Pyrrolizidine Alkaloids: The Botanical Origin of Pollen Collected during the Flowering Period of Echium vulgare and the Stability of Pyrrolizidine Alkaloids in Bee Bread
Abstract
:1. Introduction
2. Results
2.1. Botanical Composition of Bee-Collected Pollen from Two Apiaries in the Southern Alps of Switzerland
2.2. Botanical Composition of Bee-Collected Pollen from an Apiary North of the Alps
2.3. Stability of PAs and PA N-oxides in Bee Bread
3. Discussion
3.1. Pollen Spectrum in the Northern and Southern Parts of Switzerland
3.2. Tertiary PAs and Corresponding PA N-oxides in Plants, Pollen, and Bee Bread
3.3. Recommendations for Maximum PA Content in Pollen Products
3.4. PA Content in Pollen and Bee Bread
3.5. Untargeted Analysis to Determine PAs in Bee Bread
4. Materials and Methods
4.1. Collection of Pollen Samples from Two Observation Sites
4.2. Determination of Botanical Origin
4.3. Collection of Bee Bread
4.4. Reagents and Materials for liquid chromatography mass spectrometry (LC-MS)
4.5. Sample Preparation for LC-MS
4.6. Identification and Quantification of PA/PA N-oxides in Bee Bread by LC-MS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vásquez, A.; Olofsson, T.C. The lactic acid bacteria involved in the production of bee pollen and bee bread. J. Apicult. Res. Bee World 2009, 48, 189–195. [Google Scholar] [CrossRef]
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Błażejak, S.; Chlebowska-Śmigiel, A.; Wolska, I. Pollen and bee bread as new health-oriented products: A review. Trends Food. Sci. Technol. 2018, 71, 170–180. [Google Scholar] [CrossRef]
- Campos, M.G.R.; Frigerio, C.; Lopes, J.; Bogdanov, S. What is the future of bee-pollen? J. ApiProd. ApiMed. Sci. 2010, 2, 131–144. [Google Scholar] [CrossRef]
- Komosinska-Vassev, K.; Olczyk, P.; Kazmierczak, J.; Mencner, L.; Olczyk, K. Bee pollen: Chemical composition and therapeutic application. Evid. Based Complement. Altern. Med. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef]
- Saraiva, L.C.F.; Cunha, F.V.M.; Léllis, D.R.O.D.; Nunes, L.C.C. Composition, biological activity and toxicity of bee pollen: State of the art. Bol. Latinoam. Caribe Plant. Med. Aromat. 2018, 17, 426–440. [Google Scholar]
- Bogdanov, S. Contaminants of bee products. Apidologie 2006, 37, 1–18. [Google Scholar] [CrossRef]
- Beales, K.; Betteridge, K.; Boppré, M.; Cao, Y.; Colegate, S.M.; Edgar, J.A. Hepatotoxic pyrrolizidine alkaloids and their N-oxides in honey and pollen. In Poisonous Plants: Global Research and Solutions; Panter, K.E., Wierenga, T.L., Pfister, J.A., Eds.; CABI: Wallingford, UK, 2007; pp. 94–100. [Google Scholar]
- Boppré, M.; Colegate, S.M.; Edgar, J.A.; Fischer, O.W. Hepatotoxic pyrrolizidine alkaloids in pollen and drying-related implications for commercial processing of bee pollen. J. Agric. Food Chem. 2008, 56, 5662–5672. [Google Scholar] [CrossRef]
- Kempf, M.; Heil, S.; Haßlauer, I.; Schmidt, L.; von der Ohe, K.; Theuring, C.; Reinhard, A.; Schreier, P.; Beuerle, T. Pyrrolizidine alkaloids in pollen and pollen products. Mol. Nutr. Food Res. 2010, 54, 292–300. [Google Scholar] [CrossRef]
- Kempf, M.; Reinhard, A.; Beuerle, T. Pyrrolizidine alkaloids (PAs) in honey and pollen-legal regulation of PA levels in food and animal feed required. Mol. Nutr. Food Res. 2010, 54, 158–168. [Google Scholar] [CrossRef]
- Dübecke, A.; Beckh, G.; Lüllmann, C. Pyrrolizidine alkaloids in honey and bee pollen. Food Addit. Contam. Part A 2011, 28, 348–358. [Google Scholar] [CrossRef]
- Mulder, P.P.J.; López, P.; Castelari, M.; Bodi, D.; Ronczka, S.; Preiss-Weigert, A.; These, A. Occurrence of pyrrolizidine alkaloids in animal- and plant-derived food: Results of a survey across Europe. Food Addit. Contam. Part A 2018, 35, 118–133. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Dietary exposure assessment to pyrrolizidine alkaloids in the European population. EFSA J. 2016, 14, 4572. [Google Scholar] [CrossRef]
- Kast, C.; Kilchenmann, V.; Reinhard, H.; Droz, B.; Lucchetti, M.A.; Dübecke, A.; Beckh, G.; Zoller, O. Chemical fingerprinting identifies Echium vulgare, Eupatorium cannabinum and Senecio spp. as plant species mainly responsible for pyrrolizidine alkaloids in bee-collected pollen. Food Addit. Contam. Part A 2018, 35, 316–327. [Google Scholar] [CrossRef]
- Picron, J.-F.; Herman, M.; Van Hoeck, E.; Goscinny, S. Monitoring of pyrrolizidine alkaloids in beehive products and derivatives on the Belgian market. Environ. Sci. Pollut. Res. Int. 2019. [Google Scholar] [CrossRef]
- Culvenor, C.C.J.; Edgar, J.A.; Jago, M.V.; Outteridge, A.; Peterson, J.E.; Smith, L.W. Hepato- and pneumotoxicity of pyrrolizidine alkaloids and derivatives in relation to molecular structure. Chem.-Biol. Interact. 1976, 12, 299–324. [Google Scholar] [CrossRef]
- Fu, P.P.; Chou, M.W.; Xia, Q.; Yang, Y.C.; Yan, J.; Doerge, D.R.; Chan, P.C. Genotoxic pyrrolizidine alkaloids and pyrrolizidine alkaloid N-oxides—Mechanisms leading to DNA adduct formation and tumorigenicity. J. Environ. Sci. Health Part C 2001, 19, 353–385. [Google Scholar] [CrossRef]
- Chou, M.W.; Wang, Y.P.; Yan, J.; Yang, Y.C.; Berger, R.D.; Williams, L.D.; Doerge, D.R.; Fu, P.P. Riddelliine N-oxide is a phytochemical and mammalian metabolite with genotoxic activity that is comparable to the parent pyrrolizidine alkaloid riddelliine. Toxicol. Lett. 2003, 145, 239–247. [Google Scholar] [CrossRef]
- Wiedenfeld, H. Plants containing pyrrolizidine alkaloids: Toxicity and problems. Food Addit. Contam. Part A 2011, 28, 282–292. [Google Scholar] [CrossRef]
- Molyneux, R.J.; Gardner, D.L.; Colegate, S.M.; Edgar, J.A. Pyrrolizidine alkaloid toxicity in livestock: A paradigm for human poisoning? Food Addit. Contam. Part A 2011, 28, 293–307. [Google Scholar] [CrossRef]
- Hartmann, T.; Witte, L. Chemistry, Biology and Chemoecology of the Pyrrolizidine Alkaloids. In Alkaloids: Chemical and Biological Perspectives; Pelletier, S.W., Ed.; Pergamon Press: Oxford, UK, 1995; Volume 9, pp. 155–233. [Google Scholar]
- European Food Safety Authority (EFSA). Scientific Opinion on Pyrrolizidine alkaloids in food and feed. EFSA J. 2011, 9, 2406. [Google Scholar] [CrossRef]
- Kast, C.; Lucchetti, M.A. Pyrrolizidine alkaloids—A case study of Swiss honey. In Chemical Hazards in Foods of Animal Origin. Food Safety Assurance and Veterinary Public Health; Smulders, F.J.M., Rietjens, I.M.C.M., Rose, M.D., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019; Volume 7, pp. 529–542. [Google Scholar]
- Lucchetti, M.A.; Glauser, G.; Kilchenmann, V.; Dübecke, A.; Beckh, G.; Praz, C.; Kast, C. Pyrrolizidine alkaloids from Echium vulgare in honey originate primarily from floral nectar. J. Agric. Food Chem. 2016, 64, 5267–5273. [Google Scholar] [CrossRef]
- Kast, C.; Dübecke, A.; Kilchenmann, V.; Bieri, K.; Böhlen, M.; Zoller, O.; Beckh, G.; Lüllmann, C. Analysis of Swiss honeys for pyrrolizidine alkaloids. J. Apicult. Res. 2014, 53, 75–83. [Google Scholar] [CrossRef]
- Wille, M.; Wille, H. Die Pollenversorgung der Bienenvölker in den Jahren 1978-1980. Vergleichende Untersuchungen, botanische Aspekte. Schweiz. Bienenztg. 1981, 104, 227–241. [Google Scholar]
- Boppré, M.; Colegate, S.M.; Edgar, J.A. Pyrrolizidine Alkaloids of Echium vulgare honey found in pure pollen. J. Agric. Food Chem. 2005, 53, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Keller, I.; Fluri, P.; Imdorf, A. Pollen nutrition and colony development in honey bees: Part 1. Bee World 2005, 86, 3–10. [Google Scholar] [CrossRef]
- Gehring, E.; Kast, C.; Kilchenmann, V.; Bieri, K.; Gehrig, R.; Pezzatti, G.B.; Conedera, M. Impact of the Asian chestnut gall wasp, Dryocosmus kuriphilus (Hymenoptera, Cynipidae), on the chestnut component of honey in the Southern Swiss Alps. J. Econ. Entomol. 2018, 111, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Toppel, G.; Witte, L.; Riebesehl, B.; Borstel, K.V.; Hartmann, T. Alkaloid patterns and biosynthetic capacity of root cultures from some pyrrolizidine alkaloid producing Senecio species. Plant Cell Rep. 1987, 6, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, C.; Huckauf, A.; Dübecke, A.; Kaltner, F.; Zimmermann, M.; Rahaus, I.; Beuerle, T. Uncertainties in the determination of pyrrolizidine alkaloid levels in naturally contaminated honeys and comparison of results obtained by different analytical approaches. Food Addit. Contam. Part A 2018, 7, 1366–1383. [Google Scholar] [CrossRef]
- Kaltner, F.; Rychlik, M.; Gareis, M.; Gottschalk, C. Influence of storage on the stability of toxic pyrrolizidine alkaloids and their N-oxides in peppermint tea, hay, and honey. J. Agric. Food Chem. 2018, 66, 5221–5228. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Risks for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements. EFSA J. 2017, 15, 4908. [Google Scholar] [CrossRef]
- Cairns, E.; Hashmi, M.A.; Singh, A.J.; Eakins, G.; Lein, M.; Keyzers, R. Structure of echivulgarine, a pyrrolizidine alkaloid isolated from the pollen of Echium vulgare. J. Agric. Food Chem. 2015, 63, 7421–7427. [Google Scholar] [CrossRef] [PubMed]
- Imdorf, A.; Wille, M. Polleneintrag eines Bienenvolkes aufgrund des Rückbehaltes in der Pollenfalle. 2. Teil: Detaillierte Analysen des Pollenrückbehaltes in der Falle. Schweiz. Bienenztg. 1983, 106, 184–195. [Google Scholar]
- Von Der Ohe, W.; Persano Oddo, L.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized methods of melissopalynology. Apidologie 2004, 35, 18–25. [Google Scholar] [CrossRef]
- Lucchetti, M.A.; Kilchenmann, V.; Glauser, G.; Praz, C.; Kast, C. Nursing protects honeybee larvae from secondary metabolites of pollen. Proc. Biol. Sci. 2018, 285, 20172849. [Google Scholar] [CrossRef] [PubMed]
- Cymerman Craig, J.; Purushothaman, K.K. Improved preparation of tertiary amine N-oxides. J. Org. Chem. 1970, 35, 1721–1722. [Google Scholar] [CrossRef]
- Lucchetti, M.A. Pyrrolizidine Alkaloids: Occurrence in bee products and impact on honeybees (Apis mellifera L.). Ph.D. Thesis, University of Neuchâtel, Neuchâtel, Switzerland, 2017. [Google Scholar]
Sample Availability: No samples are available from the authors. |
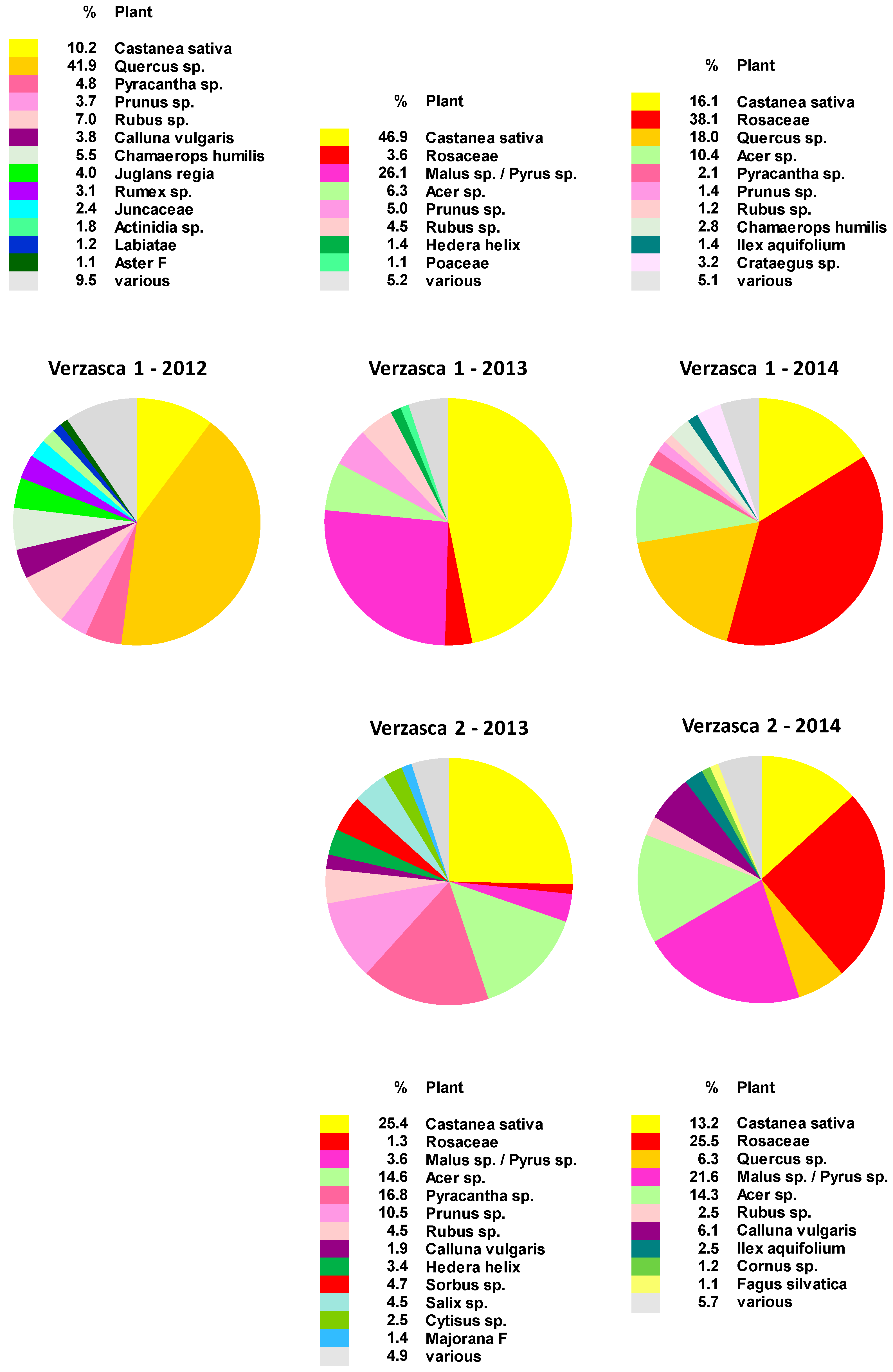


| Location | Year | Echium-Type Pollen | |
|---|---|---|---|
| % of Total Pollen | (g) | ||
| Verzasca 1 | 2012 | 0.003 | 0.01 |
| Verzasca 1 | 2013 | 0.013 | 0.04 |
| Verzasca 1 | 2014 | 0.004 | 0.01 |
| Verzasca 2 | 2013 | 0.004 | 0.01 |
| Verzasca 2 | 2014 | 0.005 | 0.02 |
| Basel | 2012 | 0.012 | 0.10 |
| Basel | 2013 | 0.001 | 0.01 |
| PA-Types | PA Conc. Range in Bee Bread from Apiary 1 (ng/g) | PA Conc. Range in Bee Bread from Apiary 2 (ng/g) |
|---|---|---|
| Intermedine-type | 116–221 | 28–56 |
| Intermedine N-oxide -type | nd–62 | nd–3 |
| Retrorsine | nd | nd–2 |
| Retrorsine N-oxide | nd | nd–2 |
| 7-Acetyl-intermedine-type | nd–10 | nd–11 |
| Senecionine | nd | nd–2 |
| Echimidine N-oxide | 2–26 | nd–1 |
| Di-acetyl-intermedine-type * | 1–13 | nd–14 |
| Di-acetyl-intermedine N-oxide -type * | 7–18 | 12–26 |
| Echimidine | 98–167 | 2–5 |
| Vulgarine * | 65–118 | nd |
| Vulgarine N-oxide * | 7–36 | nd |
| Acetyl-echimidine/vulgarine * | 8–19 | nd–1 |
| Acetyl-echimidine N-oxide * | 3–6 | nd–1 |
| Acetyl-vulgarine N-oxide * | 3–13 | nd |
| Echivulgarine | 1176–1950 | 8–36 |
| Echivulgarine N-oxide * | 32–248 | nd–2 |
| Pyrrolizindine Alkaloid | Sum Formula | Rt (min.) | Quantifier MH+ | Qualifiers |
|---|---|---|---|---|
| Intermedine-type | C15H25NO5 | 7.2–7.5 | 300.1805 | 156.1020, 138.0913, 120.0808 |
| Intermedine N-oxide-type | C15H25NO6 | 7.6–8.2 | 316.1755 | 172.0968, 155.0941, 136.0757 |
| Retrorsine | C18H25NO6 | 8.8 | 352.1755 | 324.1806, 220.1331, 138.0913 |
| Retrorsine N-oxide | C18H25NO7 | 8.9 | 368.1704 | 340.1755, 220.1331, 136.0757 |
| 7-Acetyl-intermedine-type | C17H27NO6 | 9.0 | 342.1911 | 198.1125, 180.1019, 120.0808 |
| Senecionine | C18H25NO5 | 10.5 | 336.1805 | 308.1880, 220.1331, 138.0913 |
| Echimidine N-oxide | C20H31NO8 | 11.2 | 414.2122 | 396.2017, 352.1755, 254.1387 |
| Di-acetyl-intermedine-type * | C19H29NO7 | 11.3 | 384.2017 | 324.1806, 240.1230, 180.1019 |
| Di-acetyl-intermedine N-oxide-type * | C19H29NO8 | 11.3 | 400.1966 | 340.1755, 180.1019, 136.0757 |
| Echimidine | C20H31NO7 | 11.4 | 398.2173 | 238.1457, 220.1331, 138.0913 |
| Vulgarine * | C20H31NO7 | 11.5 | 398.2173 | 380.2068, 254.1387, 240.1230 |
| Vulgarine N-oxide * | C20H31NO8 | 11.8 | 414.2122 | 314.1598, 256.1179, 172.0968 |
| Acetyl-echimidine/vulgarine * | C22H33NO8 | 12.3 | 440.2279 | 422.2173, 380.2068, 138.0913 |
| Acetyl-echimidine N-oxide * | C22H33NO9 | 12.4 | 456.2228 | 438.2122, 338.1598, 220.1331 |
| Acetyl-vulgarine N-oxide * | C22H33NO9 | 12.8 | 456.2228 | 438.2122, 298.1285, 180.1019 |
| Echivulgarine | C25H37NO8 | 13.8 | 480.2592 | 380.2068, 322.1649, 220.1331 |
| Echivulgarine N-oxide * | C25H37NO9 | 13.8 | 496.2541 | 396.2017, 338.1598, 220.1331 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kast, C.; Kilchenmann, V.; Reinhard, H.; Bieri, K.; Zoller, O. Pyrrolizidine Alkaloids: The Botanical Origin of Pollen Collected during the Flowering Period of Echium vulgare and the Stability of Pyrrolizidine Alkaloids in Bee Bread. Molecules 2019, 24, 2214. https://doi.org/10.3390/molecules24122214
Kast C, Kilchenmann V, Reinhard H, Bieri K, Zoller O. Pyrrolizidine Alkaloids: The Botanical Origin of Pollen Collected during the Flowering Period of Echium vulgare and the Stability of Pyrrolizidine Alkaloids in Bee Bread. Molecules. 2019; 24(12):2214. https://doi.org/10.3390/molecules24122214
Chicago/Turabian StyleKast, Christina, Verena Kilchenmann, Hans Reinhard, Katharina Bieri, and Otmar Zoller. 2019. "Pyrrolizidine Alkaloids: The Botanical Origin of Pollen Collected during the Flowering Period of Echium vulgare and the Stability of Pyrrolizidine Alkaloids in Bee Bread" Molecules 24, no. 12: 2214. https://doi.org/10.3390/molecules24122214
APA StyleKast, C., Kilchenmann, V., Reinhard, H., Bieri, K., & Zoller, O. (2019). Pyrrolizidine Alkaloids: The Botanical Origin of Pollen Collected during the Flowering Period of Echium vulgare and the Stability of Pyrrolizidine Alkaloids in Bee Bread. Molecules, 24(12), 2214. https://doi.org/10.3390/molecules24122214







