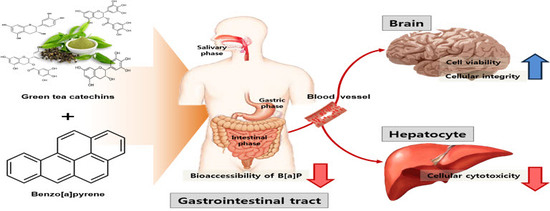
Catechins Controlled Bioavailability of Benzo[a]pyrene (B[α]P) from the Gastrointestinal Tract to the Brain towards Reducing Brain Toxicity Using the In Vitro Bio-Mimic System Coupled with Sequential Co-Cultures
Abstract
:1. Introduction
2. Results and Discussion
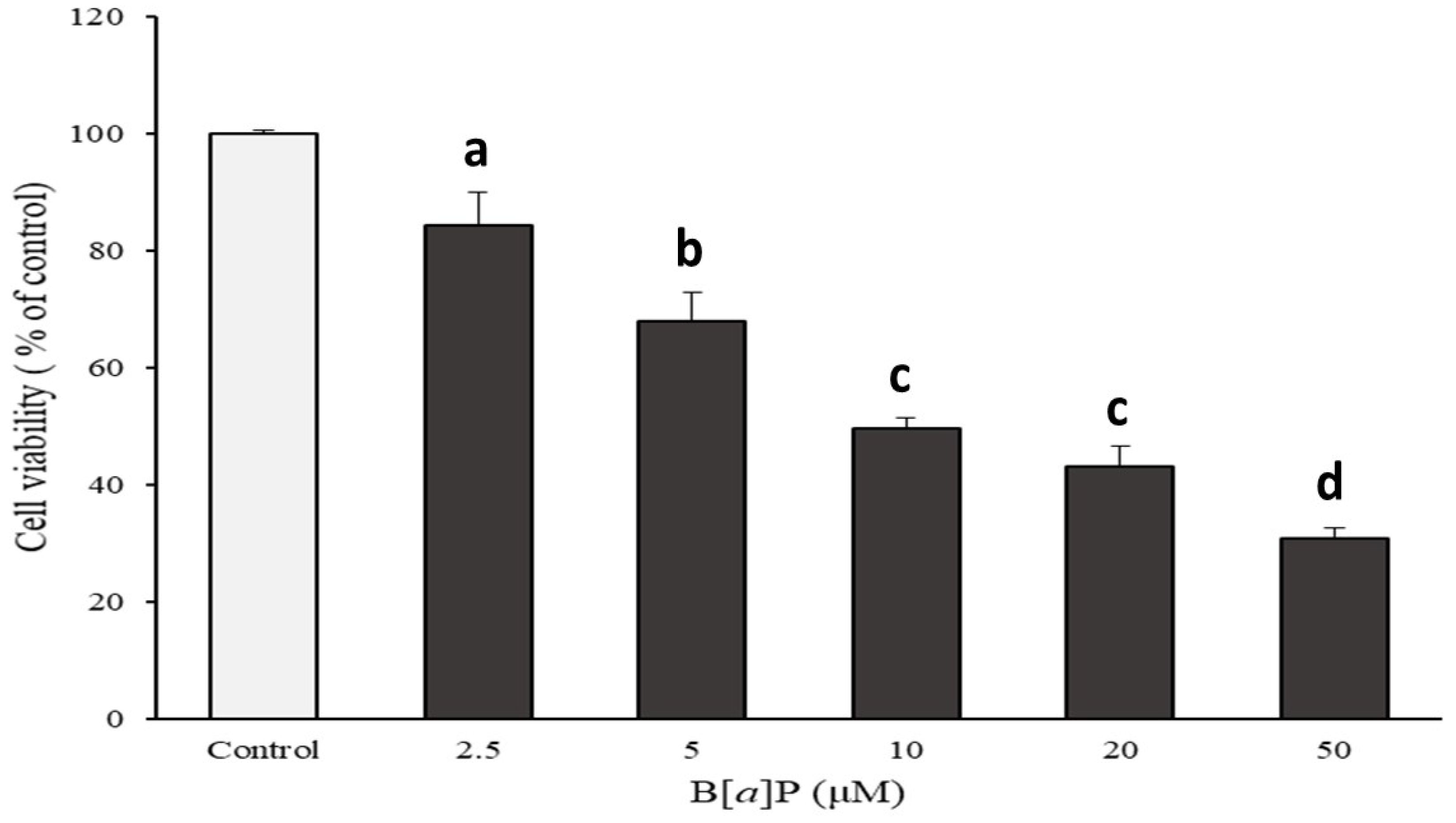
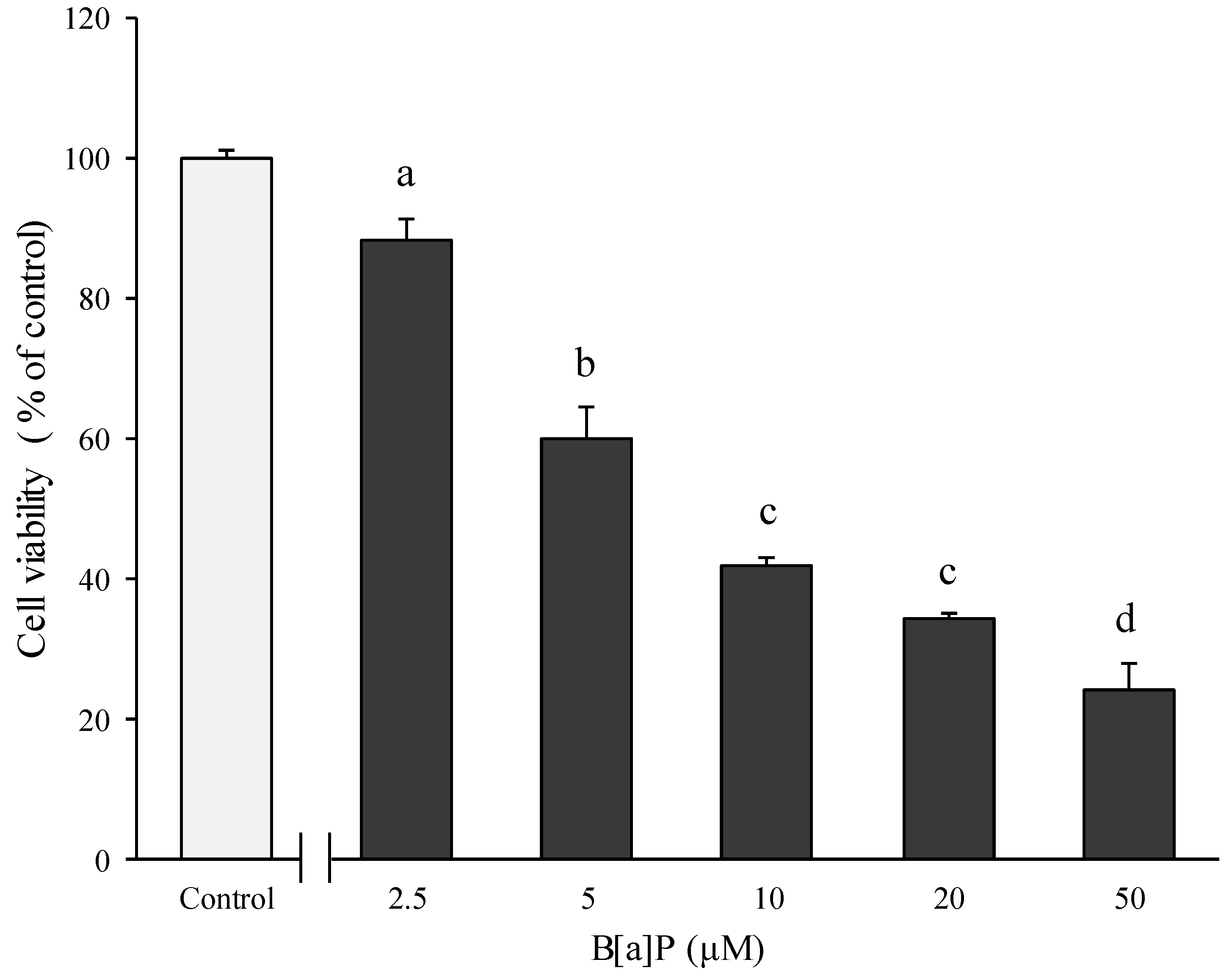
2.1. Preventive Effect of Catechins on Cell Cytotoxicity Induced by B[α]P in HepG2 and HBMECs
2.2. Preventive Effect of Catechins on Cellular Integrity of HBMECs Induced by B[α]P Exposure
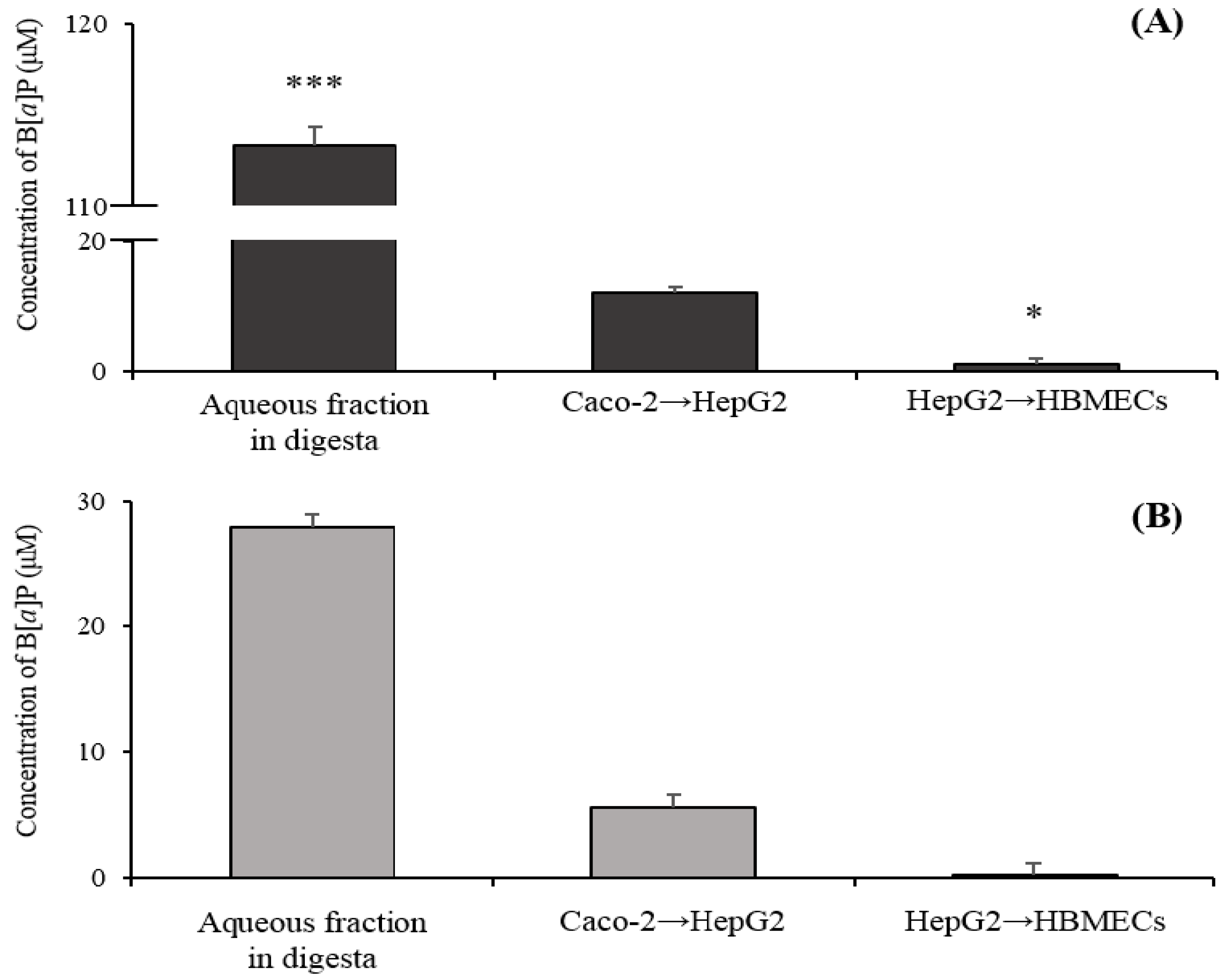
2.3. Transport of B[α]P from the Gastrointestinal Tract to the Brain by the In Vitro Bio-Mimic Model System Coupled with a Sequential Cell Culture that Included Caco-2, HepG2, and HBMECs
3. Materials and Methods
3.1. Chemicals and Standard Reagents
3.2. Cell Cultures
3.3. Measurement of Cell Viability
3.4. Transport of B[α]P from the Gastrointestinal Tract to the Brain by the In Vitro Bio-Mimic Model System Coupled with Caco-2 Cell, HepG2, and HBMECs
3.5. Analysis of B[α]P by UPLC
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Park, S.-Y.; Lee, S.-M.; Ye, S.-K.; Yoon, S.-H.; Chung, M.-H.; Choi, J. Benzo [a] pyrene-induced DNA damage and p53 modulation in human hepatoma HepG2 cells for the identification of potential biomarkers for PAH monitoring and risk assessment. Toxicol. Lett. 2006, 167, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Perfetti, T.; Rumple, M.; Rodgman, A.; Doolittle, D. “IARC group 2A Carcinogens” reported in cigarette mainstream smoke. Food Chem. Toxicol. 2000, 38, 371–383. [Google Scholar] [CrossRef]
- Kazerouni, N.; Sinha, R.; Hsu, C.-H.; Greenberg, A.; Rothman, N. Analysis of 200 food items for benzo [a] pyrene and estimation of its intake in an epidemiologic study. Food Chem. Toxicol. 2001, 39, 423–436. [Google Scholar] [CrossRef]
- Perera, F.; Tang, D.; Whyatt, R.; Lederman, S.A.; Jedrychowski, W. DNA damage from polycyclic aromatic hydrocarbons measured by benzo [a] pyrene-DNA adducts in mothers and newborns from Northern Manhattan, the World Trade Center Area, Poland, and China. Cancer Epidem. Biomar. 2005, 14, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Stumpe-Viksna, I.; Morozovs, A.; Bartkevics, V.; Kukare, A. Levels of benzo (a) pyrene (BaP) in fish, smoked according to different procedures. Proc. Latvia Univ. Agric. 2008, 21, 24–29. [Google Scholar]
- Lee, B.M.; Shim, G.A. Dietary exposure estimation of benzo[a]pyrene and cancer risk assessment. J. Toxicol. Environ. Health A 2007, 70, 1391–1394. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee. Evaluation of certain food contaminants: Sixty-fourth report of the Joint FAO/WHO Expert Committee on Food Additives. 2006. Available online: https://apps.who.int/iris/bitstream/handle/10665/43258/WHO_TRS_930_eng.pdf (accessed on 20 May 2019).
- Burczynski, M.E.; Penning, T.M. Genotoxic polycyclic aromatic hydrocarbon ortho-quinones generated by aldo-keto reductases induce CYP1A1 via nuclear translocation of the aryl hydrocarbon receptor. Cancer Res. 2000, 60, 908–915. [Google Scholar]
- Miller, K.P.; Ramos, K.S. Impact of cellular metabolism on the biological effects of benzo[a]pyrene and related hydrocarbons. Drug Metab. Rev. 2001, 33, 1–35. [Google Scholar] [CrossRef]
- Omidian, K.; Rafiei, H.; Bandy, B. Polyphenol inhibition of benzo[a]pyrene-induced oxidative stress and neoplastic transformation in an in vitro model of carcinogenesis. Food Chem. Toxicol. 2017, 106, 165–174. [Google Scholar] [CrossRef]
- Baird, W.M.; Hooven, L.A.; Mahadevan, B. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ. Mol. Mutagen. 2005, 45, 106–114. [Google Scholar] [CrossRef]
- Nesnow, S.; Nelson, G.; Padgett, W.T.; George, M.H.; Moore, T.; King, L.C.; Adams, L.D.; Ross, J.A. Lack of contribution of covalent benzo[a]pyrene-7,8-quinone-DNA adducts in benzo[a]pyrene-induced mouse lung tumorigenesis. Chem Biol Interact 2010, 186, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Chepelev, N.L.; Moffat, I.D.; Bowers, W.J.; Yauk, C.L. Neurotoxicity may be an overlooked consequence of benzo[a]pyrene exposure that is relevant to human health risk assessment. Mutat. Res. Rev. Mutat. Res. 2015, 764, 64–89. [Google Scholar] [CrossRef] [PubMed]
- Wormley, D.D.; Ramesh, A.; Hood, D.B. Environmental contaminant-mixture effects on CNS development, plasticity, and behavior. Toxicol. Appl. Pharmacol. 2004, 197, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Grova, N.; Valley, A.; Turner, J.D.; Morel, A.; Muller, C.P.; Schroeder, H. Modulation of behavior and NMDA-R1 gene mRNA expression in adult female mice after sub-acute administration of benzo(a)pyrene. Neurotoxicology 2007, 28, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.R.; Das, S.K.; Ramesh, A.; Shockley, D.C.; Mukherjee, S. Benzo(a)pyrene-induced acute neurotoxicity in the F-344 rat: Role of oxidative stress. J. Appl. Toxicol. 2006, 26, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Dutta, K.; Ghosh, D.; Nazmi, A.; Kumawat, K.L.; Basu, A. A common carcinogen benzo[a]pyrene causes neuronal death in mouse via microglial activation. PLoS ONE 2010, 5, e9984. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D. Dietary polyphenols as modulators of brain functions: Biological actions and molecular mechanisms underpinning their beneficial effects. Oxid Med Cell Longev 2012, 2012, 914273. [Google Scholar] [CrossRef]
- Ananingsih, V.K.; Sharma, A.; Zhou, W. Green tea catechins during food processing and storage: A review on stability and detection. Food Res. Int. 2013, 50, 469–479. [Google Scholar] [CrossRef]
- Cao, P.; Cai, J.; Gupta, R.C. Effect of Green Tea Catechins and Hydrolyzable Tannins on Benzo [a] pyrene-Induced DNA Adducts and Structure− Activity Relationship. Chem. Res. Toxicol. 2010, 23, 771–777. [Google Scholar] [CrossRef]
- Shim, S.-M.; Ferruzzi, M.G.; Kim, Y.-C.; Janle, E.M.; Santerre, C.R. Impact of phytochemical-rich foods on bioaccessibility of mercury from fish. Food Chem. 2009, 112, 46–50. [Google Scholar] [CrossRef]
- Girard, C.; Charette, T.; Leclerc, M.; Shapiro, B.J.; Amyot, M. Cooking and co-ingested polyphenols reduce in vitro methylmercury bioaccessibility from fish and may alter exposure in humans. Sci. Total Environ. 2018, 616–617, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.-H.; Lee, S.; Shim, S.-M. Screening bioactive components affecting the capacity of bile acid binding and pancreatic lipase inhibitory activity. Appl. Biol. Chem. 2016, 59, 475–479. [Google Scholar] [CrossRef]
- Jeong, K.-H.; Cho, S.-Y.; Hong, Y.-D.; Chung, J.-O.; Kim, K.-S.; Shim, S.-M. Transport of gallocatechin gallate and catechin gallate in high-temperature-processed green tea extract from gastrointestinal tract to brain by an in vitro bio-mimic model system coupled with sequential cell cultures. J. Funct. Foods 2018, 47, 83–90. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, J.H.; Cho, M.H.; Choe, E.S.; Kim, K.S.; Shim, S.M. Impact of commercial cigarette smoke condensate on brain tissue co-cultured with astrocytes and blood-brain barrier endothelial cells. J. Toxicol. Environ. Health A 2017, 80, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-K.; Yoon, J.-S.; Song, M.; Choi, H.-S.; Shin, C.-Y.; Kim, Y.-J.; Ryu, W.-I.; Lee, H.-S.; Ryu, J.-C. Gene expression analysis identifies DNA damage-related markers of benzo[a]pyrene exposure in HepG2 human hepatocytes. Toxicol. Env. Health Sci. 2012, 4, 19–29. [Google Scholar] [CrossRef]
- Ueno, M.; Masutani, H.; Arai, R.J.; Yamauchi, A.; Hirota, K.; Sakai, T.; Inamoto, T.; Yamaoka, Y.; Yodoi, J.; Nikaido, T. Thioredoxin-dependent redox regulation of p53-mediated p21 activation. J. Biol. Chem. 1999, 274, 35809–35815. [Google Scholar] [CrossRef] [PubMed]
- Ovesen, J.L.; Schnekenburger, M.; Puga, A. Aryl hydrocarbon receptor ligands of widely different toxic equivalency factors induce similar histone marks in target gene chromatin. Toxicol. Sci. 2011, 121, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Muto, S.; Fujita, K.-i.; Yamazaki, Y.; Kamataki, T. Inhibition by green tea catechins of metabolic activation of procarcinogens by human cytochrome P450. Mutat. Res.-Fund. Mol. Mech. Mutagenesis 2001, 479, 197–206. [Google Scholar] [CrossRef]
- Palermo, C.; Hernando, J.M.; Dertinger, S.; Kende, A.; Gasiewicz, T. Identification of potential aryl hydrocarbon receptor antagonists in green tea. Chem. Res. Toxicol. 2003, 16, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sharma, V.; Sehgal, A.; Jain, M. Protective effects of green and white tea against benzo(a)pyrene induced oxidative stress and DNA damage in murine model. Nutr. Cancer 2012, 64, 300–306. [Google Scholar] [CrossRef]
- Suzuki, T.; Hara, H. Role of flavonoids in intestinal tight junction regulation. J. Nutr. Biochem. 2011, 22, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Khanna, D. Green tea catechins: Defensive role in cardiovascular disorders. Chin. J. Nat. Med. 2013, 11, 345–353. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, J.; Cai, Y.; Huang, J.; You, L. Catechin attenuates traumatic brain injury-induced blood-brain barrier damage and improves longer-term neurological outcomes in rats. Exp. Physiol. 2017, 102, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Hirose, S.; Nagaoka, S.; Yanase, E. Interaction between tea polyphenols and bile acid inhibits micellar cholesterol solubility. J. Agric. Food Chem. 2015, 64, 204–209. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Shi, B.; Yao, K. Interactions of gallotannins with proteins, amino acids, phospholipids and sugars. Food Chem. 2006, 95, 250–254. [Google Scholar] [CrossRef]
- He, Q.; Lv, Y.; Yao, K. Effects of tea polyphenols on the activities of α-amylase, pepsin, trypsin and lipase. Food Chem. 2007, 101, 1178–1182. [Google Scholar] [CrossRef]
- Russell, M. In Vitro Model for Intestinal Uptake of benzo[a]pyrene. 1999. Available online: https://escholarship.org/uc/item/5k06t6rr (accessed on 5 June 2019).
- Brand, W.; Schutte, M.E.; Williamson, G.; van Zanden, J.J.; Cnubben, N.H.; Groten, J.P.; van Bladeren, P.J.; Rietjens, I.M. Flavonoid-mediated inhibition of intestinal ABC transporters may affect the oral bioavailability of drugs, food-borne toxic compounds and bioactive ingredients. Biomed. Pharmacother. 2006, 60, 508–519. [Google Scholar] [CrossRef]
- Romiti, N.; Tramonti, G.; Donati, A.; Chieli, E. Effects of grapefruit juice on the multidrug transporter P-glycoprotein in the human proximal tubular cell line HK-2. Life Sci. 2004, 76, 293–302. [Google Scholar] [CrossRef]
- Zhang, S.; Morris, M.E. Effect of the flavonoids biochanin A and silymarin on the P-glycoprotein-mediated transport of digoxin and vinblastine in human intestinal Caco-2 cells. Pharm. Res. 2003, 20, 1184–1191. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, K.-H.; Lee, H.J.; Park, T.-S.; Shim, S.-M. Catechins Controlled Bioavailability of Benzo[a]pyrene (B[α]P) from the Gastrointestinal Tract to the Brain towards Reducing Brain Toxicity Using the In Vitro Bio-Mimic System Coupled with Sequential Co-Cultures. Molecules 2019, 24, 2175. https://doi.org/10.3390/molecules24112175
Jeong K-H, Lee HJ, Park T-S, Shim S-M. Catechins Controlled Bioavailability of Benzo[a]pyrene (B[α]P) from the Gastrointestinal Tract to the Brain towards Reducing Brain Toxicity Using the In Vitro Bio-Mimic System Coupled with Sequential Co-Cultures. Molecules. 2019; 24(11):2175. https://doi.org/10.3390/molecules24112175
Chicago/Turabian StyleJeong, Kang-Hyun, Hyun Jeong Lee, Tae-Sik Park, and Soon-Mi Shim. 2019. "Catechins Controlled Bioavailability of Benzo[a]pyrene (B[α]P) from the Gastrointestinal Tract to the Brain towards Reducing Brain Toxicity Using the In Vitro Bio-Mimic System Coupled with Sequential Co-Cultures" Molecules 24, no. 11: 2175. https://doi.org/10.3390/molecules24112175
APA StyleJeong, K.-H., Lee, H. J., Park, T.-S., & Shim, S.-M. (2019). Catechins Controlled Bioavailability of Benzo[a]pyrene (B[α]P) from the Gastrointestinal Tract to the Brain towards Reducing Brain Toxicity Using the In Vitro Bio-Mimic System Coupled with Sequential Co-Cultures. Molecules, 24(11), 2175. https://doi.org/10.3390/molecules24112175