Formation of Polyethylene Glycol Particles Using a Low-Temperature Supercritical Assisted Atomization Process
Abstract
:1. Introduction
2. Results and Discussion
2.1. Operation Parameters for LTSAA
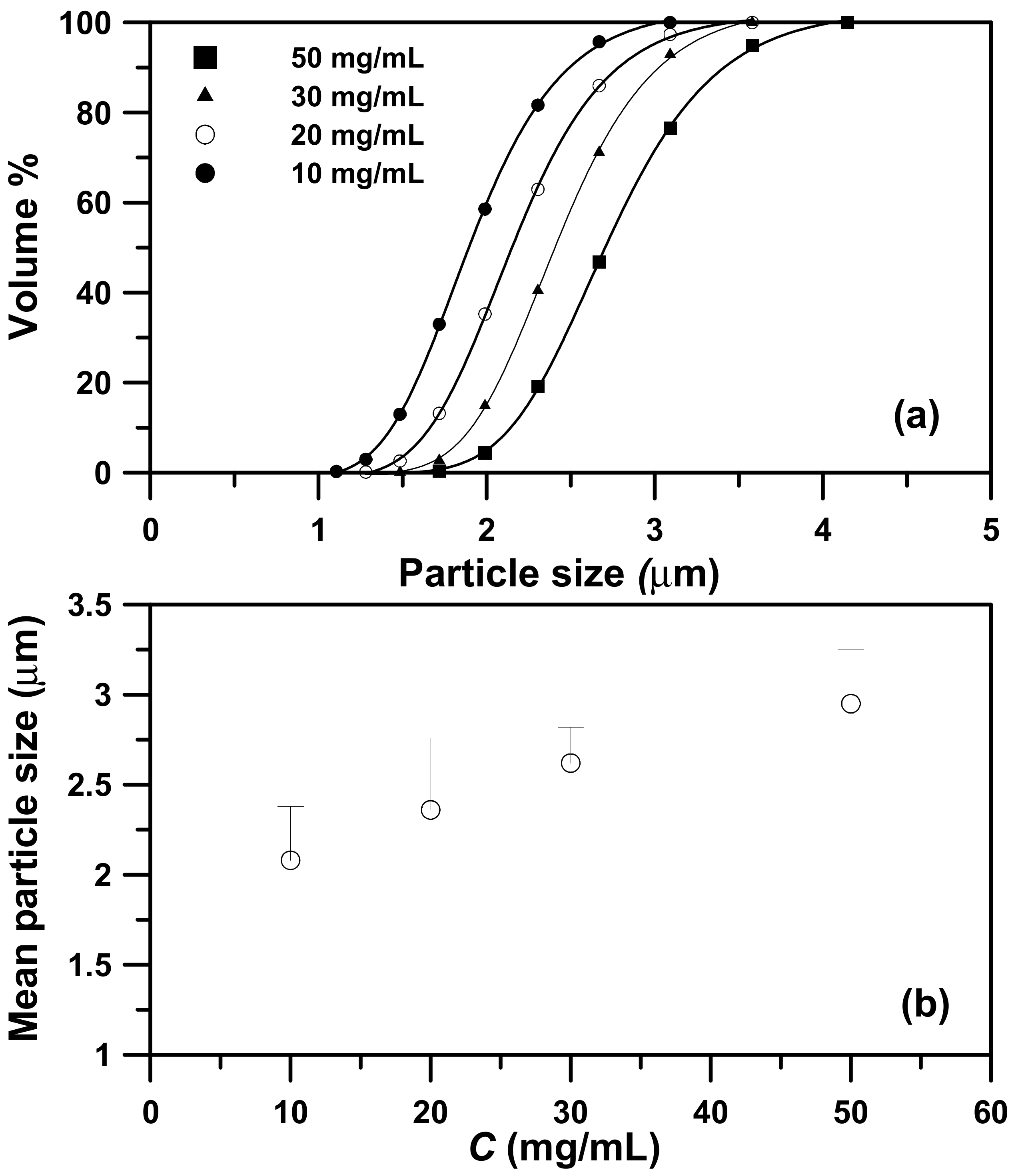
2.2. Effects of the Precipitator Temperature and Concentration of the PEG Solution on the PEG Particle Size
2.3. Effects of the Phase Behavior and Molecular Weight of PEG on the PEG Particle Size
2.4. Solid Characterization
3. Materials and Methods
3.1. Materials
3.2. Production of PEG Particles
3.3. Solid-State Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Committee on Food Additives. Evaluation of certain food additives. Twenty-third Report of the Joint FAO/WHO Expert. TRS 931 1980, 648, 17–18. [Google Scholar]
- Noyes, A.A.; Whitney, W.R. The rate of solution of solid substances in their own solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef]
- Wu, H.-T.; Yang, C.-P.; Huang, S.-C. Dissolution enhancement of indomethacin-chitosan hydrochloride composite particles produced using supercritical assisted atomization. J. Taiwan Inst. Chem. Eng. 2016, 67, 98–105. [Google Scholar] [CrossRef]
- Dos Santos, K.M.; Barbosa, R.M.; Vargas, F.G.A.; de Azevedo, E.P.; Lins, A.C.D.S.; Camara, C.A.; Aragão, C.F.S.; Moura, T.F.L.E.; Raffin, F.N. Development of solid dispersions of β-lapachone in PEG and PVP by solvent evaporation method. Drug Dev. Ind. Pharm. 2018, 44, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, N.; Katare, O.P.; Singh, B. Studies on dissolution enhancement and mathematical modeling of drug release of a poorly water-soluble drug using water-soluble carriers. Eur. J. Pharm. Biopharm. 2007, 65, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.Q.M. A review of thermal methods used for the analysis of the crystal form, solution thermodynamics and glass transition behaviour of polyethylene glycols. Thermochim. Acta 1995, 248, 189–203. [Google Scholar] [CrossRef]
- Owens III, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Weuts, I.; Kempen, D.; Verreck, G.; Decorte, A.; Heymans, K.; Peeters, J.; Brewster, M.; Van den Mooter, G. Study of the physicochemical properties and stability of solid dispersions of loperamide and PEG6000 prepared by spray drying. Eur. J. Pharm. Biopharm. 2005, 59, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Barron, M.K.; Young, T.J.; Johnston, K.P.; Williams 3rd, R.O. Investigation of processing parameters of spray freezing into liquid to prepare polyethylene glycol polymeric particles for drug delivery. AAPS PharmSciTech 2003, 4, E12. [Google Scholar] [CrossRef]
- Hao, J.; Whitaker, M.J.; Serhatkulu, G.; Shakesheff, K.M.; Howdle, S.M. Supercritical fluid assisted melting of poly(ethylene glycol): A new solvent-free route to microparticles. J. Mater. Chem. 2005, 15, 1148–1153. [Google Scholar] [CrossRef]
- Nalawade, S.P.; Picchioni, F.; Janssen, L.P.B.M. Batch production of micron size particles from poly(ethylene glycol) using supercritical CO2 as a processing solvent. Chem. Eng. Sci. 2007, 62, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Adami, R.; Liparoti, S.; Reverchon, E. A new supercritical assisted atomization configuration, for the micronization of thermolabile compounds. Chem. Eng. J. 2011, 173, 55–61. [Google Scholar] [CrossRef]
- Liparoti, S.; Adami, R.; Reverchon, E. PEG micronization by supercritical assisted atomization, operated under reduced pressure. J. Supercrit. Fluids 2012, 72, 46–51. [Google Scholar] [CrossRef]
- Reverchon, E. Supercritical-assisted atomization to produce micro- and/or nanoparticles of controlled size and distribution. Ind. Eng. Chem. Res. 2002, 41, 2405–2411. [Google Scholar] [CrossRef]
- Reverchon, E.; Antonacci, A. Polymer microparticles production by supercritical assisted atomization. J. Supercrit. Fluids 2007, 39, 444–452. [Google Scholar] [CrossRef]
- Wu, H.-T.; Yang, M.-W. Precipitation kinetics of PMMA sub-micrometric particles with a supercritical assisted-atomization process. J. Supercrit. Fluids 2011, 59, 98–107. [Google Scholar] [CrossRef]
- Wu, H.-T.; Yang, M.-W. Precipitation kinetics of PMMA-co-BMA sub-micrometric particles with compressed CO2 assisted-atomization process. Powder Technol. 2012, 228, 91–99. [Google Scholar] [CrossRef]
- Wu, H.-T.; Yang, M.-W.; Huang, S.-C. Sub-micrometric polymer particles formation by a supercritical assisted-atomization process. J. Taiwan Inst. Chem. Eng. 2014, 45, 1992–2001. [Google Scholar] [CrossRef]
- Mathias, P.M.; Klotz, H.C.; Prausnitz, J.M. Equation-of-State mixing rules for multicomponent mixtures: the problem of invariance. Fluid Phase Equilibria 1991, 67, 31–44. [Google Scholar] [CrossRef]
- Han, F.; Xue, Y.; Tian, Y.; Zhao, X.; Chen, L. Vapor–liquid equilibria of the carbon-dioxide + acetone system at pressures from (2.36 to 11.77) MPa and temperatures from (333.15 to 393.15) K. J. Chem. Eng. Data 2005, 50, 36–39. [Google Scholar] [CrossRef]
- Bamberger, A.; Maurer, G. High-pressure (vapour + liquid) equilibria in (carbon dioxide + acetone or 2-propanol) at temperatures from 293 K to 333 K. J. Chem. Thermodyn. 2000, 32, 685–700. [Google Scholar] [CrossRef]
- Chiu, H.-Y.; Lee, M.-J.; Lin, H.-M. Vapor−liquid phase boundaries of binary mixtures of carbon dioxide with ethanol and acetone. J. Chem. Eng. Data 2008, 53, 2393–2402. [Google Scholar] [CrossRef]
- Chang, C.J.; Day, C.-Y.; Ko, C.-M.; Chiu, K.-L. Densities and P-x-y diagrams for carbon dioxide dissolution in methanol, ethanol, and acetone mixtures. Fluid Phase Equilibria 1997, 131, 243–258. [Google Scholar] [CrossRef]
- Reverchon, E.; Adami, R.; Scognamiglio, M.; Fortunato, G.; Della Porta, G. Beclomethasone microparticles for wet Inhalation, produced by supercritical assisted atomization. Ind. Eng. Chem. Res. 2010, 49, 12747–12755. [Google Scholar] [CrossRef]
- Cai, M.-Q.; Guan, Y.-X.; Yao, S.-J.; Zhu, Z.-Q. Supercritical fluid assisted atomization introduced by hydrodynamic cavitation mixer (SAA-HCM) for micronization of levofloxacin hydrochloride. J. Supercrit. Fluids 2008, 43, 524–534. [Google Scholar] [CrossRef]
- Liparoti, S.; Adami, R.; Reverchon, E. Supercritical Assisted Atomization: effect of operative conditions on PVP microparticle size and morphology. J. Supercrit. Fluids 2015, 97, 31–35. [Google Scholar] [CrossRef]
- Reverchon, E.; Antonacci, A. Chitosan microparticles production by supercritical fluid processing. Ind. Eng. Chem. Res. 2006, 45, 5722–5728. [Google Scholar] [CrossRef]
- Reverchon, E.; Spada, A. Erythromycin micro-particles produced by supercritical fluid atomization. Powder Technol. 2004, 141, 100–108. [Google Scholar] [CrossRef]
- Wang, Q.; Guan, Y.-X.; Yao, S.-J.; Zhu, Z.-Q. Microparticle formation of sodium cellulose sulfate using supercritical fluid assisted atomization introduced by hydrodynamic cavitation mixer. Chem. Eng. J. 2010, 159, 220–229. [Google Scholar] [CrossRef]
- Wu, H.-T.; Huang, S.-C.; Yang, C.-P.; Chien, L.-J. Precipitation parameters and the cytotoxicity of chitosan hydrochloride microparticles production by supercritical assisted atomization. J. Supercrit. Fluids 2015, 102, 123–132. [Google Scholar] [CrossRef]
- Wu, H.-T.; Lee, H.-K.; Chen, H.-C.; Chien, L.-J. Precipitation kinetics and biological properties of chitosan microparticles produced using supercritical assisted atomization. Chem. Eng. Res. Des. 2015, 104, 615–625. [Google Scholar] [CrossRef]
- Wu, H.-T.; Su, Y.-C.; Wang, Y.-M.; Tsai, H.-M. Characterisation and aerosolisation of mannitol particles produced using supercritical assisted atomization. Chem. Eng. Res. Des. 2018, 137, 308–318. [Google Scholar] [CrossRef]
- Pielichowski, K.; Flejtuch, K.; Pielichowska, K. Differential scanning calorimetry studies on poly(ethylene glycol) with different molecular weights for thermal energy storage materials. Polym. Adv. Technol. 2002, 13, 690–696. [Google Scholar] [CrossRef]
- Corrigan, D.O.; Healy, A.M.; Corrigan, O.I. The effect of spray drying solutions of polyethylene glycol (PEG) and lactose/PEG on their physicochemical properties. Int. J. Pharm. 2002, 235, 193–205. [Google Scholar] [CrossRef]
- Ford, J.L.; Stewart, A.F.; Dubois, J.-L. The properties of solid dispersions of indomethacin or phenylbutazone in polyethylene glycol. Int. J. Pharm. 1986, 28, 11–22. [Google Scholar] [CrossRef]
- Craig, D.Q.M.; Newton, J.M. Characterisation of polyethylene glycols using differential scanning calorimetry. Int. J. Pharm. 1991, 74, 33–41. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |











| Components | TC (K) | PC (bar) | ω |
|---|---|---|---|
| carbon dioxide | 304.1 | 73.8 | 0.239 |
| acetone | 508.1 | 47.0 | 0.304 |
| 36.356 | 20.235 | ||
| −0.05798 | −0.03273 | ||
| −5700.2 | −3131.5 | ||
| Run | TP | C | Ts | R | dnoa | d4,3 b | PDI |
|---|---|---|---|---|---|---|---|
| K | mg/mL | K | FCO2/Fl | μm | μm | d4,3/dno | |
| 1 c | 293 | 10 | 323 | 2.0 | 2.9 ± 0.3 | 3.2 ± 0.3 | 1.1 |
| 2 d | 283 | 10 | 323 | 2.0 | 2.5 ± 0.2 | 2.9 ± 0.2 | 1.2 |
| 3 | 278 | 10 | 323 | 2.0 | 1.7 ± 0.2 | 2.0 ± 0.3 | 1.1 |
| 4 | 278 | 20 | 323 | 2.0 | 2.0 ± 0.3 | 2.3 ± 0.4 | 1.4 |
| 5 | 278 | 30 | 323 | 2.0 | 1.9 ± 0.2 | 2.5 ± 0.2 | 1.3 |
| 6 | 278 | 50 | 323 | 2.0 | 2.6 ± 0.2 | 2.9 ± 0.3 | 1.1 |
| 7 | 278 | 10 | 323 | 0.8 | 1.7 ± 0.1 | 2.6 ± 0.3 | 1.5 |
| 8 | 278 | 10 | 323 | 1.5 | 1.6 ± 0.2 | 2.2 ± 0.1 | 1.4 |
| 9 | 278 | 10 | 323 | 2.8 | 1.7 ± 0.1 | 2.5 ± 0.4 | 1.5 |
| 10 | 273 | 10 | 313 | 0.8 | 2.3 ± 0.2 | 2.9 ± 0.2 | 1.3 |
| 11 | 273 | 10 | 313 | 1.5 | 2.2 ± 0.2 | 2.5 ± 0.2 | 1.1 |
| 12 | 273 | 10 | 313 | 2.0 | 2.0 ± 0.3 | 2.2 ± 0.3 | 1.1 |
| 13 | 273 | 10 | 313 | 2.4 | 1.9 ± 0.4 | 2.1 ± 0.1 | 1.1 |
| 14 | 273 | 10 | 313 | 2.8 | 2.6 ± 0.4 | 3.0 ± 0.2 | 1.2 |
| 15 | 285 | 10 | 333 | 1.0 | 2.0 ± 0.3 | 2.5 ± 0.2 | 1.3 |
| 16 | 285 | 10 | 333 | 1.5 | 1.5 ± 0.2 | 1.7 ± 0.2 | 1.1 |
| 17 | 285 | 10 | 333 | 2.0 | 1.6 ± 0.5 | 2.2 ± 0.2 | 1.3 |
| 18 | 285 | 10 | 333 | 2.5 | 1.6 ± 0.5 | 2.3 ± 0.1 | 1.4 |
| 19 e | 278 | 10 | 323 | 2.0 | 2.5 ± 0.3 | 2.9 ± 0.5 | 1.2 |
| 20 f | 278 | 10 | 323 | 2.0 | 2.6 ± 0.3 | 3.2 ± 0.3 | 1.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-T.; Tsai, H.-M.; Li, T.-H. Formation of Polyethylene Glycol Particles Using a Low-Temperature Supercritical Assisted Atomization Process. Molecules 2019, 24, 2235. https://doi.org/10.3390/molecules24122235
Wu H-T, Tsai H-M, Li T-H. Formation of Polyethylene Glycol Particles Using a Low-Temperature Supercritical Assisted Atomization Process. Molecules. 2019; 24(12):2235. https://doi.org/10.3390/molecules24122235
Chicago/Turabian StyleWu, Hsien-Tsung, Hong-Ming Tsai, and Tsung-Hsuan Li. 2019. "Formation of Polyethylene Glycol Particles Using a Low-Temperature Supercritical Assisted Atomization Process" Molecules 24, no. 12: 2235. https://doi.org/10.3390/molecules24122235
APA StyleWu, H.-T., Tsai, H.-M., & Li, T.-H. (2019). Formation of Polyethylene Glycol Particles Using a Low-Temperature Supercritical Assisted Atomization Process. Molecules, 24(12), 2235. https://doi.org/10.3390/molecules24122235





