Rubus ulmifolius Schott as a Novel Source of Food Colorant: Extraction Optimization of Coloring Pigments and Incorporation in a Bakery Product
Abstract
:1. Introduction
2. Results and Discussion
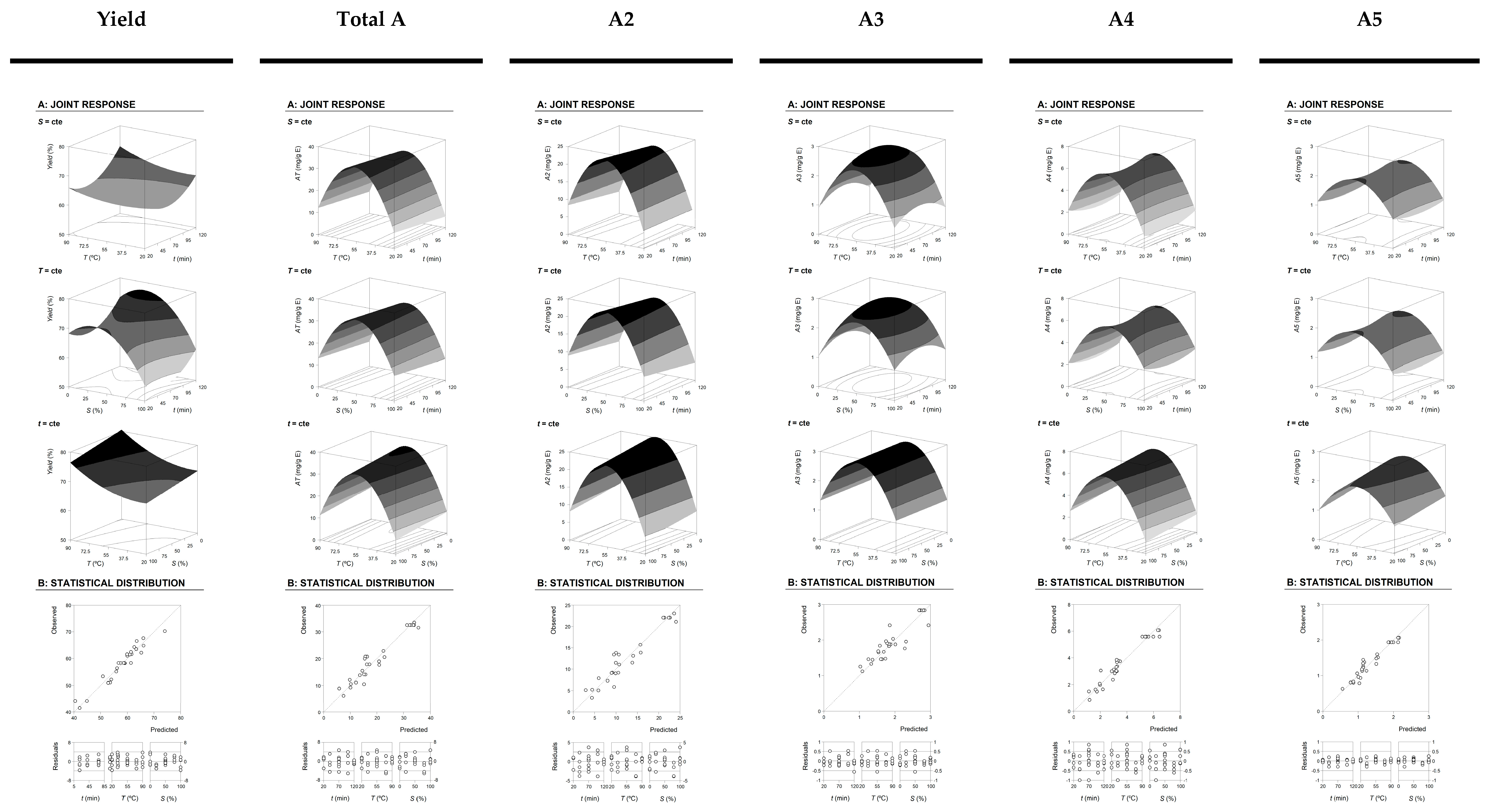
2.1. Extraction Optimization by RSM Method
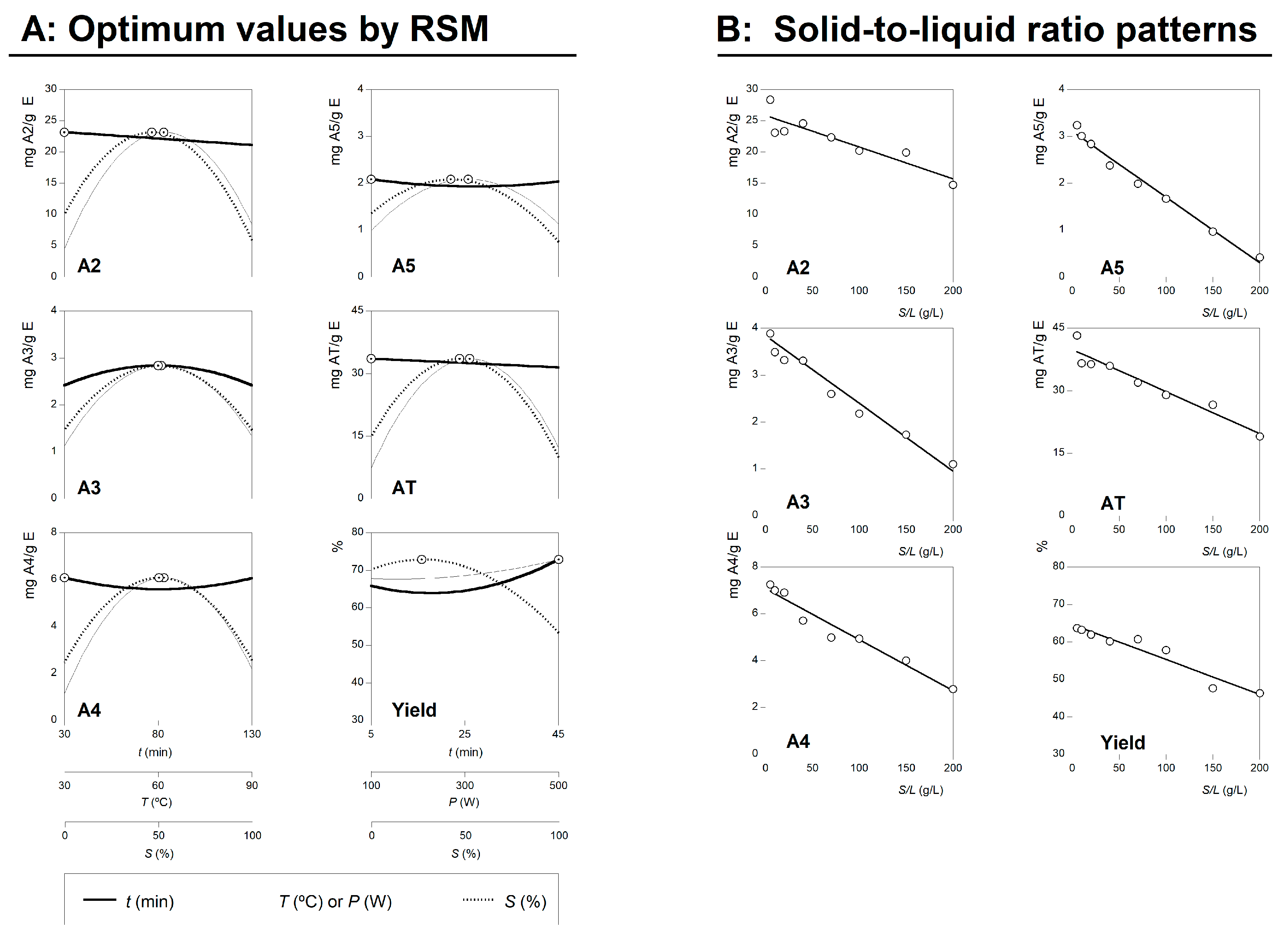
2.1.1. Numerical Optimal Conditions that Maximize the Extraction and Experimental Verification of Predictive Models
2.1.2. Dose-Response Analysis of the Solid-to-Liquid Ratio Effect at the Optimal Conditions
2.2. Coloring Potential, Cytotoxicity and Antimicrobial Activity of the Rich Anthocyanin Extract
3. Material and Methods
3.1. Samples
3.2. Extraction Technique
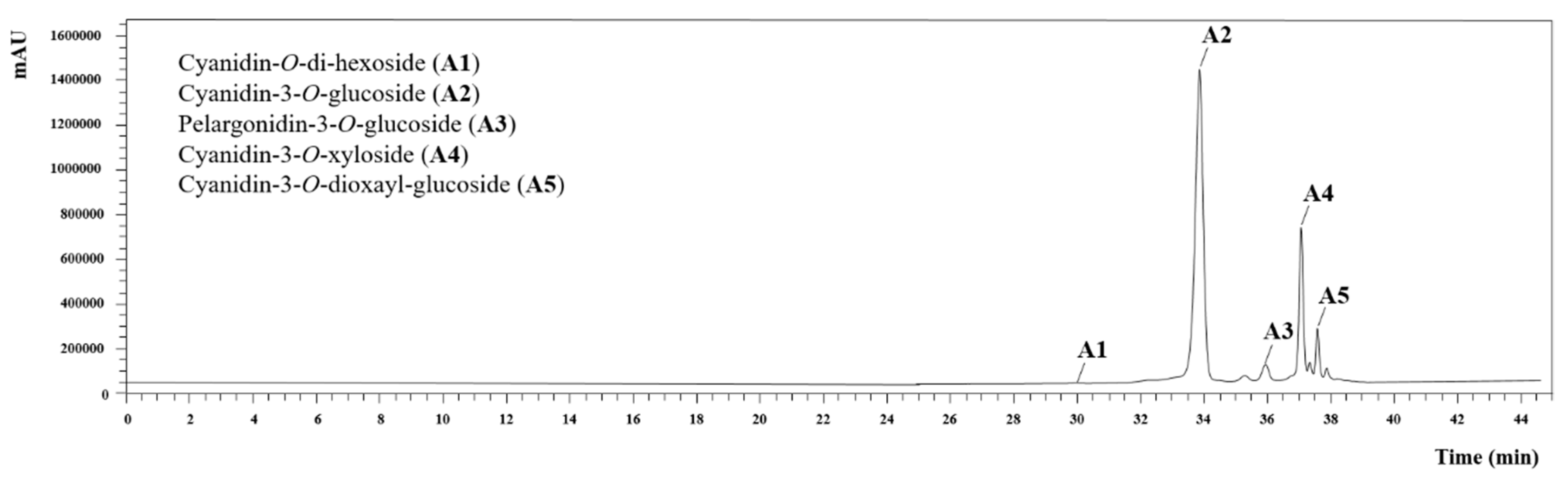
3.3. Identification and Quantification of Anthocyanin Compounds
3.4. Response Format Values for the Results Presentation
3.5. Experimental Design, Model Analysis and Statistical Evaluation
3.5.1. RSM Experimental Design
3.5.2. Mathematical Model
3.5.3. Procedure to Optimize the Variables to a Maximum Response
3.5.4. Dose-Response Analysis of the Solid to Liquid Ratio
3.6. Numerical Methods, Statistical Analysis, and Graphical Illustrations
- The measurement of the coefficients was achieved using the nonlinear least-square (quasi-Newton) method provided by the macro “Solver”, by minimization of the sum of the quadratic differences between the observed and model-predicted values.
- The significance of the coefficients was obtained via “SolverAid” macro to determine the parametric confidence intervals. The terms that were not statistically significant (p-value > 0.05) were excluded to simplify the model.
- The model reliability was confirmed by applying the following criteria: a) the Fisher F-test (α = 0.05) was used to determine the consistency of the constructed models to describe the obtained data; b) the “SolverStat” macro was used to make an assessment of the parameter and model prediction uncertainties; c) R² was determined to explain the variability proportion of the dependent variable obtained by the model.
3.7. Preparation of the Optimal Extract Rich in Anthocyanin Compounds
3.8. Evaluation of Coloring Potential of the Rich Anthocyanin Extract
3.9. Bioactivity Evaluation of the Rich Anthocyanin Extract
3.9.1. Cytotoxic and Hepatotoxic Potential
3.9.2. Antimicrobial Activity
3.10. Incorporation of the Rich Anthocyanin Extract in a Bakery Product
3.10.1. Donuts preparation
3.10.2. Evaluation of the Color Parameters in Donut Samples
3.10.3. Evaluation of the Nutritional and Chemical Properties of the Donuts
Nutritional Value
Chemical Composition
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arici, M.; Karasu, S.; Baslar, M.; Toker, O.; Sagdic, O.; Karaagacli, M. Tulip petal as a novel natural food colorant source: Extraction optimization and stability studies. Ind. Crops Prod. 2016, 91, 215–222. [Google Scholar] [CrossRef]
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends Food Sci. Technol. 2016, 52, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Prado, M.; Godoy, H. Corantes artificiais em alimentos. Braz. J. Food Nutr. 2003, 14, 237–250. [Google Scholar]
- Martins, N.; Ferreira, I.C.F.R. Mountain food products: A broad spectrum of market potential to be exploited. Trends Food Sci. Technol. 2017, 67, 12–18. [Google Scholar] [CrossRef]
- Zhang, N.; Ju, Z.; Zuo, T. Time for food: The impact of diet on gut microbiota and human health. Nutrition 2018, 51–52, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Blackhall, M.L.; Berry, R.; Davies, N.W.; Walls, J.T. Optimized extraction of anthocyanins from Reid Fruits Prunus avium ‘Lapins’ cherries. Food Chem. 2018, 256, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Pereira, E.; Jabeur, I.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Optimization of heat- and ultrasound-assisted extraction of anthocyanins from Hibiscus sabdariffa calyces for natural food colorants. Food Chem. 2019, 275, 309–321. [Google Scholar] [CrossRef]
- Backes, E.; Pereira, C.; Barros, L.; Prieto, M.A.; Genena, A.K.; Barreiro, M.F.; Ferreira, I.C.F.R. Recovery of bioactive anthocyanin pigments from Ficus carica L. peel by heat, microwave, and ultrasound based extraction techniques. Food Res. Int. 2018, 113, 197–209. [Google Scholar] [CrossRef]
- López, C.J.; Caleja, C.; Prieto, M.A.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Optimization and comparison of heat and ultrasound assisted extraction techniques to obtain anthocyanin compounds from Arbutus unedo L. Fruits. Food Chem. 2018, 264, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Mojica, L.; Berhow, M.; Gonzalez de Mejia, E. Black bean anthocyanin-rich extracts as food colorants: Physicochemical stability and antidiabetes potential. Food Chem. 2017, 229, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.R.C.; Murador, D.C.; de Souza Mesquita, L.M.; de Rosso, V.V. Bioavailability of anthocyanins: Gaps in knowledge, challenges and future research. J. Food Compos. Anal. 2018, 68, 31–40. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Culver, C.A. Alternatives to Those Artificial FD&C Food Colorants. Annu. Rev. Food Sci. Technol. 2012, 3, 59–77. [Google Scholar]
- Rodriguez-Amaya, D.B. Update on natural food pigments—A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2018. [Google Scholar] [CrossRef]
- Joaquín-Cruz, E.; Dueñas, M.; García-Cruz, L.; Salinas-Moreno, Y.; Santos-Buelga, C.; García-Salinas, C. Anthocyanin and phenolic characterization, chemical composition and antioxidant activity of chagalapoli (Ardisia compressa K.) fruit: A tropical source of natural pigments. Food Res. Int. 2015, 70, 151–157. [Google Scholar]
- Kaume, L.; Howard, L.R.; Devareddy, L. The blackberry fruit: A review on its composition and chemistry, metabolism and bioavailability, and health benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. [Google Scholar] [CrossRef]
- Reidel, R.; Melai, B.; Cioni, P.; Flamini, G.; Pistelli, L. Aroma Profile of Rubus ulmifolius Flowers and Fruits During Different Ontogenetic Phases. Chem. Biodivers. 2016, 13, 1776–1784. [Google Scholar] [CrossRef]
- Cassidy, A. Berry anthocyanin intake and cardiovascular health. Mol. Aspects Med. 2018, 61, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Mane, S.; Bremner, D.H.; Tziboula-Clarke, A.; Lemos, M.A. Effect of ultrasound on the extraction of total anthocyanins from Purple Majesty potato. Ultrasonics Sonochem. 2015, 27, 509–514. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, E.A.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Pinela, J.; Prieto, M.A.; Carvalho, A.M.; Barreiro, M.F.; Oliveira, M.B.P.; Barros, L.; Ferreira, I.C.F.R. Microwave-assisted extraction of phenolic acids and flavonoids and production of antioxidant ingredients from tomato: A nutraceutical-oriented optimization study. Sep. Pur. Technol. 2016, 164, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Oludemi, T.; Barros, L.; Prieto, M.A.; Heleno, S.A.; Barreiro, M.F.; Ferreira, I.C.F.R. Extraction of triterpenoids and phenolic compounds from: Ganoderma lucidum: Optimization study using the response surface methodology. Food Funct. 2018, 9, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-J.; Lu, H.; Yu, X.-L.; Song, W.-B.; Zhang, S.-W.; Fen, L.-Y.; Xuan, L.-J. Anti-inflammatory secoiridoid glycosides from Gentianella azurea. Bioorg. Med. Chem. Lett. 2014, 24, 5260–5264. [Google Scholar] [CrossRef] [PubMed]
- Pertuzatti, P.B.; Barcia, M.T.; Rebello, L.P.G.; Gómez-Alonso, S.; Duarte, R.M.T.; Duarte, M.C.T.; Godoy, H.T.; Hermosín-Gutiérrez, I. Antimicrobial activity and differentiation of anthocyanin profiles of rabbiteye and highbush blueberries using HPLC–DAD–ESI-MSn and multivariate analysis. J. Funct. Foods 2016, 26, 506–516. [Google Scholar] [CrossRef]
- Mollica, A.; Stefanucci, A.; Macedonio, G.; Locatelli, M.; Luisi, G.; Novellino, E.; Zengin, G. Chemical composition and biological activity of Capparis spinosa L. from Lipari Island. S. Afr. J. Bot. 2018. [Google Scholar] [CrossRef]
- Albano, M.; Alves, F.C.B.; Andrade, B.F.M.T.; Barbosa, L.N.; Pereira, A.F.M.; da Cunha, M.d.L.R.d.S.; Rall, V.L.M.; Júnior, A.F. Antibacterial and anti-staphylococcal enterotoxin activities of phenolic compounds. Innov. Food Sci. Emer. Technol. 2016, 38, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Mingo, E.; Silván, J.M.; Martinez-Rodriguez, A.J. Selective antibacterial effect on Campylobacter of a winemaking waste extract (WWE) as a source of active phenolic compounds. LWT Food Sci. Technol. 2016, 68, 418–424. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, T.; Wei, C.; Lan, W.; Zhao, Y.; Pan, Y.; Wu, V.C. Antibacterial effect and mechanism of anthocyanin rich Chinese wild blueberry extract on various foodborne pathogens. Food Control 2018, 94, 155–161. [Google Scholar] [CrossRef]
- Pearline, D.; Kamat, N.; Thiagarajan, P. Rosa sinensis (Hibiscus)-a versatile Indian origin plant. J. Chem. Pharm. Sci. 2015, 8, 970–974. [Google Scholar]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C.F.R. Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, H.; Fang, T.; Lin, Q.; Song, H.; Liu, B.; Chen, L. Red raspberry and its anthocyanins: Bioactivity beyond antioxidant capacity. Trends Food Sci. Technol. 2017, 66, 153–165. [Google Scholar] [CrossRef]
- Dellai, A.; Laajili, S.; Le Morvan, V.; Robert, J.; Bouraoui, A. Antiproliferative activity and phenolics of the Mediterranean seaweed Laurencia obusta. Ind. Crops Prod. 2013, 47, 252–255. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Pereira, E.; Pires, T.C.S.P.; Alves, M.J.; Pereira, O.R.; Barros, L.; Ferreira, I.C.F.R. Rubus ulmifolius Schott fruits: A detailed study of its nutritional, chemical and bioactive properties. Food Res. Int. 2019, 119, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Prieto, M.A.; Barros, L.; Rodrigues, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Optimization of microwave-assisted extraction of ergosterol from Agaricus bisporus L. by-products using response surface methodology. Food Bioprod. Process. 2016, 100, 25–35. [Google Scholar] [CrossRef]
- Vieira, V.; Prieto, M.A.; Barros, L.; Coutinho, J.A.P.; Ferreira, O.; Ferreira, I.C.F.R. Optimization and comparison of maceration and microwave extraction systems for the production of phenolic compounds from Juglans regia L. for the valorization of walnut leaves. Ind. Crops Prod. 2017, 107, 341–352. [Google Scholar] [CrossRef]
- Prieto, M.A.; Vázquez, J.A. In vitro determination of the lipophilic and hydrophilic antioxidant capacity of unroasted coffee bean extracts and their synergistic and antagonistic effects. Food Res. Int. 2014, 62, 1183–1196. [Google Scholar] [CrossRef] [Green Version]
- Murado García, M.A.; Prieto Lage, M.Á. Dose-Response Analysis in the Joint Action of Two Effectors. A New Approach to Simulation, Identification and Modelling of Some Basic Interactions. PLoS ONE 2013, 8, e61391. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.F.R.; Martins, A.; Pintado, M. Antimicrobial activity of wild mushroom extracts against clinical isolates resistant to different antibiotics. J. Appl. Microbiol. 2012, 113, 466–475. [Google Scholar] [CrossRef]
- Latimer, G.W., Jr. (Ed.) AOAC Official Methods of Analysis of AOAC INTERNATIONAL, 20th ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- Chahdoura, H.; Barreira, J.C.; Fernández-Ruiz, V.; Morales, P.; Calhelha, R.C.; Flamini, G.; Sokovic, M.; Ferreira, I.C.; Achour, L. Bioactivity, proximate, mineral and volatile profiles along the flowering stages of Opuntia microdasys (Lehm.): Defining potential applications. Food Funct. 2016, 7, 1458–1467. [Google Scholar] [PubMed]
Sample Availability: Samples of the fruits are available from the authors. |


| A) Experimental Design And Response Results | ||||||||||||
| Coded and Natural | Extract | Individual Compounds Content | Total | |||||||||
| X1 | X2 | X3 | X1:t min | X2:T °C | X3:S % | Yield % | A2 mg/g E | A3 mg/g E | A4 mg/g E | A5 mg/g E | AT mg/g E | |
| 1 | −1 | −1 | −1 | 40.3 | 34.2 | 20.3 | 59.89 | 10.68 | 1.58 | 2.04 | 1.14 | 15.44 |
| 2 | −1 | −1 | 1 | 40.3 | 34.2 | 79.7 | 53.52 | 9.97 | 1.83 | 3.27 | 1.14 | 16.21 |
| 3 | −1 | 1 | −1 | 40.3 | 75.8 | 20.3 | 61.33 | 15.71 | 1.95 | 3.25 | 1.54 | 22.45 |
| 4 | −1 | 1 | 1 | 40.3 | 75.8 | 79.7 | 55.83 | 10.01 | 1.83 | 3.35 | 1.14 | 16.33 |
| 5 | 1 | −1 | −1 | 99.7 | 34.2 | 20.3 | 62.55 | 13.83 | 2.28 | 3.24 | 1.52 | 20.86 |
| 6 | 1 | −1 | 1 | 99.7 | 34.2 | 79.7 | 52.80 | 10.76 | 1.78 | 3.48 | 1.17 | 17.20 |
| 7 | 1 | 1 | −1 | 99.7 | 75.8 | 20.3 | 63.47 | 15.79 | 2.31 | 3.21 | 1.52 | 22.83 |
| 8 | 1 | 1 | 1 | 99.7 | 75.8 | 79.7 | 56.12 | 9.53 | 1.75 | 3.23 | 1.14 | 15.65 |
| 9 | 1.68 | 0 | 0 | 120 | 55 | 50 | 66.06 | 24.06 | 2.94 | 6.41 | 2.13 | 35.55 |
| 10 | −1.68 | 0 | 0 | 20 | 55 | 50 | 60.88 | 23.63 | 1.85 | 6.33 | 2.16 | 33.97 |
| 11 | 0 | −1.68 | 0 | 70 | 20 | 50 | 59.06 | 4.36 | 1.08 | 1.19 | 0.86 | 7.50 |
| 12 | 0 | 1.68 | 0 | 70 | 90 | 50 | 63.31 | 8.04 | 1.33 | 1.75 | 1.06 | 12.18 |
| 13 | 0 | 0 | −1.68 | 70 | 55 | 0 | 61.59 | 5.95 | 1.25 | 1.64 | 0.97 | 9.82 |
| 14 | 0 | 0 | 1.68 | 70 | 55 | 100 | 44.82 | 9.56 | 1.64 | 2.95 | 1.03 | 15.18 |
| 15 | −1.68 | −1.68 | −1.68 | 20 | 20 | 0 | 65.19 | 10.55 | 1.73 | 2.20 | 1.23 | 15.72 |
| 16 | −1.68 | −1.68 | 1.68 | 20 | 20 | 100 | 40.44 | 5.89 | 1.37 | 1.96 | 0.87 | 10.08 |
| 17 | −1.68 | 1.68 | −1.68 | 20 | 90 | 0 | 59.97 | 14.02 | 2.01 | 3.21 | 1.56 | 20.81 |
| 18 | −1.68 | 1.68 | 1.68 | 20 | 90 | 100 | 53.96 | 9.90 | 1.60 | 3.09 | 0.56 | 15.15 |
| 19 | 1.68 | −1.68 | −1.68 | 120 | 20 | 0 | 65.99 | 2.91 | 1.03 | 1.15 | 0.79 | 5.87 |
| 20 | 1.68 | −1.68 | 1.68 | 120 | 20 | 100 | 42.16 | 4.43 | 1.69 | 3.01 | 1.11 | 10.24 |
| 21 | 1.68 | 1.68 | −1.68 | 120 | 90 | 0 | 74.03 | 8.97 | 1.54 | 1.98 | 1.11 | 13.60 |
| 22 | 1.68 | 1.68 | 1.68 | 120 | 90 | 100 | 50.83 | 9.15 | 1.53 | 2.86 | 1.00 | 14.54 |
| 23 | 0 | 0 | 0 | 70 | 55 | 50 | 61.33 | 21.33 | 2.80 | 5.15 | 2.00 | 31.27 |
| 24 | 0 | 0 | 0 | 70 | 55 | 50 | 57.47 | 22.34 | 3.00 | 6.45 | 2.13 | 33.92 |
| 25 | 0 | 0 | 0 | 70 | 55 | 50 | 58.93 | 22.34 | 2.69 | 5.37 | 1.86 | 32.26 |
| 26 | 0 | 0 | 0 | 70 | 55 | 50 | 57.47 | 22.60 | 2.73 | 5.45 | 1.94 | 32.73 |
| 27 | 0 | 0 | 0 | 70 | 55 | 50 | 58.56 | 21.09 | 2.68 | 5.62 | 1.87 | 31.26 |
| 28 | 0 | 0 | 0 | 70 | 55 | 50 | 56.64 | 22.63 | 3.09 | 5.98 | 1.94 | 33.65 |
| B) Parametric Values and Optimal Variable Conditions | ||||||||||||
| Extract | Individual Compounds Content | Total | ||||||||||
| Yield | A2 | A3 | A4 | A5 | AT | |||||||
| B1) Parametric Information and Statistical Information | ||||||||||||
| Intercept | b0 | 58.312 ± 0.834 | 22.091 ± 0.654 | 2.837 ± 0.105 | 5.587 ± 0.177 | 1.934 ± 0.054 | 32.502 ± 0.868 | |||||
| Linear effect | b1 | 0.983 ± 0.344 | −0.573 ± 0.325 | ns | ns | ns | −0.569 ± 0.432 | |||||
| b2 | 1.577 ± 0.344 | 1.177 ± 0.325 | 0.062 ± 0.043 | 0.185 ± 0.073 | 0.030 ± 0.022 | 1.454 ± 0.432 | ||||||
| b3 | −5.182 ± 0.344 | −0.595 ± 0.325 | ns | 0.215 ± 0.073 | −0.081 ± 0.022 | −0.475 ± 0.432 | ||||||
| Quadratic effect | b11 | 1.711 ± 0.584 | ns | −0.150 ± 0.073 | 0.170 ± 0.124 | 0.046 ± 0.038 | ns | |||||
| b22 | 0.902 ± 0.584 | −5.938 ± 0.539 | −0.571 ± 0.073 | −1.563 ± 0.124 | −0.372 ± 0.038 | −8.458 ± 0.715 | ||||||
| b33 | −1.918 ± 0.584 | −5.387 ± 0.539 | −0.487 ± 0.073 | −1.271 ± 0.124 | −0.358 ± 0.038 | −7.518 ± 0.715 | ||||||
| Interactive effect | b12 | 0.337 ± 0.244 | ns | ns | −0.079 ± 0.052 | ns | ns | |||||
| b13 | −0.712 ± 0.244 | 0.371 ± 0.231 | 0.039 ± 0.031 | 0.107 ± 0.052 | 0.058 ± 0.016 | 0.576 ± 0.306 | ||||||
| b23 | 0.806 ± 0.244 | ns | −0.034 ± 0.031 | −0.053 ± 0.052 | −0.048 ± 0.016 | ns | ||||||
| Additional complex effect | b1122 | ns | ns | ns | ns | ns | ns | |||||
| b1133 | ns | ns | ns | ns | ns | ns | ||||||
| b2233 | −0.469 ± 0.363 | 2.256 ± 0.259 | 0.268 ± 0.046 | 0.542 ± 0.077 | 0.128 ± 0.024 | 3.220 ± 0.344 | ||||||
| Statistics (R2) | 0.9336 | 0.9276 | 0.8908 | 0.9411 | 0.9294 | 0.9330 | ||||||
| B2) Optimal Variable Conditions for Response Maximization | ||||||||||||
| Individual optimal conditions | Time | 120.00 ± 3.60 | 20.00 ± 0.20 | 69.99 ± 3.50 | 20.00 ± 1.60 | 20.00 ± 1.00 | 20.00 ± 1.00 | |||||
| Temperature | 90.00 ± 9.00 | 57.07 ± 0.57 | 56.13 ± 2.25 | 57.11 ± 4.00 | 56.20 ± 3.37 | 56.79 ± 1.14 | ||||||
| Solvent | 26.97 ± 2.16 | 46.62 ± 0.93 | 49.94 ± 4.49 | 50.34 ± 5.03 | 42.42 ± 2.12 | 47.14 ± 0.94 | ||||||
| Response | 72.91 ± 3.54 | 23.18 ± 3.54 | 2.84 ± 3.54 | 6.09 ± 3.54 | 2.09 ± 3.54 | 33.59 ± 3.54 | ||||||
| Global optimal conditions | Time | 20.00 ± 0.60 | ||||||||||
| Temperature | 56.87 ± 3.41 | |||||||||||
| Solvent | 46.07 ± 3.69 | |||||||||||
| Response | 62.08 ± 3.54 | 23.18 ± 3.54 | 2.42 ± 3.54 | 6.06 ± 3.54 | 2.08 ± 3.54 | 33.58 ± 3.54 | ||||||
| Coded Values | Natural Values | ||
|---|---|---|---|
| t (min) | T (°C) | S (%) | |
| −1.68 | 20 | 20 | 0 |
| −1 | 40.3 | 37.2 | 20.3 |
| 0 | 70 | 55 | 50 |
| +1 | 99.7 | 72.8 | 79.7 |
| +1.68 | 120 | 90 | 100 |
| Anthocyanins (mg AT/g E) | L* | a* | b* | Conversion Color to RGB Values |
|---|---|---|---|---|
| 33.2 ± 0.8 | 24.8 ± 0.1 | 31.7 ± 0.5 | 7.6 ± 0.2 |
| A) Antimicrobial Activity | |||||||||
| Extract | Ampicillin | Imipenem | Vancomycin | ||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||
| Gram-Negative Bacteria (mg/mL) | |||||||||
| Escherichia coli | 5 | >20 | <0.15 | <0,.5 | <0.0078 | <0.0078 | n.t. | n.t. | |
| Klebsiella pneumoniae | 20 | >20 | 10 | 20 | <0.0078 | <0.0078 | n.t. | n.t. | |
| Morganella morganii | 2.5 | >20 | 20 | >20 | <0.0078 | <0.0078 | n.t. | n.t. | |
| Proteus mirabilis | 10 | >20 | <0.15 | <0.15 | <0.0078 | <0.0078 | n.t. | n.t. | |
| Pseudomonas aeruginosa | >20 | >20 | >20 | >20 | 0.5 | 1 | n.t. | n.t. | |
| Gram-Positive Bacteria (mg/mL) | |||||||||
| Enterococcus faecalis | 10 | >20 | <0.15 | <0.15 | n.t. | n.t. | <0.0078 | <0.0078 | |
| Listeria monocytogenes | 5 | >20 | <0.15 | <0.15 | <0.0078 | <0.0078 | n.t. | n.t. | |
| MRSA | 2.5 | >20 | <0.15 | <0.15 | n.t. | n.t. | 0.25 | 0.5 | |
| B) Citotoxicity Activity | |||||||||
| Rich extract | Ellipticin | ||||||||
| Cytotoxicity Activity (GI50, µg/mL) | |||||||||
| HeLa | 301 ± 19 | 1.91 ± 0.06 | |||||||
| NCI H460 | 337 ± 11 | 1.0 ± 0.1 | |||||||
| MCF7 | 328 ± 13 | 0.91 ± 0.04 | |||||||
| HepG2 | 286 ± 13 | 1.1 ± 0.2 | |||||||
| Hepatotoxicity (GI50, µg/mL) | |||||||||
| PLP2 | >400 | 3.2 ± 0.7 | |||||||
| DCT0 | DRAET0 | p-Value | DCT3 | DRAET3 | p-Value | |
|---|---|---|---|---|---|---|
| Color parameters | ||||||
| L* | 76 ± 1 | 57,5 ± 0.4 | <0,01 | 77.0 ± 0.3 | 57.0 ± 0.3 | <0.01 |
| a* | −0.1 ± 0.2 | 10.8 ± 0.4 | <0,01 | 0.03 ± 0.0 | 10.2 ± 0.1 | <0.01 |
| b* | 19.7 ± 0.3 | 10.9 ± 0.5 | <0,01 | 20.0 ± 0.3 | 10.4 ± 0.6 | <0.01 |
| Nutritional value | ||||||
| Moisture (g/100 g fw) | 22.13 ± 0.8 | 28.02 ± 0.1 | < 0.01 | 22.35 ± 0.3 | 27.19 ± 0.5 | < 0.01 |
| Proteins (g/100 g fw) | 6.9 ± 0.1 | 6.936 ± 0.003 | 0.134 | 6.8 ± 0.2 | 6.82 ± 0.04 | 0.165 |
| Ash (g/100 g fw) | 0.97 ± 0.02 | 0.96 ± 0.02 | 0.334 | 0.97 ± 0.02 | 0.97 ± 0.02 | 0.430 |
| Fat (g/100 g fw) | 5.0 ± 0.1 | 4.6 ± 0.1 | 0.133 | 4.7 ± 0.1 | 4.9 ± 0.1 | 0.058 |
| Carbohydrates (g/100 g fw) | 64.91 ± 0.04 | 59.5 ± 0.1 | < 0.01 | 65.2 ± 0.1 | 60.10 ± 0.03 | < 0.01 |
| Energy (kcal/100 g fw) | 332.8 ± 0.4 | 307.2 ± 0.4 | < 0.01 | 330.1 ± 0.8 | 311.9 ± 0.5 | < 0.01 |
| pH | 6.40 ± 0.14 | 5.23 ± 0.02 | - | - | - | - |
| Free sugars (g/100 g fw) | ||||||
| Fructose | 0.07 ± 0.01 | 0.63 ± 0.04 | <0.01 | 0.07 ± 0.01 | 0.58 ± 0.01 | <0.01 |
| Glucose | 0.06 ± 0.01 | 0.51 ± 0.04 | <0.01 | 0.07 ± 0.01 | 0.53 ± 0.04 | <0.01 |
| Sucrose | 17.5 ± 0.6 | 16.2 ± 0.3 | <0.01 | 17.6 ± 0.6 | 15.2 ± 0.3 | <0.01 |
| Threhalose | 1.6 ± 0.1 | 1.92 ± 0.04 | <0.01 | 1.7 ± 0.1 | 1.78 ± 0.1 | 0.004 |
| Total | 19.2 ± 0.7 | 19.3 ± 0.2 | 0.619 | 19.4 ± 0.7 | 18.1 ± 0.3 | <0.01 |
| Fatty acids (%) | ||||||
| C6:0 | 0.28 ± 0.01 | 0.32 ± 0.01 | 0.367 | 0.29 ± 0.01 | 0.337 ± 0.001 | 0.609 |
| C8:0 | 0.18 ± 0.01 | 0.198 ± 0.004 | 0.289 | 0.179 ± 0.002 | 0.205 ± 0.001 | 0.275 |
| C10:0 | 0.41 ± 0.02 | 0.465 ± 0.003 | 0.163 | 0.41 ± 0.01 | 0.482 ± 0.005 | 0.287 |
| C11:0 | 0.019 ± 0.001 | 0.019 ± 0.001 | 1.000 | 0.020 ± 0.001 | 0.021 ± 0.001 | 0.998 |
| C12:0 | 0.49 ± 0.02 | 0.585 ± 0.002 | 0.144 | 0.48 ± 0.01 | 0.56 ± 0.01 | 0.743 |
| C13:0 | 0.022 ± 0.001 | 0.022 ± 0.001 | 1.000 | 0.019 ± 0.001 | 0.023 ± 0.001 | 0.432 |
| C14:0 | 1.4 ± 0.1 | 1.589 ± 0.003 | 0.132 | 1.40 ± 0.01 | 1.61 ± 0.01 | 0.338 |
| C14:1 | 0.12 ± 0.01 | 0.131 ± 0.001 | 0.165 | 0.114 ± 0.001 | 0.134 ± 0.001 | 0.070 |
| C15:0 | 0.17 ± 0.01 | 0.183 ± 0.003 | 0.367 | 0.17 ± 0.1 | 0.187 ± 0.004 | 0.559 |
| C16:0 | 10.4 ± 0.3 | 10.8 ± 0.1 | 0.215 | 10.39 ± 0.07 | 10.87 ± 0.04 | 0.537 |
| C16:1 | 0.29 ± 0.01 | 0.314 ± 0.003 | 0.498 | 0.291 ± 0.001 | 0.312 ± 0.001 | 0.898 |
| C17:0 | 0.13 ± 0.01 | 0.14 ± 0.01 | 0.639 | 0.130 ± 0.001 | 0.140 ± 0.004 | 0.116 |
| C17:1 | 0.039 ± 0.001 | 0.045 ± 0.001 | 0.116 | 0.039 ± 0.001 | 0.045 ± 0.002 | 0.116 |
| C18:1n9t | 3.7 ± 0.1 | 3.83 ± 0.01 | 0.155 | 3.71 ± 0.02 | 3.76 ± 0.01 | 0.609 |
| C18:1n9c | 19 ± 1 | 18.4 ± 0.6 | 0.373 | 18.8 ± 0.5 | 19.25 ± 0.03 | 0.133 |
| C18:2n6 | 62 ± 1 | 60.9 ± 0.5 | 0.471 | 61.6 ± 0.3 | 60.0 ± 0.1 | 0.236 |
| C18:3n3 | 0.530 ± 0.001 | 0.72 ± 0.01 | 0.242 | 0.508 ± 0.001 | 0.716 ± 0.003 | 0.219 |
| C20:0 | 0.219 ± 0.001 | 0.221 ± 0.001 | 0.116 | 0.22 ± 0.01 | 0.219 ± 0.001 | 0.151 |
| C20:1 | 0.158 ± 0.002 | 0.161 ± 0.002 | 0.897 | 0.153 ± 0.001 | 0.15 ± 0.01 | 0.152 |
| C20:2 | 0.137 ± 0,001 | 0.135 ± 0.001 | 0.921 | 0.149 ± 0.004 | 0.127 ± 0.004 | 0.811 |
| C20:3n3 | 0.024 ± 0.002 | 0.030 ± 0.003 | 0.710 | 0.03 ± 0.01 | 0.037 ± 0.001 | 0.422 |
| C22:0 | 0.61 ± 0.01 | 0.589 ± 0.004 | 0.377 | 0.613 ± 0.001 | 0.571 ± 0.001 | 0.989 |
| C24:0 | 0.20 ± 0.01 | 0.239 ± 0.003 | 0.197 | 0.27 ± 0.02 | 0.20 ± 0.01 | 0.202 |
| SFA | 14.5 ± 0.4 | 15.4 ± 0.1 | 0.231 | 14.6 ± 0.1 | 15.4 ± 0.1 | 0.458 |
| MUFA | 23 ± 1 | 22.8 ± 0.6 | 0.387 | 23.1 ± 0.4 | 23.66 ± 0.02 | 0.128 |
| PUFA | 62 ± 1 | 61.7 ± 0.5 | 0.467 | 62.3 ± 0.3 | 60.9 ± 0.1 | 0.253 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, L.P.; Pereira, E.; Prieto, M.A.; Simal-Gandara, J.; Pires, T.C.S.P.; Alves, M.J.; Calhelha, R.; Barros, L.; Ferreira, I.C.F.R. Rubus ulmifolius Schott as a Novel Source of Food Colorant: Extraction Optimization of Coloring Pigments and Incorporation in a Bakery Product. Molecules 2019, 24, 2181. https://doi.org/10.3390/molecules24112181
da Silva LP, Pereira E, Prieto MA, Simal-Gandara J, Pires TCSP, Alves MJ, Calhelha R, Barros L, Ferreira ICFR. Rubus ulmifolius Schott as a Novel Source of Food Colorant: Extraction Optimization of Coloring Pigments and Incorporation in a Bakery Product. Molecules. 2019; 24(11):2181. https://doi.org/10.3390/molecules24112181
Chicago/Turabian Styleda Silva, Liliana Primo, Eliana Pereira, Miguel A. Prieto, Jesus Simal-Gandara, Tânia C.S.P. Pires, Maria José Alves, Ricardo Calhelha, Lillian Barros, and Isabel C.F.R. Ferreira. 2019. "Rubus ulmifolius Schott as a Novel Source of Food Colorant: Extraction Optimization of Coloring Pigments and Incorporation in a Bakery Product" Molecules 24, no. 11: 2181. https://doi.org/10.3390/molecules24112181
APA Styleda Silva, L. P., Pereira, E., Prieto, M. A., Simal-Gandara, J., Pires, T. C. S. P., Alves, M. J., Calhelha, R., Barros, L., & Ferreira, I. C. F. R. (2019). Rubus ulmifolius Schott as a Novel Source of Food Colorant: Extraction Optimization of Coloring Pigments and Incorporation in a Bakery Product. Molecules, 24(11), 2181. https://doi.org/10.3390/molecules24112181