From Elemental Sulfur to Hydrogen Sulfide in Agricultural Soils and Plants
Abstract
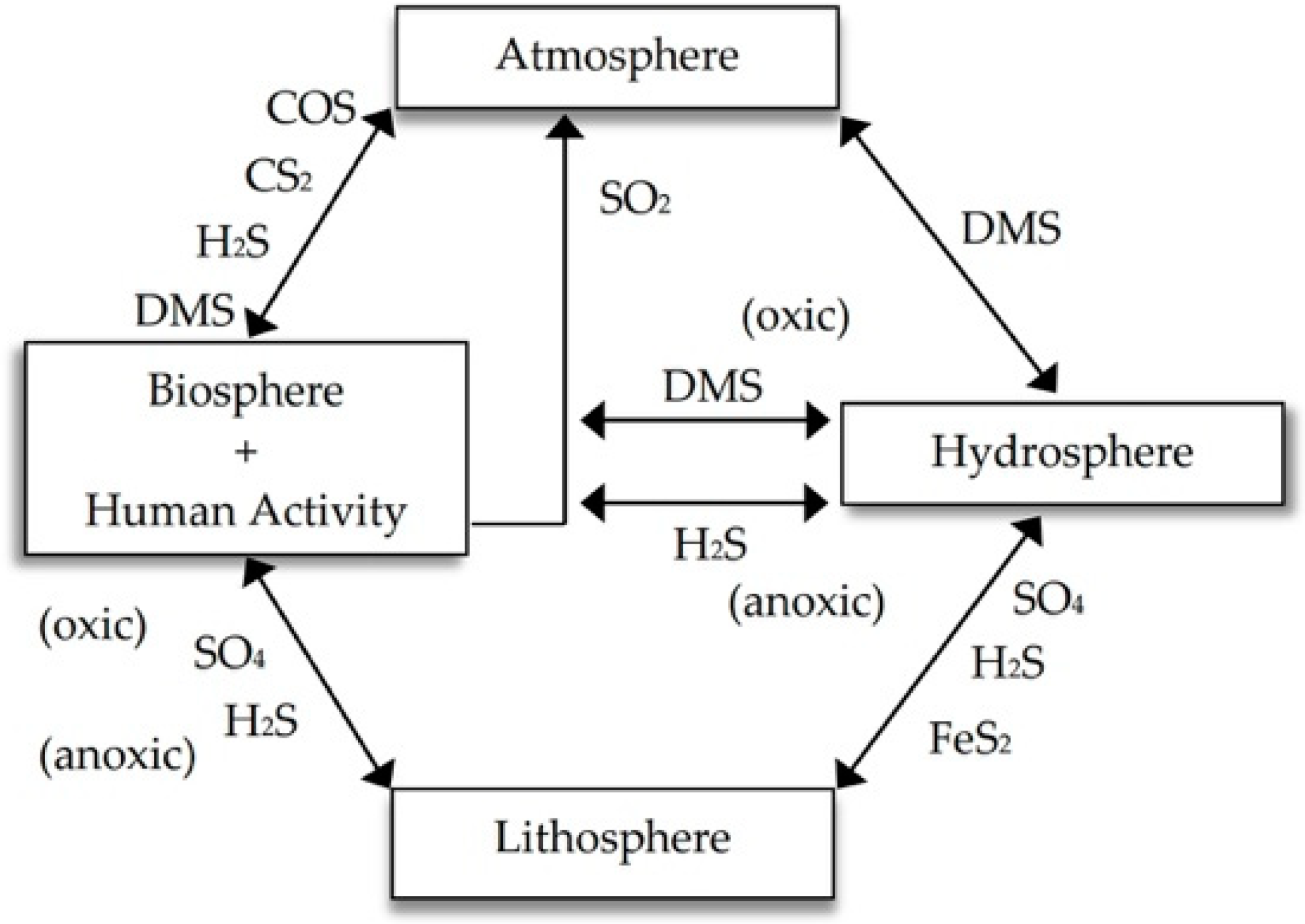
1. Introduction
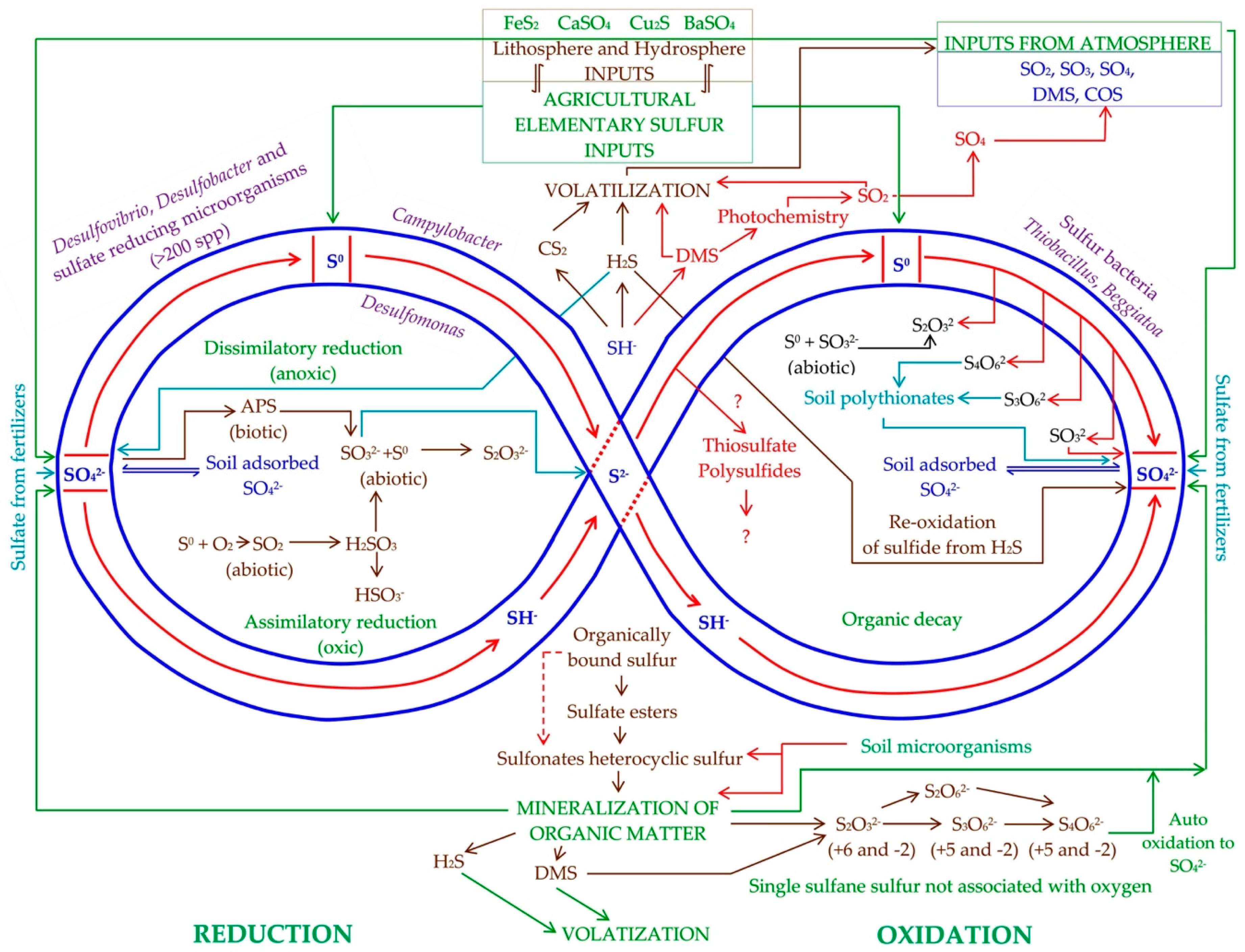
2. Transformations of Elemental Sulfur in Soil
3. Absorption and Assimilation of Sulfur in Plants
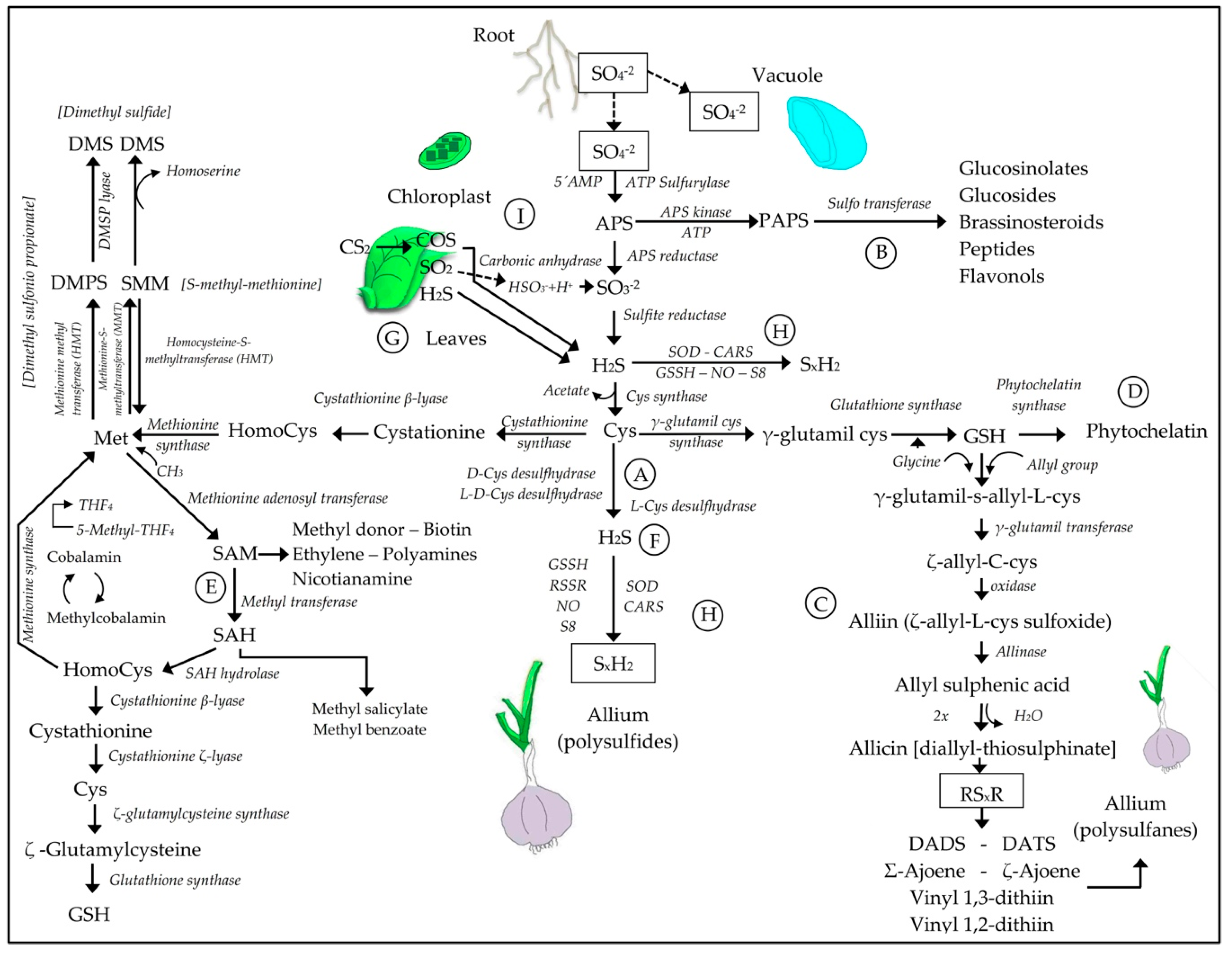
3.1. Sulfur Absorption and Transport
3.2. Sulfur Assimilation and the Synthesis of H2S
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Kok, L.J.; Castro, A.; Durenkamp, M.; Koralewska, A.; Posthumus, F.S.; Stuiver, C.E.E.; Yang, L.; Stulen, I. Pathways of plant sulfur uptake and metabolism—An overview. In Proceedings of the 1st Sino-German Workshop on Aspects of Sulfur Nutrition of Plants, Shenyang, China, 23–27 May 2004; Schnug, E., de Kok, L.J., Eds.; FAL Agricultural Research: Braunschweig, Germany, 2005; pp. 5–13, ISBN 3-86576-007-4. [Google Scholar]
- Huxtable, R.J. Biochemistry of Sulfur; Springer: Boston, MA, USA, 1986; ISBN 978-1-4757-9438-0. [Google Scholar]
- Rendig, V.V.; Oputa, C.; McComb, E.A. Effects of sulfur deficiency on non-protein nitrogen, soluble sugars, and N/S ratios in young corn (Zea mays L.) plants. Plant Soil 1976, 44, 423–437. [Google Scholar] [CrossRef]
- Reuveny, Z.; Dougall, D.K.; Trinity, P.M. Regulatory coupling of nitrate and sulfate assimilation pathways in cultured tobacco cells. Proc. Natl. Acad. Sci. USA 1980, 77, 6670–6672. [Google Scholar] [CrossRef] [PubMed]
- Rennenberg, H. The fate of excess sulfur in higher plants. Annu. Rev. Plant Physiol. 1984, 35, 121–153. [Google Scholar] [CrossRef]
- Andreae, M.O. Ocean-atmosphere interactions in the global biogeochemical sulfur cycle. Mar. Chem. 1990, 30, 1–29. [Google Scholar] [CrossRef]
- Tolocka, M.P.; Turpin, B. Contribution of organosulfur compounds to organic aerosol mass. Environ. Sci. Technol. 2012, 46, 7978–7983. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, R.; Norton, R. Soil and fertilizer sulfur. Better Crop. 2013, 97, 7–9. [Google Scholar]
- Chao, T.T.; Harward, M.E.; Fang, S.C. Cationic effects on sulfate adsorption by soils. Soil Sci. Soc. Am. J. 1963, 27, 35–38. [Google Scholar] [CrossRef]
- Chao, T.T.; Harward, M.E.; Fang, S.C. Adsorption and desorption phenomena of sulfate ions in soils. Soil Sci. Soc. Am. J. 1962, 26, 234–237. [Google Scholar] [CrossRef]
- Przygocka-Cyna, K.; Biber, M.; Przygocka-Cyna, K.; Grzebisz, W.; Pluta, M.; Grzebisz, W. Mineral density of onion bulbs as affected by fertilizers based on elemental sulfur. J. Elem. 2016, 21, 485–499. [Google Scholar] [CrossRef]
- González-Morales, S.; Pérez-Labrada, F.; García-Enciso, E.L.; Leija-Martínez, P.; Medrano-Macías, J.; Dávila-Rangel, I.E.; Juárez-Maldonado, A.; Rivas-Martínez, E.N.; Benavides-Mendoza, A. Selenium and sulfur to produce Allium functional crops. Molecules 2017, 22, 558. [Google Scholar] [CrossRef]
- Tea, I.; Genter, T.; Naulet, N.; Boyer, V.; Lummerzheim, M.; Kleiber, D. Effect of foliar sulfur and nitrogen fertilization on wheat storage protein composition and dough mixing properties. Cereal Chem. J. 2004, 81, 759–766. [Google Scholar] [CrossRef]
- TSI Sulphur Fertilizer Types. Available online: https://www.sulphurinstitute.org/fertilizer/sulphate.cfm (accessed on 5 May 2019).
- Johnson, D.W.; Cole, D.W. Anion mobility in soils: Relevance to nutrient transport from forest ecosystems. Environ. Int. 1980, 3, 79–90. [Google Scholar] [CrossRef]
- Hutt, L.P. Taxonomy, Physiology and Biochemistry of the Sulfur Bacteria; Plymouth University: Plymouth, UK, 2017. [Google Scholar]
- Montesinos-Pereira, D.; de la Torre-González, A.; Blasco, B.; Ruiz, J.M. Hydrogen sulphide increase the tolerance to alkalinity stress in cabbage plants (Brassica oleracea L. ’Bronco’). Sci. Hortic. (Amsterdam) 2018, 235, 349–356. [Google Scholar] [CrossRef]
- Steiger, A.K.; Zhao, Y.; Pluth, M.D. Emerging roles of carbonyl sulfide in chemical biology: Sulfide transporter or gasotransmitter? Antioxid. Redox Signal. 2018, 28, 1516–1532. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Macías, J.; Leija-Martínez, P.; González-Morales, S.; Juárez-Maldonado, A.; Benavides-Mendoza, A. Use of iodine to biofortify and promote growth and stress tolerance in crops. Front. Plant Sci. 2016, 7, 1146. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.W. Quantum-chemical calculations of sulfur-rich compounds. Top. Curr. Chem. 2003, 231, 1–29. [Google Scholar] [CrossRef]
- Mayer, R. Elemental sulfur and its reactions. In Organic Chemistry of Sulfur; Oae, S., Ed.; Plenum Press: New York, NY, USA, 1977; p. 681. ISBN 978-1-4684-2049-4. [Google Scholar]
- Reusch, W. Nucleophilicity of Sulfur Compounds. Available online: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Thiols_and_Sulfides/Nucleophilicity_of_Sulfur_Compounds (accessed on 5 May 2019).
- Chapman, S.J. Powdered elemental sulphur: Oxidation rate, temperature dependence and modelling. Nutr. Cycl. Agroecosyst. 1996, 47, 19–28. [Google Scholar] [CrossRef]
- Lucheta, A.R.; Lambais, M.R. Sulfur in agriculture. Rev. Bras. Ciência do Solo 2012, 36, 1369–1379. [Google Scholar] [CrossRef]
- Wörner, S.; Zecchin, S.; Dan, J.; Todorova, N.H.; Loy, A.; Conrad, R.; Pester, M. Gypsum amendment to rice paddy soil stimulated bacteria involved in sulfur cycling but largely preserved the phylogenetic composition of the total bacterial community. Environ. Microbiol. Rep. 2016, 8, 413–423. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Skogley, E.O.; Engel, R.E. Band-applied elemental sulfur to enhance the phytoavailability of phosphorus in alkaline calcareous soils. Biol. Fertil. Soils 1989, 7, 346–350. [Google Scholar] [CrossRef]
- Degryse, F.; Ajiboye, B.; Baird, R.; da Silva, R.C.; McLaughlin, M.J. Oxidation of elemental sulfur in granular fertilizers depends on the soil-exposed surface area. Soil Sci. Soc. Am. J. 2016, 80, 294. [Google Scholar] [CrossRef]
- Zhao, C.; Degryse, F.; Gupta, V.; McLaughlin, M.J. Low effective surface area explains slow oxidation of co-granulated elemental sulfur. Soil Sci. Soc. Am. J. 2016, 80, 911–918. [Google Scholar] [CrossRef]
- Lee, A.; Boswell, C.C.; Watkinson, J.H. Effect of particle size on the oxidation of elemental sulphur, thiobacilli numbers, soil sulphate, and its availability to pasture. N. Z. J. Agric. Res. 1988, 31, 179–186. [Google Scholar] [CrossRef]
- Haneklaus, S.; Bloem, E.; Schnug, E. Sulfur interactions in crop ecosystems. In Sulfur in Plants An Ecological Perspective; Hawkesford, M.J., De Kok, L.J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 16–58. ISBN 978-1-4020-5887-5. [Google Scholar]
- Gupta, V.V.S.R.; Lawrence, J.R.; Germida, J.J. Impact of elemental sulfur fertilization on agricultural soils. I. Effects on microbial biomass and enzyme activities. Can. J. Soil Sci. 1988, 68, 463–473. [Google Scholar] [CrossRef]
- Cooper, R.M.; Williams, J.S. Elemental sulphur as an induced antifungal substance in plant defence. J. Exp. Bot. 2004, 55, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Bloem, E.; Haneklaus, S.; Schnug, E. Milestones in plant sulfur research on sulfur-induced-resistance (SIR) in Europe. Front. Plant Sci. 2015, 5, 779. [Google Scholar] [CrossRef] [PubMed]
- Terán, G.E.; Benavides, A.; Hernández, F.; Quero, E. New Technologies for Horticultural Crops. In Plant Production on the Threshold of a New Century; Struik, P.C., Vredenberg, W.J., Renkema, J.A., Parlevliet, J.E., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 375–380. ISBN 0792329031. [Google Scholar]
- Almutairi, K.F.; Machado, R.M.A.; Bryla, D.R.; Strik, B.C. Chemigation with micronized sulfur rapidly reduces soil pH in a new planting of northern highbush blueberry. HortScience 2017, 52, 1413–1418. [Google Scholar] [CrossRef]
- Roy Choudhury, S.; Ghosh, M.; Mandal, A.; Chakravorty, D.; Pal, M.; Pradhan, S.; Goswami, A. Surface-modified sulfur nanoparticles: An effective antifungal agent against Aspergillus niger and Fusarium oxysporum. Appl. Microbiol. Biotechnol. 2011, 90, 733–743. [Google Scholar] [CrossRef]
- Rao, K.J.; Paria, S. Use of sulfur nanoparticles as a green pesticide on Fusarium solani and Venturia inaequalis phytopathogens. RSC Adv. 2013, 3, 10471. [Google Scholar] [CrossRef]
- Juárez-Maldonado, A.; Ortega-Ortíz, H.; Morales-Díaz, A.B.; González-Morales, S.; Morelos-Moreno, Á.; Cabrera-De la Fuente, M.; Sandoval-Rangel, A.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Nanoparticles and nanomaterials as plant biostimulants. Int. J. Mol. Sci. 2019, 20, 162. [Google Scholar] [CrossRef]
- Williams, J.S.; Cooper, R.M. Elemental sulphur is produced by diverse plant families as a component of defence against fungal and bacterial pathogens. Physiol. Mol. Plant Pathol. 2003, 63, 3–16. [Google Scholar] [CrossRef]
- Wainwright, M. Sulfur oxidation in soils. Adv. Agron. 1984, 37, 349–396. [Google Scholar] [CrossRef]
- Zhao, C.; Gupta, V.V.S.R.; Degryse, F.; McLaughlin, M.J. Effects of pH and ionic strength on elemental sulphur oxidation in soil. Biol. Fertil. Soils 2017, 53, 247–256. [Google Scholar] [CrossRef]
- Kumar, U.; Panneerselvam, P.; Gupta, V.V.S.R.; Manjunath, M.; Priyadarshinee, P.; Sahoo, A.; Dash, S.R.; Kaviraj, M.; Annapurna, K. Diversity of sulfur-oxidizing and sulfur-reducing microbes in diverse ecosystems. In Advances in Soil Microbiology: Recent Trends and Future Prospects. Microorganisms for Sustainability; Adhya, T., Lal, B., Mohapatra, B., Paul, D., Das, S., Eds.; Springer: Singapore, 2018; Volume 3, pp. 65–89. ISBN 978-981-10-6178-3. [Google Scholar]
- Li, G.; Zhao, Y.; Li, P.; Zhang, F.; Qu, P.; Li, B.; Gao, Q.; Wang, S. Antibacterial activities of polythionates enhanced by carbonates. Medchemcomm 2015, 6, 1643–1648. [Google Scholar] [CrossRef]
- Findlay, A.J. Microbial impact on polysulfide dynamics in the environment. FEMS Microbiol. Lett. 2016, 363, fnw103. [Google Scholar] [CrossRef]
- Kimura, H. Signaling molecules: Hydrogen sulfide and polysulfide. Antioxid. Redox Signal. 2015, 22, 362–376. [Google Scholar] [CrossRef]
- Calderwood, A.; Kopriva, S. Hydrogen sulfide in plants: From dissipation of excess sulfur to signaling molecule. Nitric Oxide 2014, 41, 72–78. [Google Scholar] [CrossRef]
- Rennenberg, H. Synthesis and emission of hydrogen sulfide by higher plants. In Biogenic Sulfur in the Environment; Saltzman, E.S., Cooper, W.J., Eds.; American Chemical Society: Washington, DC, USA, 1989; pp. 44–57. [Google Scholar]
- Gigolashvili, T.; Kopriva, S. Transporters in plant sulfur metabolism. Front. Plant Sci. 2014, 5, 442. [Google Scholar] [CrossRef]
- Maruyama-Nakashita, A. Metabolic changes sustain the plant life in low-sulfur environments. Curr. Opin. Plant Biol. 2017, 39, 144–151. [Google Scholar] [CrossRef]
- Janík, R.; Bublinec, E.; Dubová, M. Sulphate concentration and S-SO42– flux in soil solutions in the West Carpathians Mountains on an example of submontane beech forest stand. J. For. Sci. 2012, 58, 35–44. [Google Scholar] [CrossRef]
- Takahashi, H.; Kopriva, S.; Giordano, M.; Saito, K.; Hell, R. Sulfur assimilation in photosynthetic organisms: Molecular functions and regulations of transporters and assimilatory enzymes. Annu. Rev. Plant Biol. 2011, 62, 157–184. [Google Scholar] [CrossRef]
- Kataoka, T.; Watanabe-Takahashi, A.; Hayashi, N.; Ohnishi, M.; Mimura, T.; Buchner, P.; Hawkesford, M.J.; Yamaya, T.; Takahashi, H. Vacuolar sulfate transporters are essential determinants controlling internal distribution of sulfate in Arabidopsis. Plant Cell 2004, 16, 2693–2704. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Inoue, E.; Saito, K.; Yamaya, T.; Takahashi, H. Phloem-localizing sulfate transporter, Sultr1;3, mediates re-distribution of sulfur from source to sink organs in Arabidopsis. Plant Physiol. 2003, 131, 1511–1517. [Google Scholar] [CrossRef]
- Kirschner, S.; Woodfield, H.; Prusko, K.; Koczor, M.; Gowik, U.; Hibberd, J.M.; Westhoff, P. Expression of SULTR2;2, encoding a low-affinity sulphur transporter, in the Arabidopsis bundle sheath and vein cells is mediated by a positive regulator. J. Exp. Bot. 2018, 69, 4897–4906. [Google Scholar] [CrossRef]
- Kataoka, T. Root-to-shoot transport of sulfate in Arabidopsis. Evidence for the role of SULTR3;5 as a component of low-affinity sulfate transport system in the root vasculature. Plant Physiol. 2004, 136, 4198–4204. [Google Scholar] [CrossRef]
- Cao, M.J.; Wang, Z.; Wirtz, M.; Hell, R.; Oliver, D.J.; Xiang, C. Bin SULTR3;1 is a chloroplast-localized sulfate transporter in Arabidopsis thaliana. Plant J. 2013, 73, 607–616. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, M.; Xia, Z. The SULTR gene family in maize (Zea mays L.): Gene cloning and expression analyses under sulfate starvation and abiotic stress. J. Plant Physiol. 2018, 220, 24–33. [Google Scholar] [CrossRef]
- Gallardo, K.; Courty, P.-E.; Le Signor, C.; Wipf, D.; Vernoud, V. Sulfate transporters in the plants response to drought and salinity: Regulation and possible functions. Front. Plant Sci. 2014, 5, 580. [Google Scholar] [CrossRef]
- Akbudak, M.A.; Filiz, E.; Kontbay, K. Genome-wide identification and cadmium induced expression profiling of sulfate transporter (SULTR) genes in sorghum (Sorghum bicolor L.). BioMetals 2018, 31, 91–105. [Google Scholar] [CrossRef]
- Aghajanzadeh, T.; Hawkesford, M.J.; De Kok, L.J. Atmospheric H2S and SO2 as sulfur sources for Brassica juncea and Brassica rapa: Regulation of sulfur uptake and assimilation. Environ. Exp. Bot. 2016, 124, 1–10. [Google Scholar] [CrossRef]
- Mazid, M.; Zeba, H.K.; Quddusi, S.; Khan, T.A.; Mohammad, F. Significance of sulphur nutrition against metal induced oxidative stress in plants. J. Stress Physiol. Biochem. 2011, 7, 165–184. [Google Scholar]
- Birke, H.; De Kok, L.J.; Wirtz, M.; Hell, R. The Role of compartment-specific cysteine synthesis for sulfur homeostasis during H2S exposure in Arabidopsis. Plant Cell Physiol. 2015, 56, 358–367. [Google Scholar] [CrossRef]
- Riemenschneider, A.; Wegele, R.; Schmidt, A.; Papenbrock, J. Isolation and characterization of a D-cysteine desulfhydrase protein from Arabidopsis thaliana. FEBS J. 2005, 272, 1291–1304. [Google Scholar] [CrossRef]
- Jing, W.W.; Li, N.; Li, X.F.; Li, D.Q.; Wang, L.L. Exchange fluxes of VOSCs between rice paddy fields and the atmosphere in the oasis of arid area in Xinjiang, China. J. Atmos. Chem. 2018, 75, 17–32. [Google Scholar] [CrossRef]
- Khan, M.N.; AlZuaibr, F.M.; Al-Huqail, A.A.; Siddiqui, M.H.; M. Ali, H.; Al-Muwayhi, M.A.; Al-Haque, H.N. Hydrogen sulfide-mediated activation of O-Acetylserine (Thiol) Lyase and l/d-Cysteine Desulfhydrase enhance dehydration tolerance in Eruca sativa Mill. Int. J. Mol. Sci. 2018, 19, 3981. [Google Scholar] [CrossRef]
- Da-Silva, C.J.; Modolo, L.V. Hydrogen sulfide: A new endogenous player in an old mechanism of plant tolerance to high salinity. Acta Bot. Brasilica 2018, 32, 150–160. [Google Scholar] [CrossRef]
- Ausma, T.; Parmar, S.; Hawkesford, M.J.; De Kok, L.J. Impact of atmospheric H2S, salinity and anoxia on sulfur metabolism in Zea mays. In Sulfur Metabolism in Higher Plants—Fundamental, Environmental and Agricultural Aspects; De Kok, L.J., Hawkesford, M.J., Haneklaus, S.H., Schnug, E., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 93–101. [Google Scholar]
- Saito, K. Sulfur assimilatory metabolism. The long and smelling road. Plant Physiol. 2004, 136, 2443–2450. [Google Scholar] [CrossRef]
- Stimler, K.; Montzka, S.A.; Berry, J.A.; Rudich, Y.; Yakir, D. Relationships between carbonyl sulfide (COS) and CO2 during leaf gas exchange. New Phytol. 2010, 186, 869–878. [Google Scholar] [CrossRef]
- Stimler, K.; Berry, J.A.; Yakir, D. Effects of carbonyl sulfide and carbonic anhydrase on stomatal conductance. Plant Physiol. 2012, 158, 524–530. [Google Scholar] [CrossRef]
- Wirtz, M.; Droux, M. Synthesis of the sulfur amino acids: Cysteine and methionine. Photosynth. Res. 2005, 86, 345–362. [Google Scholar] [CrossRef]
- Kharma, A.; Grman, M.; Misak, A.; Domínguez-Álvarez, E.; Nasim, M.; Ondrias, K.; Chovanec, M.; Jacob, C. Inorganic polysulfides and related reactive sulfur–selenium species from the perspective of chemistry. Molecules 2019, 24, 1359. [Google Scholar] [CrossRef]
- Bullock, H.A.; Luo, H.; Whitman, W.B. Evolution of dimethylsulfoniopropionate metabolism in marine phytoplankton and bacteria. Front. Microbiol. 2017, 8, 637. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, D.K. Pharmacological effects of garlic (Allium sativum L.). Annu. Rev. Biomed. Sci. 2008, 10, 6–26. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Yabe, A.; Sugino, Y.; Murakami, S.; Sai-Ngam, N.; Sumi, S.-I.; Tsuneyoshi, T.; Saito, K. Garlic γ-glutamyl transpeptidases that catalyze deglutamylation of biosynthetic intermediate of alliin. Front. Plant Sci. 2015, 5, 758. [Google Scholar] [CrossRef]
- Rennenberg, H.; Herschbach, C. A detailed view on sulphur metabolism at the cellular and whole-plant level illustrates challenges in metabolite flux analyses. J. Exp. Bot. 2014, 65, 5711–5724. [Google Scholar] [CrossRef]
- Kopriva, S.; Mugford, S.G.; Baraniecka, P.; Lee, B.-R.; Matthewman, C.A.; Koprivova, A. Control of sulfur partitioning between primary and secondary metabolism in Arabidopsis. Front. Plant Sci. 2012, 3, 163. [Google Scholar] [CrossRef]
- Li, Q.; Lancaster, J.R. Chemical foundations of hydrogen sulfide biology. Nitric Oxide 2013, 35, 21–34. [Google Scholar] [CrossRef]
- Predmore, B.L.; Lefer, D.J.; Gojon, G. Hydrogen sulfide in biochemistry and medicine. Antioxid. Redox Signal. 2012, 17, 119–140. [Google Scholar] [CrossRef]
- Jin, Z.; Sun, L.; Yang, G.; Pei, Y. Hydrogen sulfide regulates energy production to delay leaf senescence induced by drought stress in Arabidopsis. Front. Plant Sci. 2018, 9, 1722. [Google Scholar] [CrossRef]
- Guo, H.; Xiao, T.; Zhou, H.; Xie, Y.; Shen, W. Hydrogen sulfide: A versatile regulator of environmental stress in plants. Acta Physiol. Plant. 2016, 38, 16. [Google Scholar] [CrossRef]
- Chen, J.; Shang, Y.-T.; Wang, W.-H.; Chen, X.-Y.; He, E.-M.; Zheng, H.-L.; Shangguan, Z. Hydrogen sulfide-mediated polyamines and sugar changes are involved in hydrogen sulfide-induced drought tolerance in Spinacia oleracea seedlings. Front. Plant Sci. 2016, 7, 1173. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, L.-Y.; Hu, K.-D.; He, Y.-D.; Wang, S.-H.; Luo, J.-P. Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J. Integr. Plant Biol. 2008, 50, 1518–1529. [Google Scholar] [CrossRef]
- Christou, A.; Manganaris, G.A.; Papadopoulos, I.; Fotopoulos, V. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J. Exp. Bot. 2013, 64, 1953–1966. [Google Scholar] [CrossRef]
- Zhang, H.; Jiao, H.; Jiang, C.-X.; Wang, S.-H.; Wei, Z.-J.; Luo, J.-P.; Jones, R.L. Hydrogen sulfide protects soybean seedlings against drought-induced oxidative stress. Acta Physiol. Plant. 2010, 32, 849–857. [Google Scholar] [CrossRef]
- Corpas, F.J.; González-Gordo, S.; Cañas, A.; Palma, J.M. Nitric oxide and hydrogen sulfide in plants: Which comes first? J. Exp. Bot. 2019. [Google Scholar] [CrossRef]
- Hancock, J.T. Hydrogen sulfide and environmental stresses. Environ. Exp. Bot. 2019, 161, 50–56. [Google Scholar] [CrossRef]
- Gruhlke, M.C.H.; Slusarenko, A.J. The biology of reactive sulfur species (RSS). Plant Physiol. Biochem. 2012, 59, 98–107. [Google Scholar] [CrossRef]
- IUPAC Compendium of Chemical Terminology Gold Book. Release 2.3.3b. Available online: http://goldbook.iupac.org/index.html (accessed on 12 May 2019).
- Schneider, T.; Ba, L.A.; Khairan, K.; Zwergel, C.; Bach, N.D.; Bernhardt, I.; Brandt, W.; Wessjohann, L.; Diederich, M.; Jacob, C. Interactions of polysulfanes with components of red blood cells. Medchemcomm 2011, 2, 196–200. [Google Scholar] [CrossRef]
- Grman, M.; Nasim, M.; Leontiev, R.; Misak, A.; Jakusova, V.; Ondrias, K.; Jacob, C. Inorganic reactive sulfur-nitrogen species: Intricate release mechanisms or cacophony in yellow, blue and red? Antioxidants 2017, 6, 14. [Google Scholar] [CrossRef]
- Oosthuizen, C.; Arbach, M.; Meyer, D.; Hamilton, C.; Lall, N. Diallyl polysulfides from Allium sativum as immunomodulators, hepatoprotectors, and antimycobacterial agents. J. Med. Food 2017, 20, 685–690. [Google Scholar] [CrossRef]
- Anwar, A.; Gould, E.; Tinson, R.; Iqbal, J.; Hamilton, C. Redox modulation at work: Natural phytoprotective polysulfanes from alliums based on redox-active sulfur. Curr. Pharmacol. Rep. 2018, 4, 397–407. [Google Scholar] [CrossRef]
- O’Gara, E.A.; Hill, D.J.; Maslin, D.J. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl. Environ. Microbiol. 2000, 66, 2269–2273. [Google Scholar] [CrossRef]
- Pluth, M.D.; Bailey, T.S.; Hammers, M.D.; Hartle, M.D.; Henthorn, H.A.; Steiger, A.K. Natural products containing hydrogen sulfide releasing moieties. Synlett 2015, 26, 2633–2643. [Google Scholar] [CrossRef]

| Oxidation State | Representative Compound and Formula | Oxidation State | Representative Compound and Formula |
|---|---|---|---|
| +6 | Sulfate, SO42− | 0 | S0, elemental sulfur. Sulfoxide (R-S(-O)-R such as dimethyl sulfoxide (DMSO). Oxidized derivatives of sulfide and sulfenic acid (RSOH). |
| +6 and −2 | Thiosulfate, S2O32− | −1 | Disulfide (R-S-S-R) is a persulfide found in the linkages between two cysteine residues in proteins. RSSH denotes persulfides (or hydrosulfides) obtained by the action of H2S on cysteine residues (R-SH). Thioethers and thiols can be oxidized to disulfides. Major products of decomposition of persulfides are polysulfanes. Thiyl-radical RS*. |
| +5 and −2 | Polythionates (−O3S-Sn-SO3−): Dithionate, S2O62−; Trithionate, S3O62−; Tetrathionate, S4O62− | −2 | Sulfide, S2−, polysulfides, S22−, S32−, S52−; carbon disulfide (CS2); FeS2; NaHS and Na2S are sources of S2− and of its conjugated acids SH− and H2S. Polysulfides (with Sn > 2) contain S0 atoms, which allows a diversity of oxidation states. |
| +4 | Sulfur dioxide, SO2; Sulfite, SO32−; Disulfite, S2O52−; Sulfone, OS(S) the oxidation product of sulfoxides | −2 | Hydrogen sulfide (H2S), disulfane (H2S2), and polysulfanes (RSSnSR, n > 2). Polysulfanes contain S0 atoms, which allows a diversity of oxidation states. |
| +3 | Dithionite, S2O42− | −2 | Thioethers (C-S-C) such as dimethyl sulfide (DMS), CH3-S-CH3 and dimethyl disulfide (DMDS), CH3-S-S-CH3. |
| +2 | Carbonyl sulfide (COS), OCS | −2 | Thiols (R-SH) such as glutathione (GSH) and methyl mercaptan, CH3-SH. Thiols are derived from the sulfhydryl group -SH of cysteine, which enables multiple oxidation states (−2 to +6). Thiolates are derivatives of thiols in which a metal or other cation replaces H. |
| 0 | Elementary sulfur (S0), mainly S8 (cycloocta-S) | −2 | Carbon disulfide, CS2. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes-Lara, L.O.; Medrano-Macías, J.; Pérez-Labrada, F.; Rivas-Martínez, E.N.; García-Enciso, E.L.; González-Morales, S.; Juárez-Maldonado, A.; Rincón-Sánchez, F.; Benavides-Mendoza, A. From Elemental Sulfur to Hydrogen Sulfide in Agricultural Soils and Plants. Molecules 2019, 24, 2282. https://doi.org/10.3390/molecules24122282
Fuentes-Lara LO, Medrano-Macías J, Pérez-Labrada F, Rivas-Martínez EN, García-Enciso EL, González-Morales S, Juárez-Maldonado A, Rincón-Sánchez F, Benavides-Mendoza A. From Elemental Sulfur to Hydrogen Sulfide in Agricultural Soils and Plants. Molecules. 2019; 24(12):2282. https://doi.org/10.3390/molecules24122282
Chicago/Turabian StyleFuentes-Lara, Laura Olivia, Julia Medrano-Macías, Fabián Pérez-Labrada, Erika Nohemí Rivas-Martínez, Ema Laura García-Enciso, Susana González-Morales, Antonio Juárez-Maldonado, Froylán Rincón-Sánchez, and Adalberto Benavides-Mendoza. 2019. "From Elemental Sulfur to Hydrogen Sulfide in Agricultural Soils and Plants" Molecules 24, no. 12: 2282. https://doi.org/10.3390/molecules24122282
APA StyleFuentes-Lara, L. O., Medrano-Macías, J., Pérez-Labrada, F., Rivas-Martínez, E. N., García-Enciso, E. L., González-Morales, S., Juárez-Maldonado, A., Rincón-Sánchez, F., & Benavides-Mendoza, A. (2019). From Elemental Sulfur to Hydrogen Sulfide in Agricultural Soils and Plants. Molecules, 24(12), 2282. https://doi.org/10.3390/molecules24122282









