In Vitro and In Vivo Anti-Breast Cancer Activities of Some Newly Synthesized 5-(thiophen-2-yl)thieno-[2,3-d]pyrimidin-4-one Candidates
Abstract
1. Introduction
2. Results and Discussion
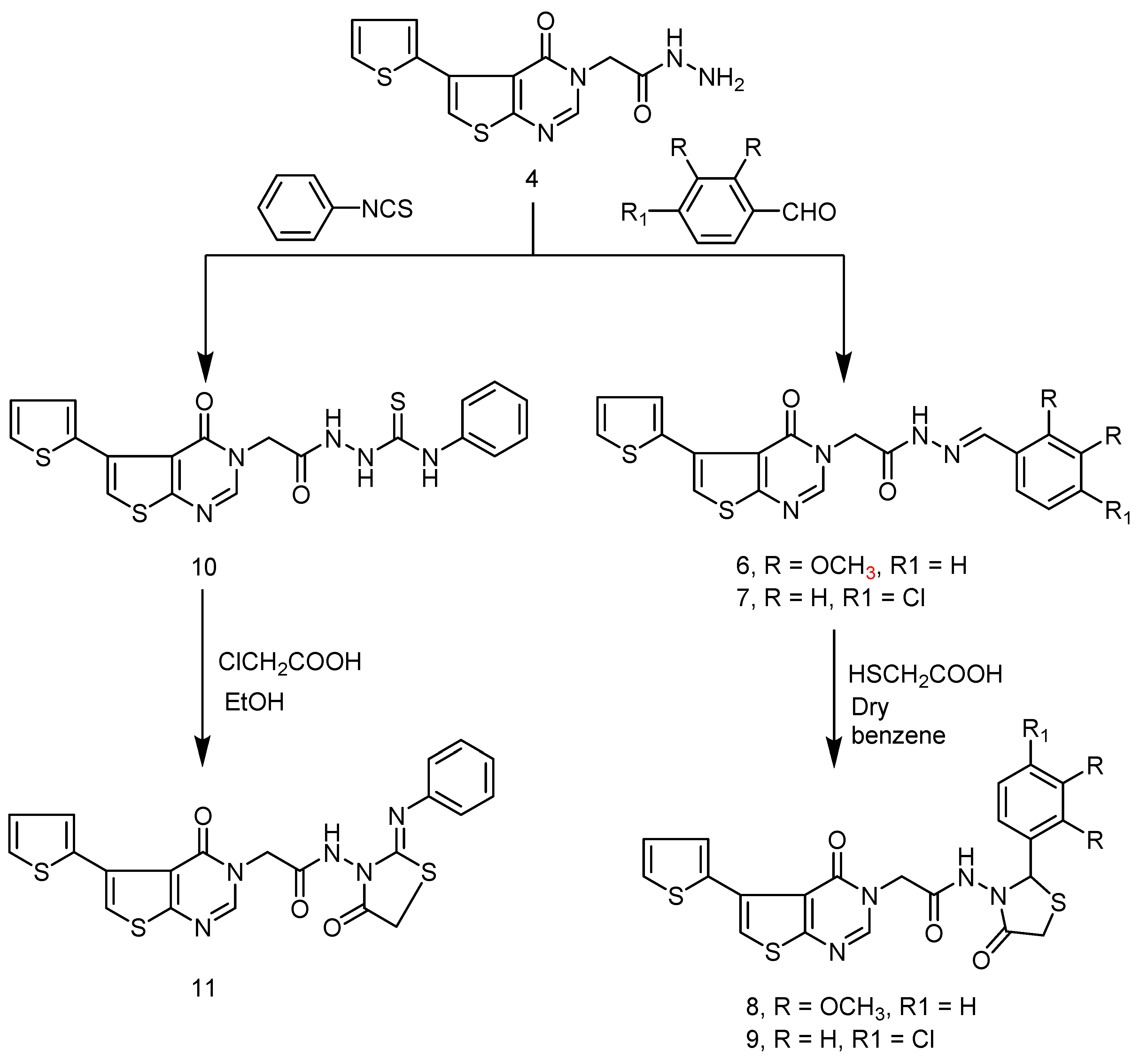
2.1. Chemistry
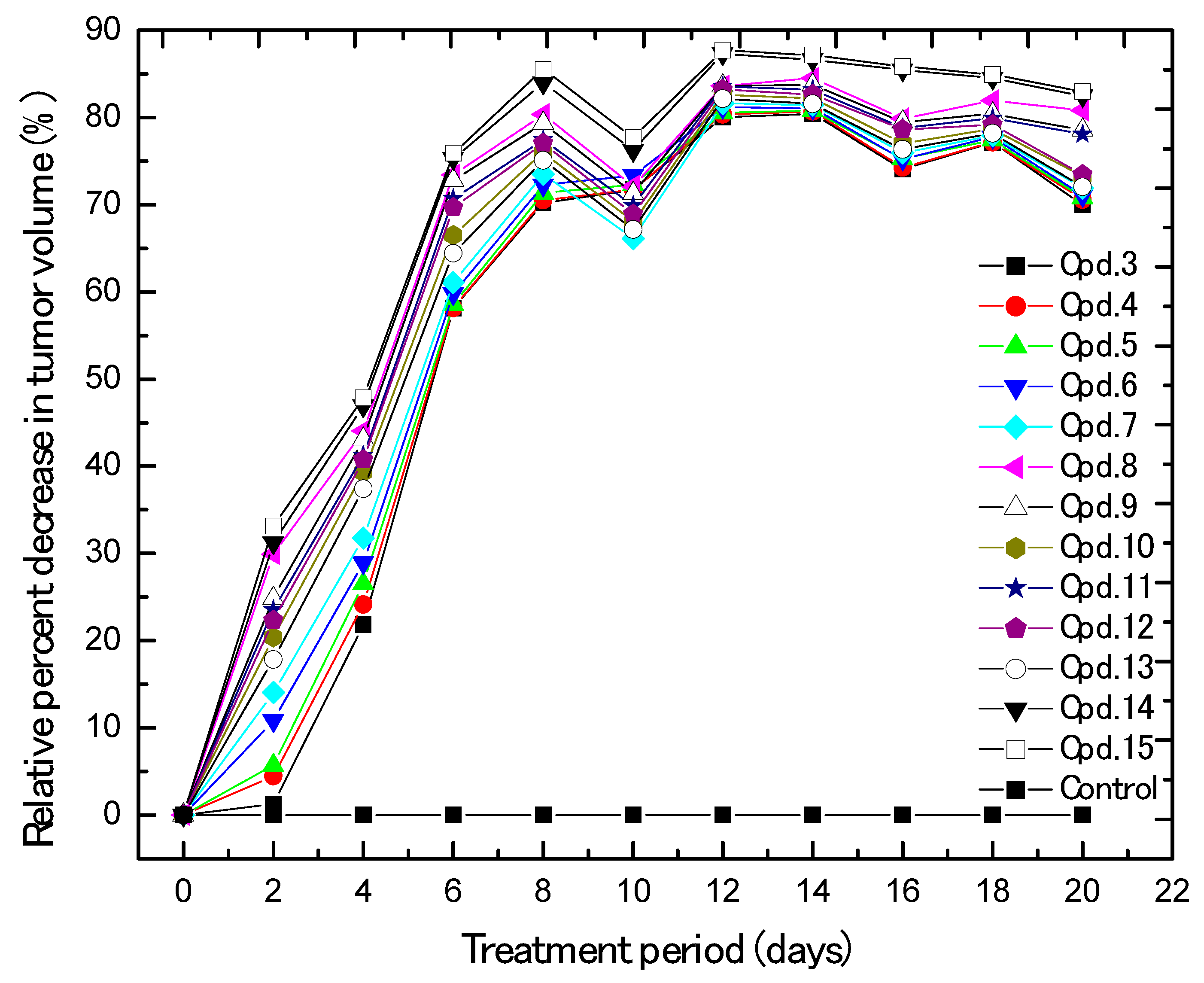
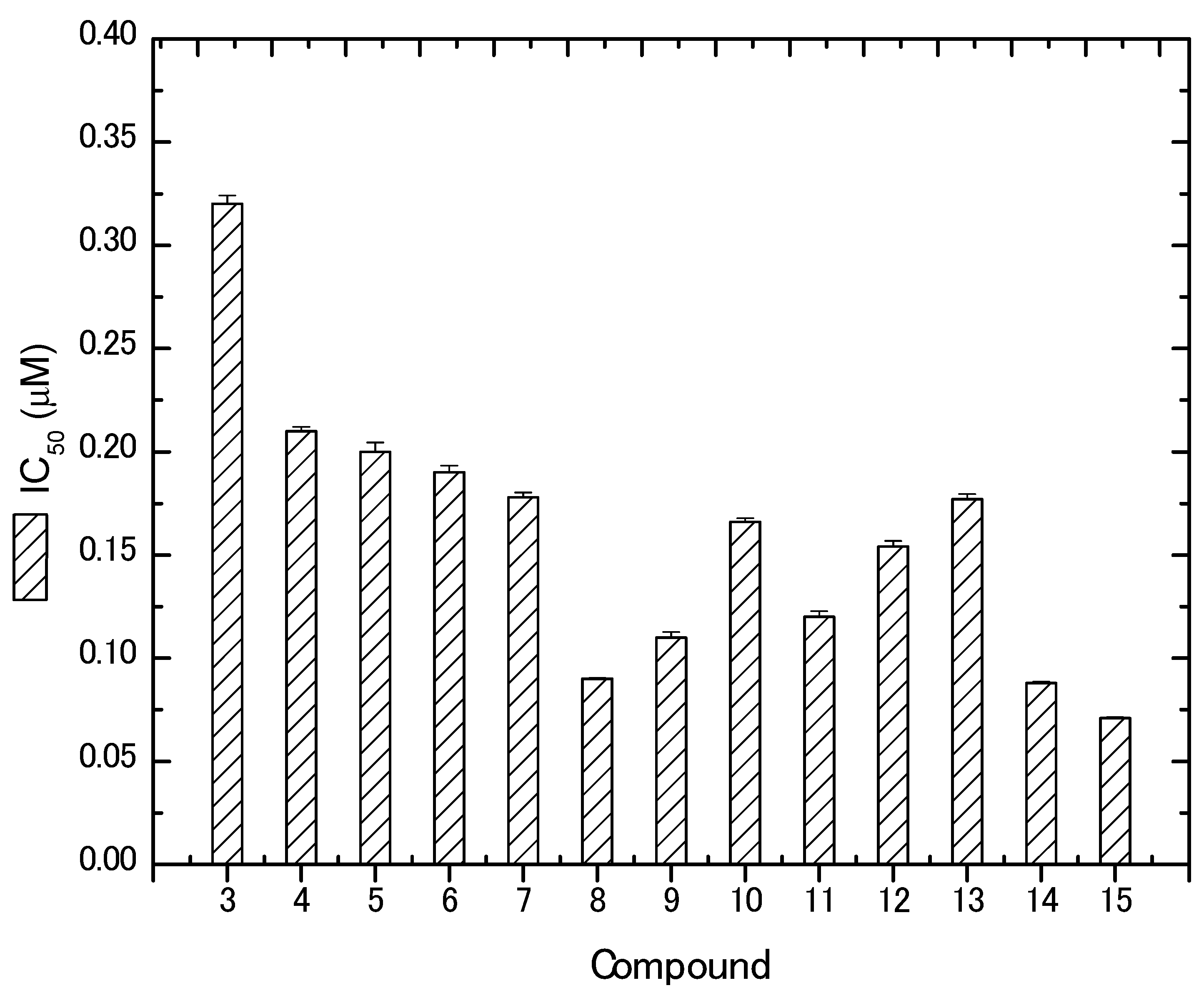
2.2. Biological Evaluation
In Vivo Xenograft Model
3. Materials and Methods
3.1. Chemistry
3.2. Biological Evaluation
3.2.1. Cytotoxic Assay
3.2.2. Human Breast Cancer Xenograft Animal Model
3.2.3. Pim-1 Kinase Inhibitory Activity
Materials and Methods
Pim-1 Kinase Protein Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amr, A.E.; Mohamed, A.M.; Mohamed, S.F.; Abdel-Hafez, N.A.; Hammam, A.G. Anticancer activities of some newly synthesized pyridine, pyrane and pyrimidine derivatives. Bioorg. Med. Chem. 2006, 14, 5481–5488. [Google Scholar] [CrossRef] [PubMed]
- Amr, A.E.; Abdalla, M.M. Anticancer activities of some synthesized 2,4,6-trisubstituted pyridine candidates. Biomed. Res. 2016, 27, 731–736. [Google Scholar]
- Mohamed, S.F.; Flefel, E.M.; Amr, A.E.; Abd El-Shafy, D.N. Anti-HSV-1 activity and mechanism of action of some new synthesized substituted pyrimidine, thiopyrimidine and thiazolopyrimidine derivatives. Eur. J. Med. Chem. 2010, 45, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Ouf, N.H.; Amr, A.E. Synthesis and anti-inflammatory activity of some pyrimidines and thienopyrimidines using 1-(2-benzo[d][1,3]dioxol-5-yl)vinyl)-4-mercapto-6-methyl- pyrimidine-5-yl)- ethan-2-one as a starting material. Mon. Chem. 2008, 139, 579–585. [Google Scholar] [CrossRef]
- Abdel-Hafez, N.A.; Mohamed, A.M.; Amr, A.E.; Abdalla, M.M. Antiarrhythmic activities of some new synthesized tricyclic and tetracyclic thienopyridine derivatives. Sci. Pharm. 2009, 77, 539–553. [Google Scholar] [CrossRef]
- Abdulla, M.M.; Amr, A.E.; Al-Omar, M.A.; Hussain, A.A.; Shalaby, A.F.A. Anti- inflammatory activity and acute toxicity (LD50) of some new synthesized pyridine-2-yl- phenyl)-2-methoxybenzamide and thieno[2,3-b]pyridine derivatives. Life Sci. J. 2013, 10, 286–297. [Google Scholar]
- Fayed, A.A.; Amr, A.E.; Al-Omar, M.A.; Mostafa, E.E. Synthesis and antimicrobial activity of some new substituted pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidinone derivatives. Russ. J. Bioorg. Chem. 2014, 40, 308–313. [Google Scholar] [CrossRef]
- Ouf, N.H.; Sakran, M.I.; Amr, A.E. Anti-inflammatory activities of some newly synthesized substituted thienochromene and Schiff base derivatives. Res. Chem. Intermed. 2015, 41, 2521–2536. [Google Scholar] [CrossRef]
- Said, S.A.; El-Sayed, H.A.; Amr, A.E.; Abdalla, M.M. Selective and orally bioavailable CHK1 inhibitors of some synthesized substituted thieno[2,3-b]pyridine candidates. Int. J. Pharm. 2015, 11, 659–671. [Google Scholar]
- Amr, A.E.; Al-Omar, M.A.; Abdalla, M.M. Biological evaluations of some synthesized pyrimidothieno[2,3-b]pyrimidine candidates as antiulcer agents. Int. J. Pharm. 2015, 11, 840–845. [Google Scholar] [CrossRef]
- Amr, A.E.; Abdalla, M.M.; Ouf, N.H. Anti-angiogenic effects of some (5-hydroxy-4- methyl-2-((1-methyl-1H-indol-3-yl)methyl)thieno[2,3-d]pyrimidine derivatives. J. Comput. Nanosci. 2017, 14, 448–453. [Google Scholar] [CrossRef]
- Nasr, M.N.; Gineinah, M.M. Pyrido[2,3-d]pyrimidines and pyrimido[5,4-5,6]pyrido[2,3-d] pyrimidines as new antiviral agents, synthesis and biologicalactivity. Arch. Pharm. Pharm. Med. Chem. 2002, 335, 289–295. [Google Scholar] [CrossRef]
- Chambhare, R.V.; Khadse, B.G.; Bobde, A.S.; Bahekar, R.H. Synthesis and preliminary evaluation of some N-[5-(2-furanyl)-2-methyl-4-oxo-4H-thieno[2,3-d]pyrimidine-3-yl]- carboxamide and 3-substituted-5-(2-furanyl)-2-methyl-3H-thieno[2,3-d]pyrimidine-4-ones as antimicrobial agents. Eur. J. Med. Chem. 2003, 38, 89–100. [Google Scholar] [CrossRef]
- Russell, R.K.; Press, J.B.; Rampulla, R.A.; McNally, J.J.; Falotico, R.; Keiser, J.A.; Bright, D.A.; Tobia, A. Thiophene systems. 9. Thienopyrimidinedione derivatives as potential antihypertensive agents. J. Med. Chem. 1988, 31, 1786–1793. [Google Scholar] [CrossRef]
- Alagarsamy, V.; Meena, S.; Ramseshu, K.V.; Solomon, V.R.; Thirumurugan, K.; Dhanabal, K.; Murugan, M. Synthesis, analgesic, anti-inflammatory, ulcerogenic index and antibacterial activities of novel 2-methylthio-3-substituted-5,6,7,8-tetrahydrobenzo thieno [2,3-d]pyrimidin- 4(3h)-ones. Eur. J. Med. Chem. 2006, 41, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Petrie, C.R.; Cottam, H.B.; Mckernan, P.A.; Robins, R.K.; Revankar, G.R. Synthesis and biological activity of 6-azacadeguomycin and certain 3,4,6-trisubstituted pyrazolo-[3,4-d]pyrimidine ribonuleosi. J. Med. Chem. 1985, 28, 1010–1016. [Google Scholar] [CrossRef]
- Mullican, M.D.; Wilson, M.W.; Connor, D.T.; Kostlan, C.R.; Schrier, D.J.; Dyer, R.D. Design of 5-(3,5-di-tert-butyl-4-hydroxyphenyl)-l,3,4-thiadiazoles-1,3,4-oxadiazoles, and 1,2,4-triazoles as orally-active, nonulcerogenic antiinflammatory agents. J. Med. Chem. 1993, 36, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Qian, X.; Zhang, R.; Song, G. Synthesis and quantitative structure-activity relationships of new 2,5-disubstituted-1,3,4-oxadiazoles. J. Agric. Food Chem. 2001, 49, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Gaonkar, S.L.; Rai, K.M.L.; Prabhuswamy, B. Synthesis and antimicrobial studies of a new series of 2-{4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl}-5-substituted-1,3,4-oxadiazoles. Eur. J. Med. Chem. 2006, 41, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.V.; Pate, P.K.; Kumari, P.; Rajani, D.P.; Chikhalia, K.H. Synthesis of benzimida zolyl-1,3,4-oxadiazol-2ylthio-n-phenyl (benzothiazolyl) acetamides as antibacterial, antifungal and antituberculosis agents. Eur. J. Med. Chem. 2012, 53, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Varvounis, G.; Giannopoulos, T. Synthesis, Chemistry, and Biological Properties of Thienopyrimidines. In Advances in Heterocyclic Chemistry; Alan, R.K., Ed.; Academic Press: Cambridge, MA, USA, 1996; pp. 193–283. [Google Scholar]
- Litvinov, V.P. The Chemistry of Thienopyrimidines. In Advances in Heterocyclic Chemistry; Alan, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2006; pp. 83–143. [Google Scholar]
- Dumaître, B.; Dodic, N. Synthesis and cyclic GMP phosphodiesterase inhibitory activity of a series of 6-phenylpyrazolo[3,4-d]pyrimidines. J. Med. Chem. 1996, 39, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbeny, M.A.; El-Ashmawy, M.B.; El-Subbagh, H.I.; El-Emam, A.A.; Badria, F.A. Synthesis, antimicrobial and antiviral evaluation of certain thienopyrimidine derivatives. Eur. J. Med. Chem. 1995, 30, 445–449. [Google Scholar] [CrossRef]
- Rizk, O.H.; Shaaban, O.G.; El-Ashmawy, I.M. Design, synthesis and biological evaluation of some novel thienopyrimidines and fused thienopyrimidines as anti-inflammatory agents. Eur. J. Med. Chem. 2012, 55, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.M.; Saleh, N.M.; Elhady, H.A. Design and synthesis of some new thiophene, thienopyrimidine and thienothiadiazine derivatives of antipyrine as potential antimicrobial agents. Eur. J. Med. Chem. 2011, 46, 4566–4572. [Google Scholar] [CrossRef] [PubMed]
- Elrazaz, E.Z.; Serya, R.A.T.; Ismail, N.S.M.; Abou El Ella, D.A.; Abouzid, K.A.M. Thieno[2,3-d]pyrimidine based derivatives as kinase inhibitors and anticancer agents. Future Journal of Pharmaceutical Sciences 2015, 1, 33–41. [Google Scholar] [CrossRef]
- Munchhof, M.J.; Beebe, J.S.; Casavant, J.M.; Cooper, B.A.; Doty, J.L.; Higdon, R.C.; Hillerman, S.M.; Soderstrom, C.I.; Knauth, E.A.; Marx, M.A.; et al. Design and SAR of thienopyrimidine and thienopyridine inhibitors of VEGFR-2 kinase activity. Bioorg. Med. Chem. Lett. 2004, 14, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Le, B.T.; Kumarasiri, M.; Adams, J.R.; Yu, M.; Milne, R.; Sykes, M.J.; Wang, S. Targeting Pim kinases for cancer treatment: Opportunities and challenges. Future Med. Chem. 2015, 7, 35–53. [Google Scholar] [CrossRef]
- Narlik-Grassow, M.; Blanco-Aparicio, C.; Carnero, A. The PIM family of serine/threonine kinases in cancer. Med. Res. Rev. 2014, 34, 136–159. [Google Scholar] [CrossRef]
- Nawijn, M.C.; Alendar, A.; Berns, A. For better or for worse: The role of PIM oncogenes in tumorigenesis. Nat. Rev. Cancer 2011, 11, 23–34. [Google Scholar] [CrossRef]
- Cuypers, H.T.; Selten, G.; Quint, W.; Zijlstra, M.; Maandag, E.R.; Boelens, W.; van Wezenbeek, P.; Melief, C.; Berns, A. Murine leukemia virus induced T-cell lymphomagenesis: Integration of proviruses in a distinct chromosomal region. Cell 1984, 37, 141–150. [Google Scholar] [CrossRef]
- Tursynbay, Y.; Zhang, J.; Li, Z.; Tokay, T.; Zhumadilov, Z.; Wu, D.; Xie, Y. PIM-1 kinase as cancer drug target: An update (Review). Biomed. Rep. 2016, 4, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Keane, N.A.; Reidy, M.; Natoni, A.; Raab, M.S.; O’Dwyer, M. Targeting the PIM kinases in multiple myeloma. Blood Cancer J. 2015, 5, e325. [Google Scholar] [CrossRef] [PubMed]
- Foulks, J.M.; Carpenter, K.J.; Luo, B.; Xu, Y.; Senina, A.; Nix, R.; Chan, A.; Clifford, A.; Wilkes, M.; Vollmer, D.; et al. A small-molecule inhibitor of pim kinases as a potential treatment for urothelial carcinomas. Neoplasia 2014, 16, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Decker, S.; Finter, J.; Forde, A.J.; Kissel, S.; Schwaller, J.; Mack, T.S.; Kuhn, A.; Gray, N.; Follo, M.; Jumaa, H.; et al. PIM kinases are essential for chronic lymphocytic leukemia cell survival (PIM2/3) and CXCR4-mediated microenvironmental interactions (PIM1). Mol. Cancer 2014, 13, 1231–1245. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zavorotinskaya, T.; Dai, Y.; Niu, X.H.; Castillo, J.; Sim, J.; Yu, J.; Wang, Y.; Langowski, J.L.; Holash, J.; et al. PIM2 is required for maintaining multiple myeloma cell growth through modulating TSC2 phosphorylation. Blood 2013, 122, 1610–1620. [Google Scholar] [CrossRef] [PubMed]
- Drygin, D.; Haddach, M.; Pierre, F.; Ryckman, D.M. Potential use of selective and nonselective pim kinase inhibitors for cancer therapy. J. Med. Chem. 2012, 55, 8199–8208. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Mao, X.; Chen, J.; Huang, B.; Jin, C.; Xu, Z.; Qiu, S. Overexpression of Pim-1 in bladder cancer. J. Exp. Clin. Cancer Res. 2010, 29, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Brault, L.; Gasser, C.; Bracher, F.; Huber, K.; Knapp, S.; Schwaller, J. PIM serine/threonine kinases in the pathogenesis and therapy of hematologic malignancies and solid cancers. Haematologica 2010, 95, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, E.A.; Sharaf-Eldin, M.A.; Wadaan, M. In vitro evaluation of cytotoxic activities of essential oil from Moringa oleifera seeds on HeLa, HepG2, MCF-7, CACO-2 and L929 cell lines. Asian Pac. J. Cancer Prev. 2015, 16, 4671–4675. [Google Scholar] [CrossRef]
- Elsayed, E.A.; Farooq, M.; Dailin, D.; El-Enshasy, H.A.; Othman, N.Z.; Malek, R.; Danial, E.; Wadaan, M. In vitro and in vivo biological screening of kefiran polysaccharide produced by lactobacillus kefiranofaciens. Biomed. Res. 2017, 28, 594–600. [Google Scholar]
- Amr, A.E.; Elsayed, E.A.; Al-Omar, M.A.; Badr Eldin, H.O.; Nossier, E.S.; Abdallah, M.M. Design, synthesis, anticancer evaluation and molecular modeling of novel estrogen derivatives. Molecules 2019, 24, 416. [Google Scholar] [CrossRef]
- Amr, A.E.; El-Naggar, M.; Al-Omar, M.A.; Elsayed, E.A.; Abdalla, M.M. In vitro and in vivo anti-breast cancer activities of some synthesized pyrazolinyl-estran-17-one candidates. Molecules 2018, 23, 1572. [Google Scholar] [CrossRef]
- McCauley, J.; Zivanovic, A.; Skropeta, D. Bioassays for anticancer activities. Methods Mol. Biol. 2013, 1055, 191–205. [Google Scholar]
- Wang, H.; Yu, D.; Agrawal, S.; Zhang, R. Experimental therapy of human prostate cancer by inhibiting MDM2 expression with novel mixed-backbone antisense oligonucleotides: In vitro and in vivo activities and mechanisms. Prostate 2003, 54, 194–205. [Google Scholar] [CrossRef]
- Naguib, B.H.; El-Nassan, H.B.; Abdelghany, T.M. Synthesis of new pyridothieno- pyrimidinone derivatives as Pim-1 inhibitors. J. Enzyme Inhibit. Med. Chem. 2017, 32, 457–467. [Google Scholar] [CrossRef]
Sample Availability: Samples of the all compounds are available from the authors. |



| Drugs | Average Weight of Animals in Grams after Days | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | 22 | |
| Control | 28 | 28 | 28 | 28 | 28.2 | 28. | 28.3 | 28.4 | 28.4 | 28.4 | 28.4 | 28.4 |
| 3 | 28 | 28 | 28 | 28 | 28.2 | 27.9 | 27.8 | 27.7 | 27.7 | 27.6 | 27.7 | 27.7 |
| 4 | 24.2 | 24.2 | 24.1 | 24.0 | 23.9 | 23.9 | 23.7 | 23.7 | 23.6 | 23.6 | 23.6 | 236. |
| 5 | 22.9 | 22.9 | 22.9 | 22.8 | 22.8 | 22.7 | 22.8 | 22.7 | 22.9 | 22.6 | 22.6 | 22.5 |
| 6 | 23.7 | 23.7 | 23.6 | 23.6 | 23.5 | 23.5 | 23.5 | 23.4 | 23.4 | 23.5 | 23.5 | 23.5 |
| 7 | 27.3 | 27.3 | 27.2 | 27.2 | 27.2 | 27.1 | 27.1 | 27.1 | 27.0 | 27.0 | 27.0 | 27.0 |
| 8 | 25.3 | 25.3 | 25.2 | 25.2 | 25.2 | 25.1 | 25.1 | 24.8 | 24.7 | 24.6 | 24.5 | 24.2 |
| 9 | 24.1 | 24.1 | 24.0 | 24.0 | 24.0 | 24.0 | 23.7 | 23.7 | 23.6 | 23.6 | 23.6 | 23.6 |
| 10 | 24.9 | 24.9 | 24.9 | 24.9 | 24.8 | 24.8 | 24.8 | 24.4 | 24.4 | 24.4 | 24.4 | 24.4 |
| 11 | 26.6 | 26.6 | 26.6 | 26.5 | 26.6 | 26.6 | 26.4 | 26.3 | 26.3 | 26.3 | 26.3 | 263. |
| 12 | 27.4 | 27.4 | 27.4 | 27.3 | 27.3 | 27.3 | 27.2 | 27.2 | 27.2 | 27.1 | 27.1 | 27.1 |
| 13 | 25.4 | 25.4 | 25.4 | 25.4 | 25.4 | 25.3 | 25.3 | 25.3 | 25.3 | 25.3 | 25.3 | 25.3 |
| 14 | 26.5 | 26.4 | 26.3 | 26.2 | 26.1 | 25.9 | 25.9 | 25.9 | 25.8 | 25.8 | 25.7 | 25.7 |
| 15 | 26.1 | 26.1 | 26.1 | 25.8 | 25.8 | 25.7 | 25.7 | 25.6 | 25.6 | 25.5 | 25.5 | 25.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amr, A.E.-G.E.; Ibrahimd, A.A.; El-Shehry, M.F.; Hosni, H.M.; Fayed, A.A.; Elsayed, E.A. In Vitro and In Vivo Anti-Breast Cancer Activities of Some Newly Synthesized 5-(thiophen-2-yl)thieno-[2,3-d]pyrimidin-4-one Candidates. Molecules 2019, 24, 2255. https://doi.org/10.3390/molecules24122255
Amr AE-GE, Ibrahimd AA, El-Shehry MF, Hosni HM, Fayed AA, Elsayed EA. In Vitro and In Vivo Anti-Breast Cancer Activities of Some Newly Synthesized 5-(thiophen-2-yl)thieno-[2,3-d]pyrimidin-4-one Candidates. Molecules. 2019; 24(12):2255. https://doi.org/10.3390/molecules24122255
Chicago/Turabian StyleAmr, Abd El-Galil E., Alhussein A. Ibrahimd, Mohamed F. El-Shehry, Hanaa M. Hosni, Ahmed A. Fayed, and Elsayed A. Elsayed. 2019. "In Vitro and In Vivo Anti-Breast Cancer Activities of Some Newly Synthesized 5-(thiophen-2-yl)thieno-[2,3-d]pyrimidin-4-one Candidates" Molecules 24, no. 12: 2255. https://doi.org/10.3390/molecules24122255
APA StyleAmr, A. E.-G. E., Ibrahimd, A. A., El-Shehry, M. F., Hosni, H. M., Fayed, A. A., & Elsayed, E. A. (2019). In Vitro and In Vivo Anti-Breast Cancer Activities of Some Newly Synthesized 5-(thiophen-2-yl)thieno-[2,3-d]pyrimidin-4-one Candidates. Molecules, 24(12), 2255. https://doi.org/10.3390/molecules24122255







