Mack, T.; Singh, G.B.; Safayhi, H. Inhibition of leukotriene B4 formation in rat peritoneal neutrophils by an ethanolic extract of the gum resin exudate of Boswellia serrata. Planta Med. 1990, 57, 203–207. [Google Scholar] [CrossRef]
- Schmidt, C.; Loos, C.; Jin, L.; Schmiech, M.; Schmidt, C.Q.; El Gaafary, M.; Syrovets, T.; Simmet, T. Acetyl-lupeolic acid inhibits Akt signaling and induces apoptosis in chemoresistant prostate cancer cells in vitro and in vivo. Oncotarget 2017, 8, 55147–55161. [Google Scholar] [CrossRef] [Green Version]
- Syrovets, T.; Büchele, B.; Gedik, E.; Slupsky, J.R.; Simmet, T. Acetyl-boswellic acids are novel catalytic inhibitors of human topoisomerases I and IIα. Mol. Pharmacol. 2000, 58, 71–81. [Google Scholar] [CrossRef]
- Syrovets, T.; Büchele, B.; Krauss, C.; Laumonnier, Y.; Simmet, T. Acetyl-boswellic acids inhibit lipopolysaccharide-mediated TNF-α induction in monocytes by direct interaction with IκB kinases. J. Immunol. 2005, 174, 498–506. [Google Scholar] [CrossRef]
- Syrovets, T.; Gscchwend, J.; Büchele, B.; Laumonnier, Y.; Zugmaier, W.; Genze, F.; Simmet, T. Inhibition of IκB kinase activity by acetyl-boswellic acids promotes apoptosis in androgen-independent PC-3 prostate cancer cells in vitro and in vivo. J. Biol. Chem. 2005, 280, 6170–6180. [Google Scholar] [CrossRef]
- Wang, H.; Syrovets, T.; Kess, D.; Buchele, B.; Hainzl, H.; Lunov, O.; Weiss, J.M.; Scharffetter-Kochanek, K.; Simmet, T. Targeting NF-kappa B with a natural triterpenoid alleviates skin inflammation in a mouse model of psoriasis. J. Immunol. 2009, 183, 4755–4763. [Google Scholar] [CrossRef]
- Safayhi, H.; Mack, T.; Sabieraj, J.O.; Anazodo, M.I.; Subramanian, L.R.; Ammon, H.P. Boswellic acids: Novel, specific, nonredox inhibitors of 5-lipoxygenase. J. Pharmacol. Exp. Ther. 1992, 261, 1143–1146. [Google Scholar]
- Tausch, L.; Henkel, A.; Siemoneit, U.; Poeckel, D.; Kather, N.; Franke, L.; Hofmann, B.; Schneider, G.; Angioni, C.; Geisslinger, G.; et al. Identification of human cathepsin G as a functional target of boswellic acids from the anti-inflammatory remedy frankincense. J. Immunol. 2009, 183, 3433–3442. [Google Scholar] [CrossRef]
- Glaser, T.; Winter, S.; Groscurth, P.; Safayhi, H.; Sailer, E.R.; Ammon, H.P.T.; Schabet, M.; Weller, M. Boswellic acids and malignant glioma: Induction of apoptosis but no modulation of drug sensitivity. Br. J. Cancer 1999, 80, 756–765. [Google Scholar] [CrossRef]
- Hoernlein, R.F.; Orlikowsky, T.; Zehrer, C.; Niethammer, D.; Sailer, E.R.; Simmet, T.; Dannecker, G.E.; Ammon, H.P.T. Acetyl-11-keto-β-boswellic acid induces apoptosis in HL-60 and CCRF-CEM cells and inhibits topoisomerase I. J. Pharmacol. Exp. Ther. 1999, 288, 613–619. [Google Scholar]
- Suhail, M.M.; Wu, W.; Cao, A.; Mondalek, F.G.; Fung, K.M.; Shih, P.T.; Fang, Y.T.; Woolley, C.; Young, G.; Lin, H.K. Boswellia sacra essential oil induces tumor cell-specific apoptosis and suppresses tumor aggressiveness in cultured human breast cancer cells. BMC Complement Altern Med. 2011, 11. [Google Scholar] [CrossRef]
- Thulin, M.; Warfa, A.M. The Frankincense Trees (Boswellia spp., Burseraceae) of Northern Somalia and Southern Arabia. Kew Bull. 1987, 42, 487–500. [Google Scholar] [CrossRef]
- Jiang, M.; Kulsing, C.; Marriott, P.J. Comprehensive 2D gas chromatography-time-of-flight mass spectrometry with 2D retention indices for analysis of volatile compounds in frankincense (Boswellia papyrifera). Anal. Bioanal. Chem. 2018, 410, 3185–3196. [Google Scholar] [CrossRef]
- Johnson, S.; DeCarlo, A.; Satyal, P.; Dosoky, N.S.; Sorensen, A.; Setzer, W.N. Organic Certification is Not Enough: The Case of the Methoxydecane Frankincense. Plants (Basel) 2019, 8, 88. [Google Scholar] [CrossRef]
- Ayla, S.; Seckin, I.; Tanriverdi, G.; Cengiz, M.; Eser, M.; Soner, B.C.; Oktem, G. Doxorubicin induced nephrotoxicity: Protective effect of nicotinamide. Int. J. Cell Biol. 2011, 2011, 390238. [Google Scholar] [CrossRef]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef]
- Gupta, I.; Parihar, A.; Malhotra, P.; Gupta, S.; Ludtke, R.; Safayhi, H.; Ammon, H.P. Effects of gum resin of Boswellia serrata in patients with chronic colitis. Planta Med. 2001, 67, 391–395. [Google Scholar] [CrossRef]
- Avtanski, D.; Poretsky, L. Phyto-polyphenols as potential inhibitors of breast cancer metastasis. Mol. Med. 2018, 24, 29. [Google Scholar] [CrossRef]
- Mocanu, M.M.; Nagy, P.; Szollosi, J. Chemoprevention of Breast Cancer by Dietary Polyphenols. Molecules 2015, 20, 22578–22620. [Google Scholar] [CrossRef] [Green Version]
- Lang, S.J.; Schmiech, M.; Hafner, S.; Paetz, C.; Steinborn, C.; Huber, R.; Gaafary, M.E.; Werner, K.; Schmidt, C.Q.; Syrovets, T.; et al. Antitumor activity of an Artemisia annua herbal preparation and identification of active ingredients. Phytomedicine 2019, 62, 152962. [Google Scholar] [CrossRef]
- Mathe, C.; Culioli, G.; Archier, P.; Vieillescazes, C. Characterization of archaeological frankincense by gas chromatography-mass spectrometry. J. Chromatogr. A 2004, 1023, 277–285. [Google Scholar] [CrossRef]
- Sterk, V.; Buchele, B.; Simmet, T. Effect of food intake on the bioavailability of boswellic acids from a herbal preparation in healthy volunteers. Planta Med. 2004, 70, 1155–1160. [Google Scholar] [CrossRef]
- Riva, A.; Morazzoni, P.; Artaria, C.; Allegrini, P.; Meins, J.; Savio, D.; Appendino, G.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. A single-dose, randomized, cross-over, two-way, open-label study for comparing the absorption of boswellic acids and its lecithin formulation. Phytomedicine 2016, 23, 1375–1382. [Google Scholar] [CrossRef] [Green Version]
- El Gaafary, M.; Buchele, B.; Syrovets, T.; Agnolet, S.; Schneider, B.; Schmidt, C.Q.; Simmet, T. An α-acetoxy-tirucallic acid isomer inhibits Akt/mTOR signaling and induces oxidative stress in prostate cancer cells. J. Pharmacol. Exp. Ther. 2015, 352, 33–42. [Google Scholar] [CrossRef]
- Estrada, A.C.; Syrovets, T.; Pitterle, K.; Lunov, O.; Buchele, B.; Schimana-Pfeifer, J.; Schmidt, T.; Morad, S.A.; Simmet, T. Tirucallic acids are novel pleckstrin homology domain-dependent Akt inhibitors inducing apoptosis in prostate cancer cells. Mol. Pharmacol. 2010, 77, 378–387. [Google Scholar] [CrossRef]
- Normenausschuss-Materialprüfung. Nachweis-, Erfassungs- und Bestimmungsgrenze. In DIN 32645; Beuth: Berlin, Germany, 1994. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |






| Compound | Regression Equation 1 | LOD 2 [ng/mg] | LOQ 2 [ng/mg] | Intraday and Interday Precision 3 (RSD [%]) | Recovery 4 [%] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low Level | Mid Level | High Level | |||||||||||
| Slope (a) | Offset (b) | R2 | Intraday | Interday | Intraday | Interday | Intraday | Interday | Mean | SD | |||
| KBA | 8.5009 | 0.0207 | 0.9992 | 0.7 | 2.6 | 7.6 | 6.8 | 6.4 | 2.0 | 7.0 | 4.5 | 97.7 | 4.9 |
| LA | 2.0181 | 0.0437 | 0.9984 | 1.9 | 7.2 | 9.3 | 6.8 | 5.8 | 5.1 | 9.8 | 4.8 | 99.8 | 3.6 |
| α-BA | 2.9393 | 0.0344 | 0.9997 | 1.0 | 6.0 | 9.9 | 7.4 | 7.2 | 7.4 | 7.7 | 3.8 | 91.2 | 4.6 |
| β-BA | 2.5022 | 0.0246 | 0.9997 | 1.6 | 5.9 | 6.4 | 6.3 | 5.9 | 4.7 | 7.1 | 3.9 | 95.1 | 1.9 |
| AKBA | 34.9050 | 0.1357 | 0.9998 | 0.4 | 1.5 | 9.4 | 7.9 | 5.8 | 6.4 | 7.8 | 2.3 | 94.7 | 5.0 |
| ALA | 20.5466 | 0.3225 | 0.9989 | 1.6 | 6.0 | 7.6 | 6.4 | 5.0 | 7.4 | 6.3 | 2.3 | 97.7 | 2.5 |
| α-ABA | 36.4455 | 0.4111 | 0.9998 | 0.9 | 3.5 | 6.9 | 6.0 | 5.4 | 6.4 | 7.5 | 2.1 | 98.7 | 1.5 |
| β-ABA | 37.0869 | 0.4314 | 0.9999 | 0.9 | 3.4 | 5.7 | 6.0 | 5.4 | 5.7 | 6.1 | 2.1 | 97.7 | 3.2 |
| Samples | Yield of Extraction (w/w) [%] | Concentrations of PTA in Boswellia oleogum Resins | IC50 [µg/mL] | Bosi | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deacetylated PTA [µg/mg] | Acetylated PTA [µg/mg] | Σ PTA [%] | MDA-MB-231 | |||||||||||||
| # | Species | Specification | Origin | KBA | LA | α-BA | β-BA | AKBA | ALA | α-ABA | β-ABA | Mean | SEM | |||
| 1 | B. sacra | Superior Hojari | Oman | 67.1 | 2.716 | 4.779 | 10.333 | 27.242 | 41.819 | 19.794 | 29.121 | 48.596 | 18.4 | 7.90 | 0.31 | 181,631 |
| 2 | B. sacra | Black Hojari | Oman | 64.2 | 2.834 | 4.683 | 7.304 | 20.174 | 29.178 | 17.971 | 17.292 | 30.624 | 13.0 | 8.70 | 1.20 | 35,612 |
| 3 | B. sacra | Royal Hojari | Oman | 63.1 | 1.221 | 2.566 | 4.142 | 13.110 | 34.373 | 16.908 | 20.736 | 35.942 | 12.9 | 8.08 | 0.22 | 168,427 |
| 4 | B. sacra | Royal Hojari | Oman | 57.7 | 1.101 | 5.810 | 6.437 | 21.131 | 32.909 | 29.762 | 25.259 | 49.536 | 17.2 | 8.84 | 0.36 | 216,446 |
| 5 | B. sacra | Red Hojari | Oman | 62.8 | 2.162 | 7.787 | 13.283 | 29.391 | 35.891 | 31.809 | 38.498 | 62.284 | 22.1 | 7.67 | 0.25 | 307,249 |
| 6 | B. sacra | Black Hojari | Oman | 49.7 | 2.294 | 3.256 | 7.668 | 24.805 | 21.773 | 10.203 | 16.939 | 31.230 | 11.8 | 8.99 | 0.07 | 13,137 |
| 7 | B. sacra | Royal Hojari | Oman | 53.6 | 1.616 | 4.067 | 7.635 | 22.360 | 37.469 | 26.093 | 27.182 | 38.505 | 16.5 | 7.62 | 0.69 | 98,912 |
| 8 | B. sacra | Superior Hojari | Oman | 61.1 | 2.100 | 4.196 | 6.705 | 18.180 | 33.344 | 25.456 | 18.955 | 32.406 | 14.1 | 8.63 | 1.16 | 68,063 |
| 9 | B. sacra | Najdi | Oman | 60.6 | 0.996 | 3.954 | 5.843 | 19.123 | 26.083 | 24.321 | 23.123 | 44.474 | 14.8 | 8.14 | 0.37 | 130,306 |
| 10 | B. sacra | Hojari | Oman | 59.3 | 1.087 | 2.950 | 4.651 | 18.614 | 21.776 | 13.042 | 18.792 | 39.895 | 12.1 | 7.33 | 0.26 | 74,933 |
| 11 | B. sacra | Sahli | Oman | 56.0 | 1.153 | 3.025 | 4.626 | 13.801 | 30.058 | 24.080 | 23.679 | 36.737 | 13.7 | 8.81 | 0.92 | 138,655 |
| Mean | 59.6 | 1.753 | 4.279 | 7.148 | 20.721 | 31.334 | 21.767 | 23.598 | 40.930 | 15.2 | 8.25 | |||||
| SD | 5.0 | 0.691 | 1.501 | 2.690 | 5.068 | 6.330 | 6.788 | 6.366 | 9.611 | 3.1 | 0.57 | |||||
| 12 | B. dalzielli | Janawhi | Burkina Faso | 73.8 | 11.663 | 5.152 | 13.250 | 17.384 | 53.425 | 14.117 | 27.349 | 31.446 | 17.4 | 11.44 | 0.82 | 134,345 |
| 13 | B. dalzielli | - | Nigeria | 68.5 | 9.859 | 9.334 | 16.226 | 20.266 | 72.197 | 21.618 | 39.248 | 37.964 | 22.7 | 8.99 | 0.58 | 303,439 |
| 14 | B. dalzielli | - | Senegal | 72.2 | 13.859 | 5.276 | 16.121 | 16.794 | 68.332 | 13.450 | 29.715 | 28.115 | 19.2 | 10.10 | 1.21 | 142,477 |
| Mean | 71.5 | 11.794 | 6.588 | 15.199 | 18.148 | 64.652 | 16.395 | 32.104 | 32.508 | 19.7 | 10.18 | |||||
| SD | 2.7 | 2.003 | 2.379 | 1.689 | 1.858 | 9.913 | 4.536 | 6.299 | 5.010 | 2.7 | 1.22 | |||||
| 15 | B. papyrifera | 1st Quality | Ethiopia | 65.7 | 4.384 | 3.689 | 10.920 | 23.645 | 43.941 | 10.575 | 21.379 | 30.049 | 14.9 | 9.47 | 0.09 | 34,172 |
| 16 | B. papyrifera | - | Eritrea | 66.4 | 3.406 | 2.375 | 5.549 | 16.434 | 27.844 | 6.591 | 14.259 | 19.871 | 9.6 | 11.65 | 1.03 | 7196 |
| 17 | B. papyrifera | - | Sudan | 70.0 | 3.187 | 3.033 | 10.331 | 31.762 | 16.809 | 9.175 | 20.171 | 46.505 | 14.1 | 10.12 | 0.80 | 32,469 |
| Mean | 67.4 | 3.659 | 3.032 | 8.933 | 23.947 | 29.531 | 8.780 | 18.603 | 32.142 | 12.9 | 10.41 | |||||
| SD | 2.3 | 0.638 | 0.657 | 2.946 | 7.668 | 13.645 | 2.021 | 3.810 | 13.439 | 2.8 | 1.12 | |||||
| 18 | B. serrata | 1st Quality | India | 59.1 | 5.330 | 4.949 | 16.373 | 49.706 | 11.324 | 6.055 | 11.693 | 36.453 | 14.2 | 8.81 | 0.85 | −9953 |
| 19 | B. serrata | 1st Quality | India | 62.0 | 22.290 | 10.157 | 25.308 | 49.748 | 18.692 | 6.581 | 11.703 | 30.560 | 17.5 | 9.81 | 1.39 | −21,112 |
| 20 | B. serrata | 1st Quality | India | 63.2 | 3.091 | 4.299 | 15.560 | 47.921 | 13.644 | 7.748 | 16.170 | 41.879 | 15.0 | 8.14 | 0.72 | −6994 |
| 21 | B. serrata | - | India | 57.1 | 5.793 | 5.548 | 13.212 | 54.020 | 8.561 | 3.867 | 7.420 | 23.143 | 12.2 | 8.29 | 0.87 | −8884 |
| 22 | B. serrata | cut | India | 58.4 | 9.797 | 5.589 | 15.345 | 42.671 | 13.257 | 5.069 | 10.118 | 29.361 | 13.1 | 7.82 | 0.15 | −9787 |
| 23 | B. serrata | ground | India | 65.8 | 6.256 | 2.493 | 8.924 | 28.286 | 9.253 | 3.440 | 6.181 | 19.756 | 8.5 | 11.62 | 0.53 | −2909 |
| 24 | B. serrata | ground | India | 55.6 | 4.047 | 3.567 | 12.167 | 38.301 | 8.068 | 4.635 | 8.421 | 23.630 | 10.3 | 10.89 | 0.96 | −4614 |
| Mean | 60.2 | 8.086 | 5.229 | 15.270 | 44.379 | 11.828 | 5.342 | 10.244 | 29.255 | 13.0 | 9.34 | |||||
| SD | 3.6 | 6.610 | 2.440 | 5.102 | 8.771 | 3.745 | 1.539 | 3.346 | 7.875 | 3.0 | 1.47 | |||||
| 25 | B. carterii | 1st Quality | Somalia | 61.1 | 0.080 | 10.850 | 11.829 | 38.053 | 0.103 | 23.494 | 19.450 | 46.412 | 15.0 | 10.38 | 0.65 | −779 |
| 26 | B. carterii | 1st Quality | Somalia | 66.7 | 0.016 | 15.168 | 15.501 | 57.312 | 0.060 | 47.071 | 33.760 | 84.567 | 25.3 | 7.28 | 0.18 | −5376 |
| 27 | B. carterii | green frankincense | Somalia | 49.4 | 0.143 | 15.406 | 28.861 | 66.660 | 0.001 | 0.073 | 0.014 | 0.130 | 11.1 | 9.42 | 0.25 | 75 |
| 28 | B. carterii | 1st Quality | Somalia (Puntland) | 59.8 | 0.865 | 19.782 | 36.476 | 83.868 | 0.006 | 0.042 | 0.033 | 0.134 | 14.1 | 9.83 | 0.94 | 95 |
| 29 | B. carterii | 2nd Quality | Somalia (Puntland) | 68.1 | 6.454 | 3.836 | 20.667 | 18.149 | 49.343 | 15.155 | 17.446 | 25.477 | 15.7 | 9.26 | 1.13 | 46,026 |
| Mean | 61.0 | 1.512 | 13.008 | 22.667 | 52.809 | 9.903 | 17.167 | 14.140 | 31.344 | 16.3 | 9.23 | |||||
| SD | 7.4 | 2.784 | 6.022 | 10.023 | 25.475 | 22.048 | 19.518 | 14.341 | 35.506 | 5.4 | 1.17 | |||||
| 30 | B. neglecta | Muqlo (black) | Somalia | 76.4 | 1.444 | 41.127 | 26.801 | 80.994 | 0.044 | 3.175 | 0.751 | 3.652 | 15.8 | 9.06 | 0.06 | 360 |
| 31 | B. neglecta | Muqlo&Gunro | Somalia | 84.4 | 1.565 | 35.831 | 26.739 | 75.827 | 0.143 | 10.829 | 2.618 | 13.088 | 16.7 | 7.89 | 0.86 | 888 |
| 32 | B. neglecta | Muqlo (black) | Somalia | 80.5 | 1.731 | 38.702 | 29.818 | 85.886 | 0.048 | 3.017 | 0.614 | 4.275 | 16.4 | 8.10 | 0.73 | 430 |
| 33 | B. neglecta | Mix | Somalia (Puntland) | 84.5 | 0.635 | 31.333 | 24.802 | 73.627 | 0.081 | 6.484 | 1.801 | 7.140 | 14.6 | 9.13 | 0.86 | 546 |
| 34 | B. neglecta | Mirafur | Somalia (Bakool/Mudug) | 49.4 | 0.240 | 7.711 | 7.217 | 20.613 | 0.025 | 1.997 | 0.490 | 2.613 | 4.1 | 8.73 | 1.53 | 71 |
| 35 | B. neglecta | 1st Quality (black) | Kenya | 42.3 | 0.062 | 0.836 | 1.920 | 3.212 | 0.018 | 0.416 | 0.081 | 0.794 | 0.7 | 12.85 | 1.14 | 5 |
| 36 | B. neglecta | 1st Quality (black) | Kenya | 68.3 | <LOQ | <LOQ | <LOQ | <LOQ | 0.002 | <LOQ | <LOQ | 0.004 | 0.0 | 8.63 | 0.60 | 0 |
| Mean | 69.4 | 0.811 | 22.220 | 16.757 | 48.594 | 0.052 | 3.703 | 0.908 | 4.510 | 9.8 | 9.20 | |||||
| SD | 17.1 | 0.752 | 18.524 | 13.088 | 38.757 | 0.048 | 3.801 | 0.958 | 4.454 | 7.8 | 1.67 | |||||
| 37 | B. rivae | 1st Quality | Ogaden | 64.6 | 0.038 | 0.049 | 0.052 | 0.307 | 0.055 | 0.099 | 0.056 | 0.194 | 0.1 | 11.10 | 0.97 | 0 |
| 38 | B. frereana | Maydi (Mujarwaal/Fas Kabir) | Somalia (Somaliland) | 90.6 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.0 | 15.72 | 3.16 | 0 |
| 39 | B. frereana | Maydi Mushaat | Somalia (Puntland) | 87.0 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.0 | 16.81 | 3.54 | 0 |
| 40 | B. frereana | Maydi Mujarwal | Somalia (Puntland) | 88.8 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.0 | 15.28 | 0.93 | 0 |
| Mean | 88.8 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.0 | 15.94 | |||||
| SD | 1.8 | 0.0 | 0.79 | |||||||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmiech, M.; Lang, S.J.; Werner, K.; Rashan, L.J.; Syrovets, T.; Simmet, T. Comparative Analysis of Pentacyclic Triterpenic Acid Compositions in Oleogum Resins of Different Boswellia Species and Their In Vitro Cytotoxicity against Treatment-Resistant Human Breast Cancer Cells. Molecules 2019, 24, 2153. https://doi.org/10.3390/molecules24112153
Schmiech M, Lang SJ, Werner K, Rashan LJ, Syrovets T, Simmet T. Comparative Analysis of Pentacyclic Triterpenic Acid Compositions in Oleogum Resins of Different Boswellia Species and Their In Vitro Cytotoxicity against Treatment-Resistant Human Breast Cancer Cells. Molecules. 2019; 24(11):2153. https://doi.org/10.3390/molecules24112153
Chicago/Turabian StyleSchmiech, Michael, Sophia J. Lang, Katharina Werner, Luay J. Rashan, Tatiana Syrovets, and Thomas Simmet. 2019. "Comparative Analysis of Pentacyclic Triterpenic Acid Compositions in Oleogum Resins of Different Boswellia Species and Their In Vitro Cytotoxicity against Treatment-Resistant Human Breast Cancer Cells" Molecules 24, no. 11: 2153. https://doi.org/10.3390/molecules24112153