Discovery of Novel UV-Filters with Favorable Safety Profiles in the 5-Arylideneimidazolidine-2,4-dione Derivatives Group
Abstract
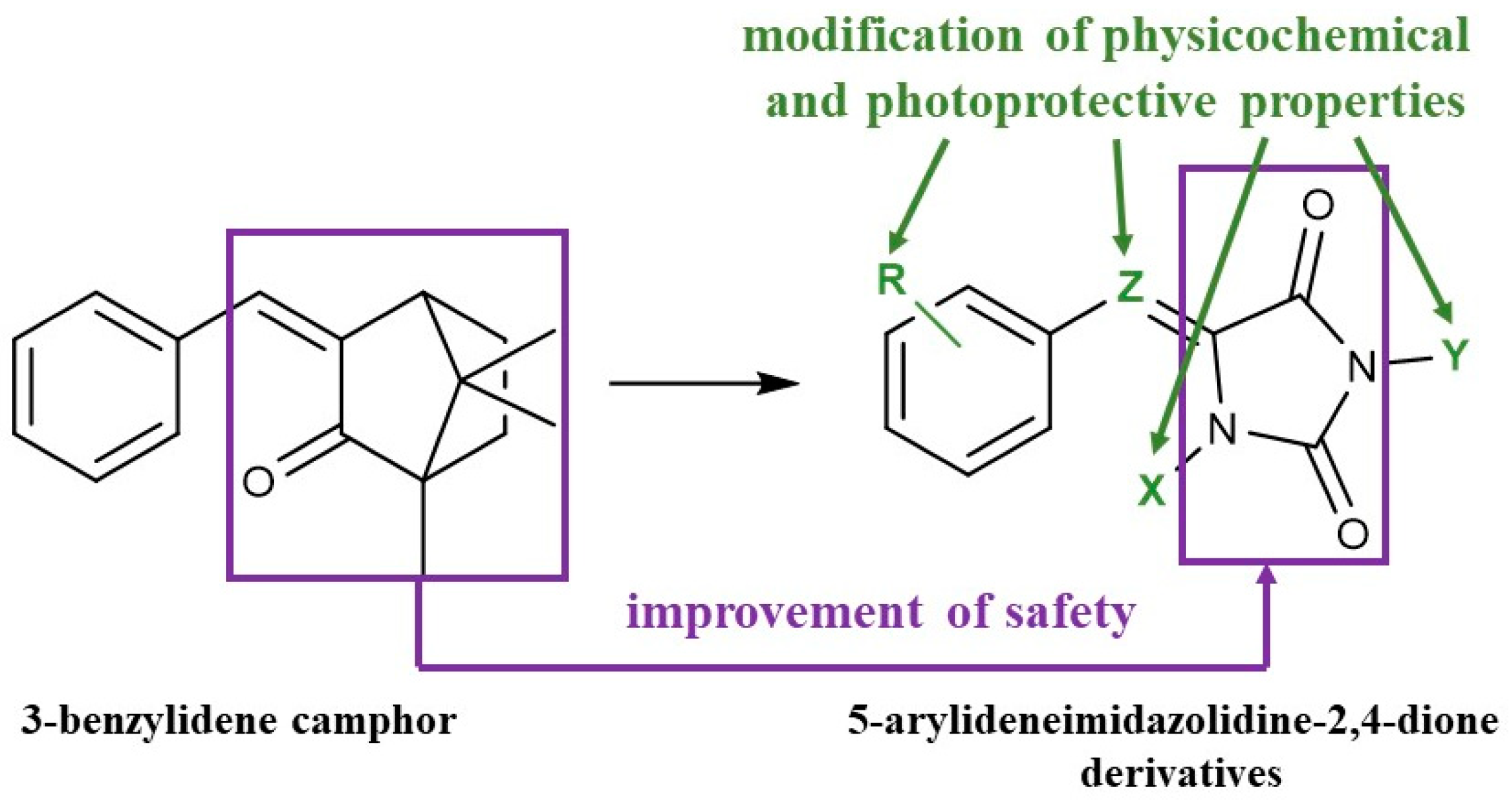
1. Introduction
2. Results and Discussion
2.1. Chemistry
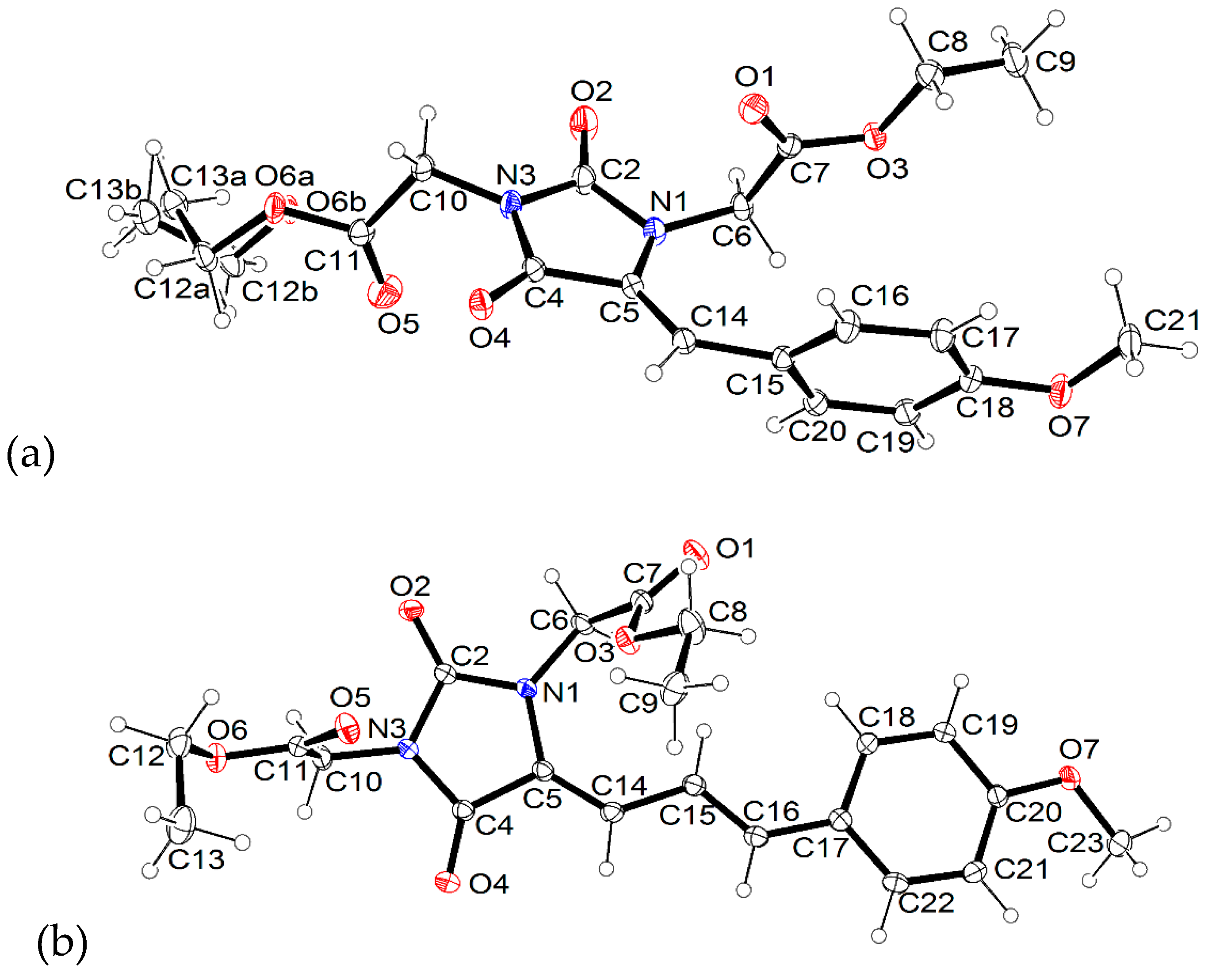
2.2. X-ray Crystallographic Studies
2.3. Ultraviolet Spectroscopic Properties
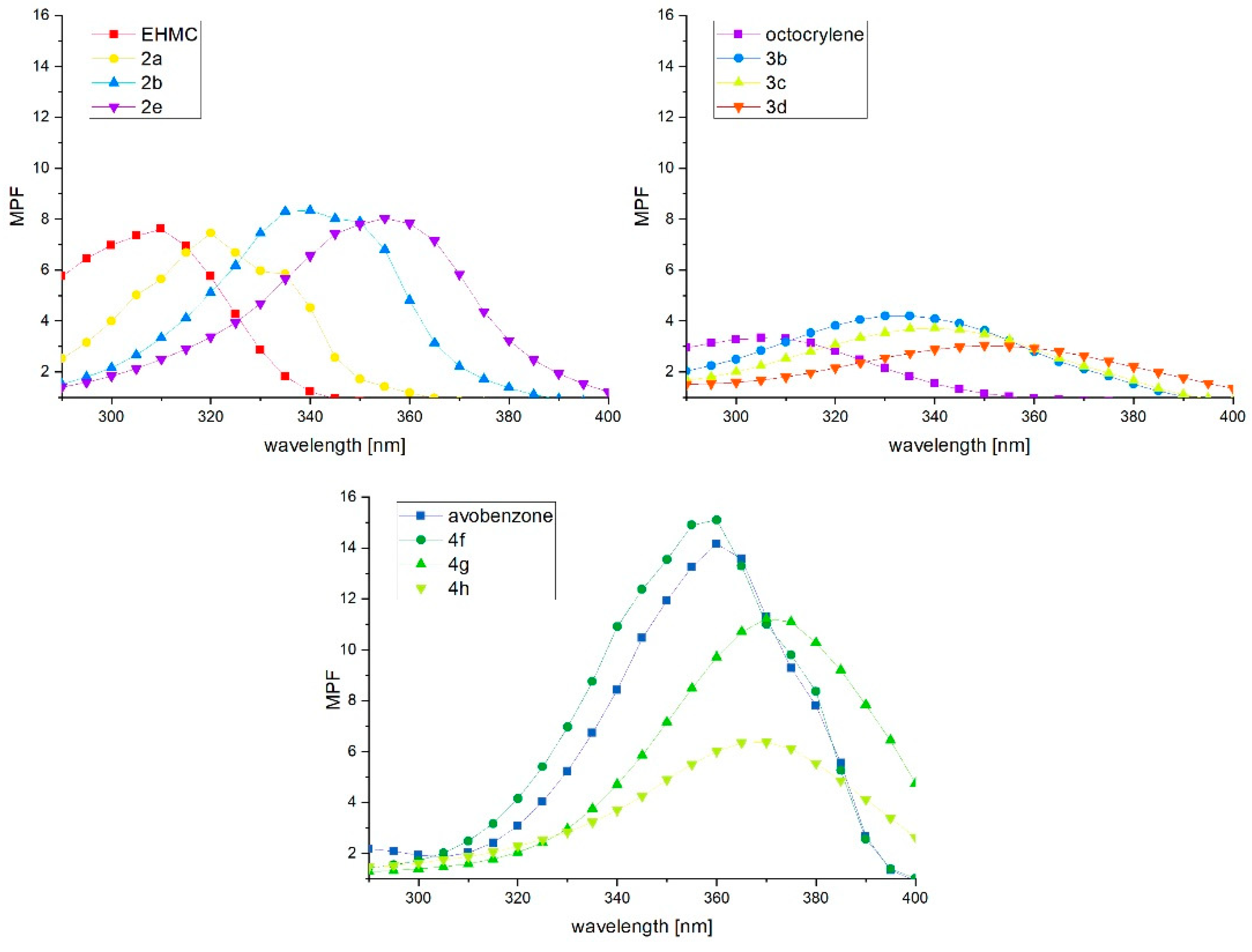
2.4. Photoprotective Activity
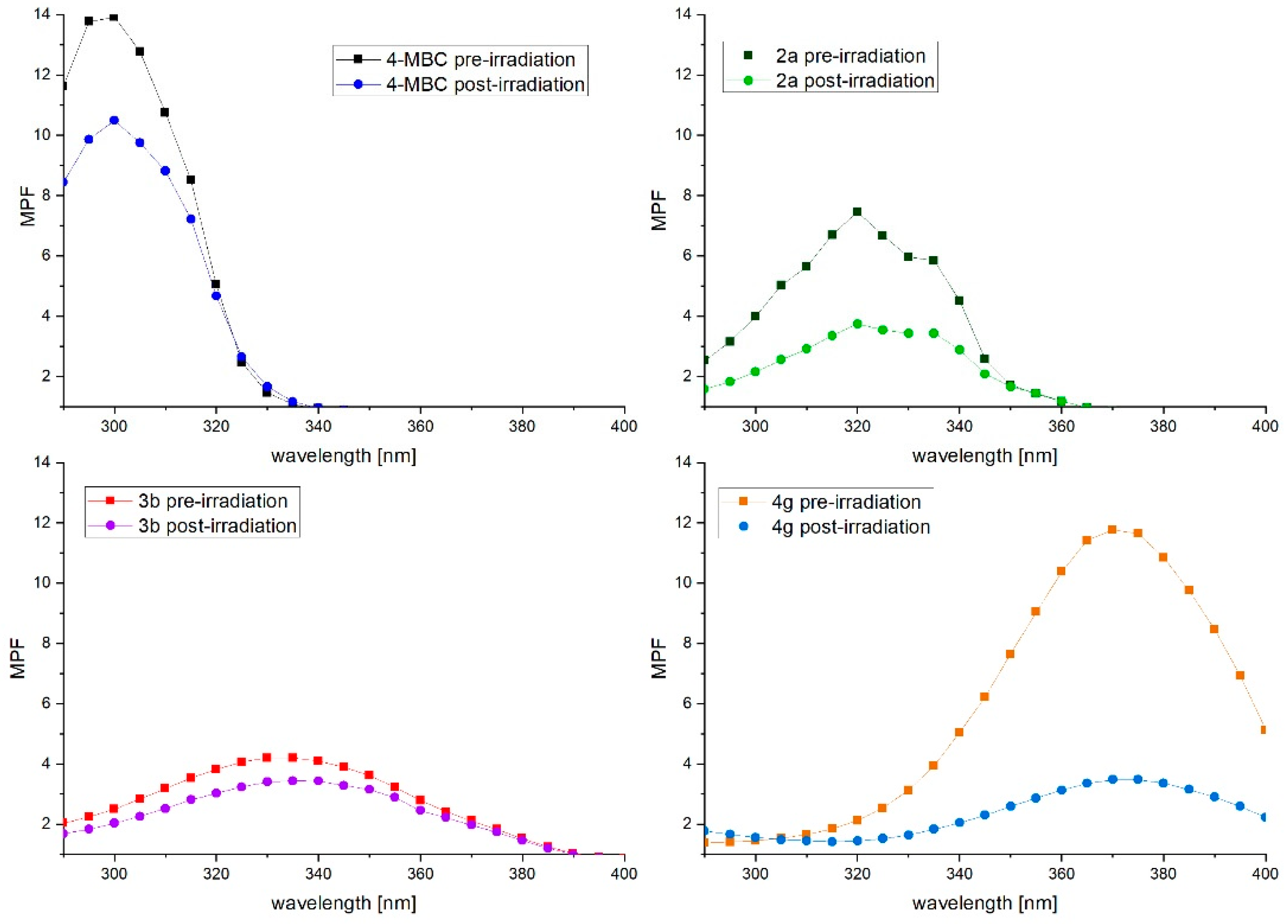
2.5. Photostability Studies
2.5.1. Photostability in Methanol Solutions
2.5.2. Photostability in Cosmetic Formulations
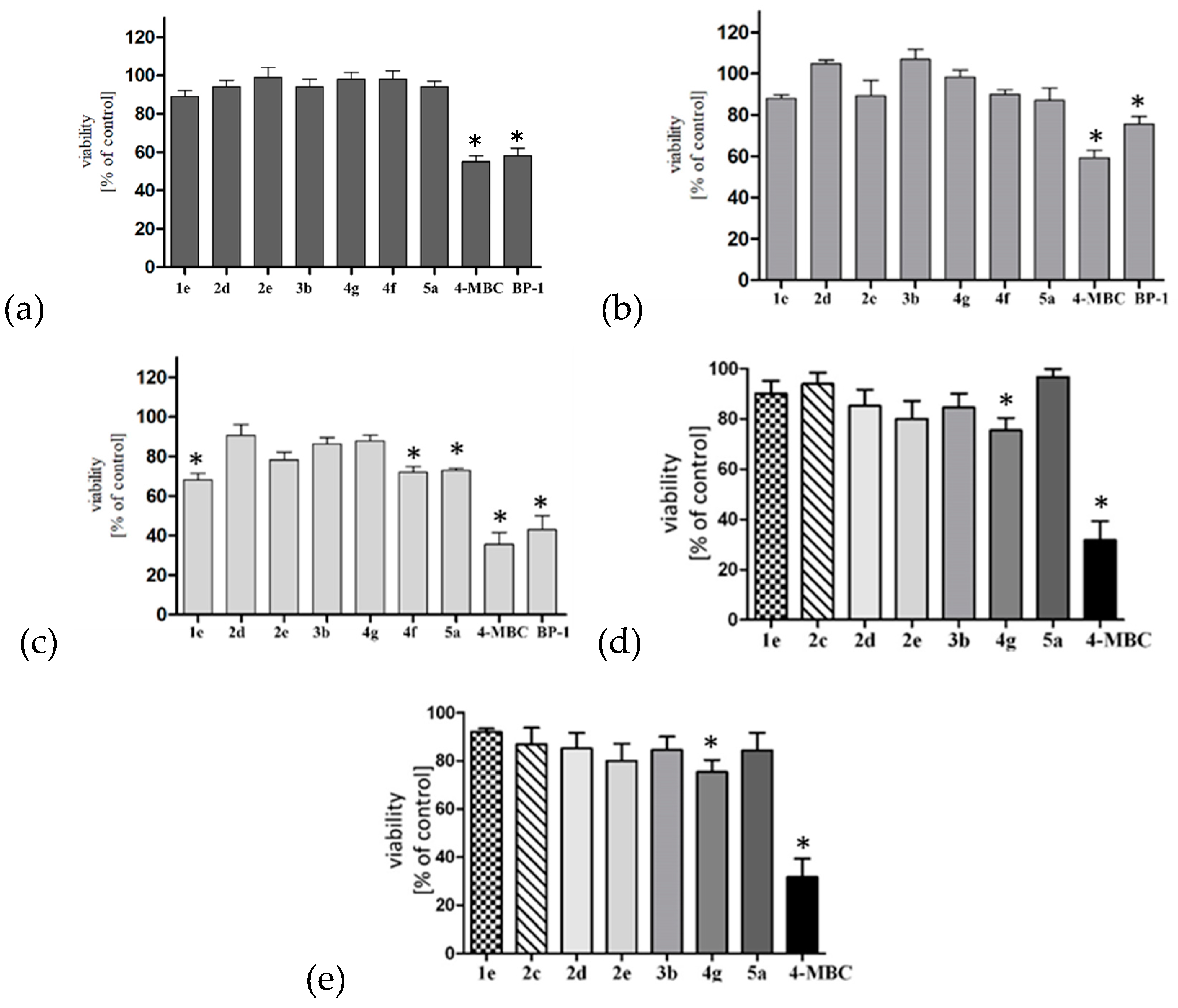
2.6. Preliminary Safety Assessment
2.6.1. Skin Panel Model
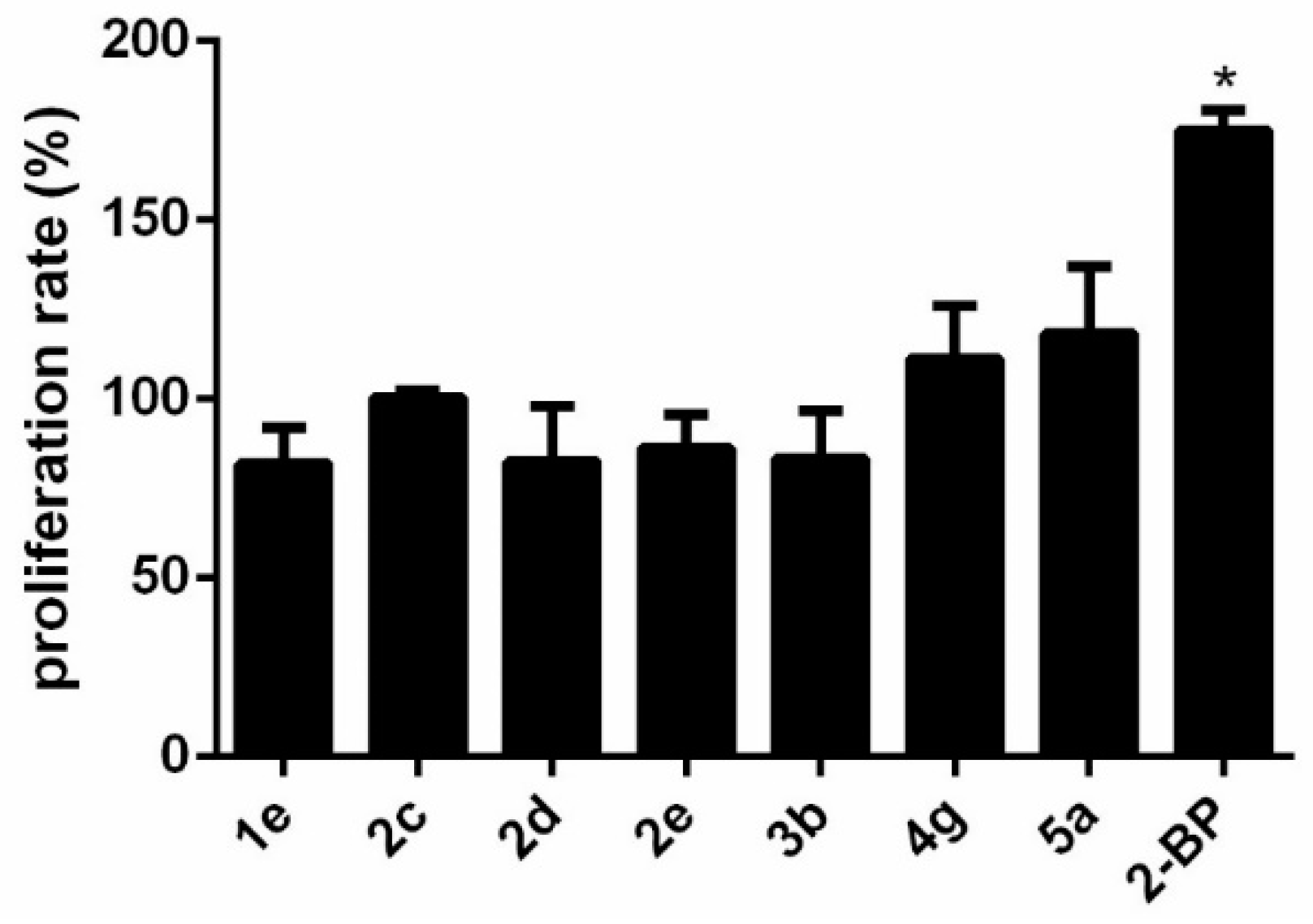
2.6.2. Hepatocytotoxicity and Neurocytotoxicity Studies
2.6.3. Estrogenic Activity
2.6.4. In Vitro Stability of Compounds in Mouse Liver Microsomes
2.6.5. Mutagenic Activity
2.7. Structure-Activity Relationship Studies
3. Materials and Methods
3.1. Chemistry
3.1.1. Preparation of (Z)-5-benzylidene- and (Z)-5-((E)-3-phenylallylidene)imidazolidine-2,4-diones (compounds 1a-1h)
3.1.2. Preparation of Ethyl (Z)-2-(4-benzylidene-2,5-dioxoimidazolidin-1-yl)acetates (compounds 2a–2e)
3.1.3. Preparation of Diethyl 2,2′-((Z)-4-benzylidene-2,5-dioxoimidazolidine-1,3-diyl) Diacetates and Diethyl 2,2′-((Z)-2,4-dioxo-5-((E)-3-phenylallylidene)imidazolidine-1,3-diyl)diacetates (compounds 3a-3e, 4f-4h)
3.1.4. Preparation of (Z)-2-(4-benzylidene-2,5-dioxoimidazolidin-1-yl)acetic acid (5a)
3.1.5. Physicochemical Properties of Tested Compounds
3.2. Crystal Structures of 3b and 4g
3.3. Ultraviolet Spectroscopy
3.4. Photoprotective Activity
3.4.1. Cosmetic Formulations
3.4.2. In Vitro Photoprotection Study
3.4.3. Photostability Study
3.5. Skin Panel Model
3.6. Hepatocytotoxicity and Neurocytotoxicity Studies
3.7. Estrogenic Activity
3.8. In Vitro Stability of Compounds in Mouse Liver Microsomes
3.9. Ames Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nash, J.F.; Tanner, P.R. Relevance of UV filter/sunscreen product photostability to human safety. Photodermatol. Photoimmunol. Photomed. 2014, 30, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.F.; Gargano, R.; Alcanfor, S.K.B.; Romeiro, L.A.S.; Martins, J.B.L. A chromophoric study of 2-ethylhexyl p-methoxycinnamate. Chem. Phys. Lett. 2011, 516, 162–165. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off. J. Eur. Union 2009, 50, 59–209. [Google Scholar]
- Nohynek, G.J.; Antignac, E.; Re, T.; Toutain, H. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol. Appl. Pharmacol. 2010, 243, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, G.J.; Schaefer, H. Benefit and risk of organic ultraviolet filters. Regul. Toxicol. Pharmacol. 2001, 33, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Hüglin, D. Advanced UV absorbers for the protection of human skin. Chim. Int. J. Chem 2016, 70, 496–501. [Google Scholar] [CrossRef]
- Tarazona, I.; Chisvert, A.; Salvador, A. Determination of benzophenone-3 and its main metabolites in human serum by dispersive liquid-liquid microextraction followed by liquid chromatography tandem mass spectrometry. Talanta 2013, 116, 388–395. [Google Scholar] [CrossRef]
- Janjua, N.R.; Mogensen, B.; Andersson, A.M.; Petersen, J.H.; Henriksen, M.; Skakkebæk, N.E.; Wulf, H.C. Systemic absorption of the sunscreens benzophenone-3, octyl-methoxycinnamate, and 3-(4-methyl-benzylidene) camphor after whole-body topical application and reproductive hormone levels in humans. J. Invest. Dermatol. 2004, 123, 57–61. [Google Scholar] [CrossRef]
- Calafat, A.M.; Wong, L.Y.; Ye, X.; Reidy, J.A.; Needham, L.L. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National health and nutrition examination survey 2003–2004. Environ. Health Perspect. 2008, 116, 893–897. [Google Scholar] [CrossRef]
- Schlumpf, M.; Kypke, K.; Wittassek, M.; Angerer, J.; Mascher, H.; Mascher, D.; Vökt, C.; Birchler, M.; Lichtensteiger, W. Exposure patterns of UV filters, fragrances, parabens, phthalates, organochlor pesticides, PBDEs, and PCBs in human milk: Correlation of UV filters with use of cosmetics. Chemosphere 2010, 81, 1171–1183. [Google Scholar] [CrossRef]
- Wang, J.; Pan, L.; Wu, S.; Lu, L.; Xu, Y.; Zhu, Y.; Guo, M.; Zhuang, S. Recent advances on endocrine disrupting effects of UV filters. Int. J. Environ. Res. Public Health 2016, 13, 782. [Google Scholar] [CrossRef] [PubMed]
- Schlumpf, M.; Schmid, P.; Durrer, S.; Conscience, M.; Maerkel, K.; Henseler, M.; Gruetter, M.; Herzog, I.; Reolon, S.; Ceccatelli, R.; et al. Endocrine activity and developmental toxicity of cosmetic UV filters—An update. Toxicology 2004, 205, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Schlumpf, M.; Cotton, B.; Conscience, M.; Haller, V.; Steinmann, B.; Lichtensteiger, W. In vitro and in vivo estrogenicity of UV screens. Environ. Health Perspect. 2001, 109, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Broniowska, Z.; Pomierny, B.; Smaga, I.; Filip, M.; Budziszewska, B. The effect of UV-filters on the viability of neuroblastoma (SH-SY5Y) cell line. Neurotoxicology 2016, 54, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Santovito, A.; Ruberto, S.; Galli, G.; Menghi, C.; Girotti, M.; Cervella, P. Induction of chromosomal aberrations and micronuclei by 2-hydroxy-4-methoxybenzophenone (oxybenzone) in human lymphocytes. Drug Chem. Toxicol. 2019, 42, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Manová, E.; Von Goetz, N.; Hungerbühler, K. Ultraviolet filter contact and photocontact allergy: Consumer exposure and risk assessment for octocrylene from personal care products and sunscreens. Br. J. Dermatol. 2014, 171, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Schauder, S.; Ippen, H. Contact and photocontact sensitivity to sunscreens. Review of a 15-year experience and of the literature. Contact Dermat. 1997, 37, 221–232. [Google Scholar] [CrossRef]
- Afonso, S.; Horita, K.; Sousa, E.; Silva, J.P.; Almeida, I.F.; Amaral, M.H.; Lobão, P.A.; Costa, P.C.; Miranda, M.S.; Esteves Da Silva, J.C.G.; et al. Photodegradation of avobenzone: Stabilization effect of antioxidants. J. Photochem. Photobiol. B 2014, 140, 36–40. [Google Scholar] [CrossRef]
- Schwack, W.; Rudolph, T. Photochemistry of dibenzoyl methane UVA filters Part 1. J. Photochem. Photobiol. B 1995, 28, 229–234. [Google Scholar] [CrossRef]
- Hanson, K.M.; Narayanan, S.; Nichols, V.M.; Bardeen, C.J. Photochemical degradation of the UV filter octyl methoxycinnamate in solution and in aggregates. Photochem. Photobiol. Sci. 2015, 14, 1607–1616. [Google Scholar] [CrossRef]
- Karlsson, I.; Hillerström, L.; Stenfeldt, A.L.; Mårtensson, J.; Börje, A. Photodegradation of dibenzoylmethanes: Potential cause of photocontact allergy to sunscreens. Chem. Res. Toxicol. 2009, 22, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission directive 2008/123/EC of 18 December 2008 amending council directive 76/768/EEC, concerning cosmetic products, for the purpose of adapting Annexes II and VII Thereto to technical progress. Off. J. Eur. Union 2008, 58, 267–268. [Google Scholar]
- European Commission. Commission Regulation (EU) 2015/1298 of July 28 2015 amending Annexes II and VI to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products. Off. J. Eur. Union 2015, 199, 22. [Google Scholar]
- Scientific Committee Opinion on 1,3,5-Triazine, 2,4,6-Tris[1,1′-Biphenyl]-4-Yl-; European Commission: Brussels, Belgium, 2011.
- Commission Regulation (EU) 2018/885 of 20 June 2018 Amending Annex VI to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products; European Commission: Brussels, Belgium, 2018.
- González, M.T.P.; Fumagalli, F.; Benevenuto, C.G.; da Silva Emery, F.; Gaspar, L.R. Novel benzophenone-3 derivatives with promising potential as UV filters: Relationship between structure, photoprotective potential and phototoxicity. Eur. J. Pharm. Sci. 2017, 101, 200–210. [Google Scholar] [CrossRef]
- Quintana Lazópulos, S.; Svarc, F.; Sagrera, G.; Dicelio, L. Absorption and photo-stability of substituted dibenzoylmethanes and chalcones as UVA filters. Cosmetics 2018, 5, 33. [Google Scholar] [CrossRef]
- Farkas, R.; Lhiaubet-Vallet, V.; Corbera, J.; Törincsi, M.; Gorchs, O.; Trullas, C.; Jiménez, O.; Miranda, M.A.; Novak, L. Synthesis of new 2-(2′-hydroxyaryl)benzotriazoles and evaluation of their photochemical behavior as potential UV-filters. Molecules 2010, 15, 6205–6216. [Google Scholar] [CrossRef] [PubMed]
- Bino, A.; Baldisserotto, A.; Scalambra, E.; Dissette, V.; Vedaldi, D.E.; Salvador, A.; Durini, E.; Manfredini, S.; Vertuani, S. Design, synthesis and biological evaluation of novel hydroxy-phenyl-1H-benzimidazoles as radical scavengers and UV-protective agents. J. Enzyme Inhib Med. Chem 2017, 32, 527–537. [Google Scholar] [CrossRef]
- Venditti, E.; Spadoni, T.; Tiano, L.; Greci, L.; Littarru, G.P.; Damiani, E. In vitro photostability and photoprotection studies of a novel “multi-active” UV-absorber. Free Radic. Biol. Med. 2008, 45, 345–354. [Google Scholar] [CrossRef]
- Handzlik, J.; Spengler, G.; Mastek, B.; Dela, A.; Molnar, J.; Amaral, L.; Kieć-Kononowicz, K. 5-arylidene(thio)hydantoin derivatives as modulators of cancer efflux pump. Acta Pol. Pharm. Drug Res. 2012, 69, 149–156. [Google Scholar]
- Ha, Y.M.; Kim, J.A.; Park, Y.J.; Park, D.; Kim, J.M.; Chung, K.W.; Lee, E.K.; Park, J.Y.; Lee, J.Y.; Lee, H.J.; et al. Analogs of 5-(substituted benzylidene)hydantoin as inhibitors of tyrosinase and melanin formation. Biochim. Biophys. Acta 2011, 1810, 612–619. [Google Scholar] [CrossRef]
- Zuliani, V.; Carmi, C.; Rivara, M.; Fantini, M.; Lodola, A.; Vacondio, F.; Bordi, F.; Plazzi, P.V.; Cavazzoni, A.; Galetti, M.; et al. 5-Benzylidene-hydantoins: Synthesis and antiproliferative activity on A549 lung cancer cell line. Eur. J. Med. Chem. 2009, 44, 3471–3479. [Google Scholar] [CrossRef] [PubMed]
- Handzlik, J.; Szymańska, E.; Alibert, S.; Chevalier, J.; Otrębska, E.; Pękala, E.; Pagès, J.M.; Kieć-Kononowicz, K. Search for new tools to combat gram-negative resistant bacteria among amine derivatives of 5-arylidenehydantoin. Bioorg Med. Chem 2013, 21, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Kieć-Kononowicz, K.; Szymańska, E. Antimycobacterial activity of 5-arylidene derivatives of hydantoin. Farmaco 2002, 57, 909–916. [Google Scholar] [CrossRef]
- Dalgleish, T.; Williams, J.M.G.; Golden, A.-M.J.; Perkins, N.; Barrett, L.F.; Barnard, P.J.; Au Yeung, C.; Murphy, V.; Elward, R.; Tchanturia, K.; et al. Benzylidene Hydantoin Derivative, Ultraviolet Ray Absorbent and External Preparation for Skin including the Same. European Patent EP0632029A1, 4 January 1995. [Google Scholar]
- Okazaki, T.; Kato, M.; Matsushita, Y.; Uehara, K. Preparation of Cinnamylidenehydantoins as Sunscreens. European Patent JP06107642, 1 October 1992. [Google Scholar]
- Żesławska, E.; Oleksyn, B.J.; Korohoda, M.J.; Śliwiński, J. Effect of chalcogen substituents (X=Se, S, O) on molecular properties of 5-p-chlorobenzylidene-3-methyl-2-X-hydantoin: X-RAY, PM-3 and database study. Phosphorus Sulfur Silicon Relat. Elem. 1997, 126, 111–124. [Google Scholar] [CrossRef]
- Żesławska, E.; Oleksyn, B.J.; Korohoda, M.J.; Stadnicka, K. The crystal and molecular structure of 3-methyl-5-p-methylbenzylidene-2-selenohydantoin. Phosphorus Sulfur Silicon Relat Elem. 2003, 178, 261–268. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Żesławska, E.; Nitek, W.; Tejchman, W. The synthesis and crystal structures of the homologues of epalrestat. J. Chem. Crystallogr. 2015, 45, 151–157. [Google Scholar] [CrossRef]
- Stuckey, R.E. The absorption spectra of organic compounds containing nitrogen. Part II. Benzylidene derivatives of hydantoin and thiohydantoin. J. Chem. Soc. 1949, 207–212. [Google Scholar] [CrossRef]
- Worldwide, F. On the theory of ultraviolet absorption by sunscreen chemicals. J. Soc. Cosmet. Chem. 1987, 207, 193–207. [Google Scholar]
- Gaspar, L.R.; Maia Campos, P.M. Evaluation of the photostability of different UV filter combinations in a sunscreen. Int. J. Pharm. 2006, 307, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.J.; Miller, I.J. The effect of molecular environment on the photochemistry of p-methoxycinnamic acid and its esters. J. Photochem. Photobiol. A 1998, 118, 93–97. [Google Scholar] [CrossRef]
- Garoli, D.; Pelizzo, M.G.; Bernardini, B.; Nicolosi, P.; Alaibac, M. Sunscreen tests: Correspondence between in vitro data and values reported by the manufacturers. J. Dermatol. Sci. 2008, 52, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Hojerová, J.; Medovcíková, A.; Mikula, M. Photoprotective efficacy and photostability of fifteen sunscreen products having the same label SPF subjected to natural sunlight. Int. J. Pharm. 2011, 408, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.P.; Ferreira, P.J.O.; Miranda, M.S.; Da Silva, J.C.G. A Theoretical study of the UV absorption of 4-methylbenzylidene camphor: From the UVB to the UVA region. Photochem. Photobiol. Sci. 2015, 14, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Paris, C.; Lhiaubet-Vallet, V.; Jiménez, O.; Trullas, C.; Miranda, M.Á. A blocked diketo form of avobenzone: Photostability, photosensitizing properties and triplet quenching by a triazine-derived UVB-filter. Photochem. Photobiol. 2009, 85, 178–184. [Google Scholar] [CrossRef]
- Eilstein, J.; Léreaux, G.; Arbey, E.; Daronnat, E.; Wilkinson, S.; Duché, D. Xenobiotic metabolizing enzymes in human skin and SkinEthic reconstructed human skin models. Exp. Dermatol. 2015, 24, 547–549. [Google Scholar] [CrossRef]
- Oesch, F.; Fabian, E.; Oesch-Bartlomowicz, B.; Werner, C.; Landsiedel, R. Drug-metabolizing enzymes in the skin of man, rat, and pig. Drug Metab. Rev. 2007, 39, 659–698. [Google Scholar] [CrossRef]
- Jiang, R.; Roberts, M.; Collins, D.; Benson, H.A.E. Absorption of sunscreens across human skin: An evaluation of commercial products for children and adults. Br. J. Clin. Pharmacol. 1999, 48, 635–637. [Google Scholar] [CrossRef]
- Okereke, C.S.; Kadry, A.M.; Abdel-Rahman, M.S.; Davis, R.A.; Friedman, M. Metabolism of benzophenone-3 in rats. Drug Metab. Dispos. 1993, 21, 788–791. [Google Scholar] [PubMed]
- Kebamo, S.; Tesema, S.; Geleta, B. The role of biotransformation in drug discovery and development. J. Drug Metab. Toxicol. 2015, 6, 196. [Google Scholar] [CrossRef]
- Kem, W.R. Hydroxy metabolites of the Alzheimer’s drug candidate 3-[(2,4-dimethoxy)benzylidene]-anabaseine dihydrochloride (GTS-21): Their molecular properties, interactions with brain nicotinic receptors, and brain penetration. Mol. Pharmacol. 2004, 65, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Sun, H. Capture hydrolysis signals in the microsomal stability assay: Molecular mechanisms of the alkyl ester drug and prodrug metabolism. Bioorg Med. Chem. Lett. 2012, 22, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zou, L.; Jin, Q.; Hou, J.; Ge, G.; Yang, L. Human carboxylesterases: A comprehensive review. Acta Pharm. Sin. B 2018, 8, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, H.; Sugihama, K.; Watanabe, Y.; Fujino, C.; Uramaru, N.; Sone, T.; Ohta, S.; Kitamura, S. Comparative study of the hydrolytic metabolism of methyl-, ethyl-, propyl-, butyl-, heptyl- and dodecylparaben by microsomes of various rat and human tissues. Xenobiotica 2013, 43, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Jewell, C.; Prusakiewicz, J.J.; Ackermann, C.; Payne, N.A.; Fate, G.; Voorman, R.; Williams, F.M. Hydrolysis of a series of parabens by skin microsomes and cytosol from human and minipigs and in whole skin in short-term culture. Toxicol. Appl. Pharmacol. 2007, 225, 221–228. [Google Scholar] [CrossRef]
- Ozaki, H.; Sugihara, K.; Tamura, Y.; Fujino, C.; Watanabe, Y.; Uramaru, N.; Sone, T.; Ohta, S.; Kitamura, S. Hydrolytic metabolism of phenyl and benzyl salicylates, fragrances and flavoring agents in foods, by microsomes of rat and human tissues. Food Chem. Toxicol. 2015, 86, 116–123. [Google Scholar] [CrossRef]
- Hanioka, N.; Isobe, T.; Ohkawara, S.; Ochi, S.; Tanaka-Kagawa, T.; Jinno, H. Hydrolysis of di(2-ethylhexyl) phthalate in humans, monkeys, dogs, rats, and mice: An in vitro analysis using liver and intestinal microsomes. Toxicol. Vitr. 2019, 54, 237–242. [Google Scholar] [CrossRef]
- Pȩkala, E.; Stadnicka, K.; Broda, A.; Zygmunt, M.; Filipek, B.; Kieć-Kononowicz, K. Synthesis, structure-activity relationship of some new anti-arrhythmic 5-arylidene imidazolidine-2,4-dione derivatives. Eur. J. Med. Chem. 2005, 40, 259–269. [Google Scholar] [CrossRef]
- Martínez-López, D.; Yu, M.L.; García-Iriepa, C.; Campos, P.J.; Frutos, L.M.; Golen, J.A.; Rasapalli, S.; Sampedro, D. Hydantoin-based molecular photoswitches. J. Org. Chem. 2015, 80, 3929–3939. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ma, L.; Zheng, H.; Chen, L.; Li, R.; He, C.; Yang, S.; Ye, X.; Chen, Z.; Li, Z.; et al. Discovery of (Z)-5-(4-methoxybenzylidene)thiazolidine-2,4-dione, a readily available and orally active glitazone for the treatment of concanavalin A-induced acute liver injury of BALB/c mice. J. Med. Chem. 2010, 53, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Thenmozhiyal, J.C.; Wong, P.T.; Chui, W.K. Anticonvulsant activity of phenylmethylenehydantoins: A structure-activity relationship study. J. Med. Chem. 2004, 47, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Lamiri, M.; Bougrin, K.; Daou, B.; Soufiaoui, M.; Nicolas, E.; Giralt, E. Microwave-assisted solvent-free regiospecific synthesis of 5-alkylidene and 5-arylidenehydantoins. Synth. Commun. 2006, 36, 1575–1584. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G. Crystal structure determination and refinement via SIR2014. J. Appl. Crystallogr. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Van De Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Determination of Sunscreen UVA Photoprotection in Vitro; ISO 24443:2012; International Organization for Standardization: London, UK, 2012.
- De Oliveira, C.A.; Peres, D.D.A.; Rugno, C.M.; Kojima, M.; Pinto, C.A.S.O.; Consiglieri, V.O.; Kaneko, T.M.; Rosado, C.; Mota, J.; Velasco, M.V.R.; et al. Functional photostability and cutaneous compatibility of bioactive UVA sun care products. J. Photochem. Photobiol. B 2015, 148, 154–159. [Google Scholar] [CrossRef]
- Waszkielewicz, A.M.; Słoczyńska, K.; Pękala, E.; Żmudzki, P.; Siwek, A.; Gryboś, A.; Marona, H. Design, synthesis, and anticonvulsant activity of some derivatives of xanthone with aminoalkanol moieties. Chem. Biol. Drug Des. 2017, 89, 339–352. [Google Scholar] [CrossRef]
- Singh, J.K.; Solanki, A.; Shirsath, V.S. Comparative In vitro intrinsic clearance of imipramine in multiple species liver microsomes: Human, rat, mouse and dog. J. Drug Metab. Toxicol. 2012, 3, 1–6. [Google Scholar] [CrossRef]
- Flückiger-Isler, S.B.; Braun, K.; Gervais, V.; Hasler-Nguyen, N.; Reimann, R.; Van Gompel, J.; Wunderlich, H.; Engelhardt, G. Assessment of the performance of the Ames II assay: A collaborative study with 19 coded compounds. Mutat Res. 2004, 558, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Flückiger-Isler, S.; Kamber, M. Direct comparison of the Ames microplate format (MPF) test in liquid medium with the standard Ames pre-incubation assay on agar plates by use of equivocal to weakly positive test compounds. Mutat. Res. 2012, 747, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Xenometrix: Ames Test, Endocrine disruptor, In Vitro Skin Absorption. Available online: http://www.xenometrix.ch (accessed on 18 December 2018).
- Umbuzeiro Gde, A.; Rech, C.; Correia, S.; Bergamasco, A.; Cardenette, G.; Flückiger-Isler, S.; Kamber, M. Comparison of the Salmonella/microsome microsuspension assay with the new microplate fluctuation protocol for testing the mutagenicity of environmental samples. Environ. Mol. Mutagen. 2010, 51, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Reifferscheid, G.; Maes, H.; Allner, B.; Badurova, J.; Belkin, S.; Bluhm, K.; Brauer, F.; Bressling, J.; Domeneghetti, S.; Elad, T.; et al. International round-robin study on the Ames fluctuation test. Environ. Mol. Mutagen. 2012, 53, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Smih, K.; Heringa, M.; Uytewaal, M.; Mayer, P. The dosing determines mutagenicity of hydrophobic compounds in the Ames II assay with metabolic transformation: Passive dosing versus solvent spiking. Mutat. Res. 2013, 7, 12–18. [Google Scholar] [CrossRef] [PubMed]


| Compound | Chemical Structure | λmax [nm] | εmax [M−1 cm−1] | Absorption Range [nm] |
|---|---|---|---|---|
| 1e | 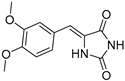 | 341 | 28440 | 290–384 |
| 2a | 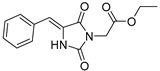 | 316 | 28460 | 290–345 |
| 2b | 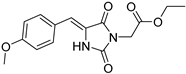 | 334 | 31920 | 290–372 |
| 2c | 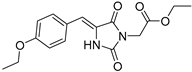 | 336 | 35610 | 290–376 |
| 2d | 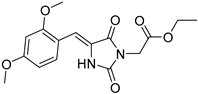 | 349 | 30700 | 290–394 |
| 2e | 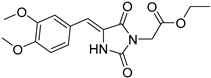 | 344 | 30740 | 290–381 |
| 3a | 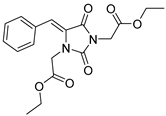 | 295 | 13980 | 290–335 |
| 3b | 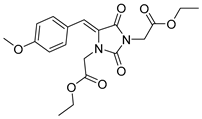 | 318 | 16310 | 290–362 |
| 3c | 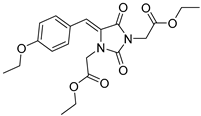 | 321 | 17010 | 290–367 |
| 3d |  | 333 | 17310 | 290–374 |
| 3e | 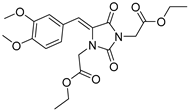 | 329 | 14200 | 290–380 |
| 4f | 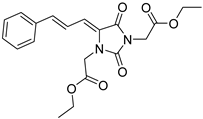 | 354 | 41477 | 295–389 |
| 4g | 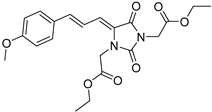 | 379 | 54996 | 290–400 |
| 4h | 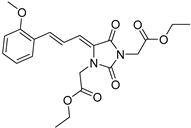 | 370 | 34987 | 290–400 |
| 5a | 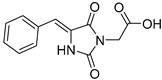 | 316 | 27310 | 290–346 |
| 4-MBC | 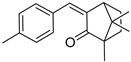 | 299 | 25550 | 290–332 |
| Octocrylene | 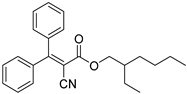 | 302 | 13650 | 290–349 |
| EHMC | 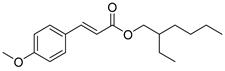 | 309 | 22990 | 290–339 |
| Avobenzone |  | 358 | 36820 | 290–393 |
| Compound | Chemical Structure | SPFin vitro | UVA PF | λc | UVA/UVB Ratio |
|---|---|---|---|---|---|
| 2a# | 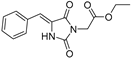 | 4.79 ± 0.02 | 2.40 ± 0.01 | 339 | 0.35 ± 0.00 |
| 2b# | 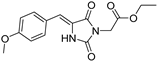 | 3.20 ± 0.07 | 4.29 ± 0.11 | 363 | 1.20 ± 0.00 |
| 2c^ |  | 3.16 ± 0.05 | 4.68 ± 0.02 | 367 | 1.36 ± 0.01 |
| 2d^ | 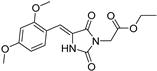 | 2.00 ± 0.11 | 4.48 ± 0.38 | 386 | 2.59 ± 0.09 |
| 2e^ | 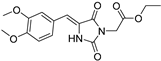 | 2.50 ± 0.08 | 4.77 ± 0.24 | 379 | 1.95 ± 0.02 |
| 3a* | 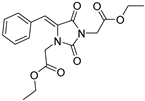 | 2.97 ± 0.45 | 1.29 ± 0.09 | 331 | 0.16 ± 0.02 |
| 3b* | 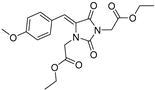 | 3.07 ± 0.04 | 2.59 ± 0.23 | 366 | 0.84 ± 0.08 |
| 3c* | 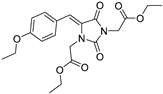 | 2.49 ± 0.08 | 2.46 ± 0.01 | 370 | 1.05 ± 0.03 |
| 3d* | 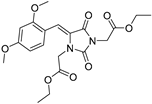 | 1.84 ± 0.02 | 2.35 ± 0.03 | 383 | 1.63 ± 0.01 |
| 3e* | 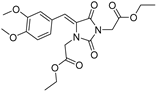 | 1.87 ± 0.08 | 2.17 ± 0.11 | 382 | 1.43 ± 0.02 |
| 4f* | 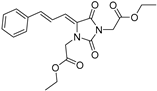 | 2.51 ± 0.17 | 8.43 ± 1.35 | 381 | 2.58 ± 0.06 |
| 4g* | 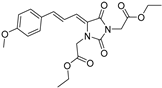 | 1.68 ± 0.04 | 6.83 ± 0.05 | 391 | 4.50 ± 0.32 |
| 4h* | 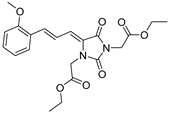 | 1.94 ± 0.04 | 4.28 ± 0.02 | 389 | 2.58 ± 0.01 |
| Octocrylene* | 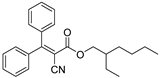 | 2.87 ± 0.16 | 1.20 ± 0.03 | 327 | 0.12 ± 0.01 |
| EHMC* |  | 4.96 ± 0.33 | 1.34 ± 0.05 | 321 | 0.05 ± 0.01 |
| 4-MBC* | 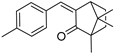 | 5.59 ± 0.27 | 1.08 ± 0.1 | 316 | 0.00 ± 0.00 |
| Avobenzone* | 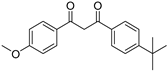 | 2.23 ± 0.03 | 7.56 ± 0.19 | 381 | 2.49 ± 0.03 |
| Compound | % of Initial SPFin vitro | % of Initial UVA PF | % of Initial AUC |
|---|---|---|---|
| 2a^ | 33.25 | 37.87 | 46.72 |
| 2a# | 55.49 | 68.96 | 62.42 |
| 2b^ | 36.51 | 24.13 | 35.28 |
| 2b# | 57.19 | 54.31 | 59.55 |
| 2c^ | 44.37 | 35.83 | 34.13 |
| 2d^ | 75.69 | 51.45 | 58.87 |
| 2e^ | 56.00 | 38.99 | 41.99 |
| 3a* | 83.31 | 103.50 | 88.19 |
| 3b* | 80.46 | 87.04 | 84.67 |
| 3b* | 72.69 | 84.08 | 76.50 |
| 3c* | 88.73 | 86.59 | 85.29 |
| 3d* | 89.92 | 97.66 | 94.01 |
| 3e* | 81.28 | 87.10 | 79.59 |
| 4f* | 62.24 | 25.98 | 27.92 |
| 4g* | 86.29 | 34.39 | 51.98 |
| 4h* | 82.22 | 62.38 | 69.19 |
| 4-MBC* | 91.05 | 101.39 | 90.89 |
| EHMC* | 80.83 | 88.76 | 78.45 |
| Compound | [M + H+] Da | t ½ (min) | Clint (µL/mg/min) | Metabolite Formation in the Presence (P) or/and Absence (A) of NADPH | Elemental Composition Change | Probable Transformation |
|---|---|---|---|---|---|---|
| 1e | 249 | 61 | 14 | M1 P | −14 | O-dealkylation |
| 2c | 319 | 33 | 52 | M1 a P | −28 | O-dealkylation |
| 2d | 335 | 15 | 59 | M1a P | −14 | O-dealkylation |
| 2e | 335 | 41 | 21 | M1 P | −14 | O-dealkylation |
| 3b | 391 | 12 | 75 | M1 P and A M2 P | −28 −14 | hydrolysis O-dealkylation |
| 4g | 417 | 3 | 328 | M1 P and A M2 P | −28 −14 | hydrolysis O-dealkylation |
| 5a | 247 | 1386 | 0.6 | ND | ||
| BP-2 | 247 | 66 | 13 | NT | NT | NT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popiół, J.; Gunia-Krzyżak, A.; Piska, K.; Żelaszczyk, D.; Koczurkiewicz, P.; Słoczyńska, K.; Wójcik-Pszczoła, K.; Krupa, A.; Kryczyk-Poprawa, A.; Żesławska, E.; et al. Discovery of Novel UV-Filters with Favorable Safety Profiles in the 5-Arylideneimidazolidine-2,4-dione Derivatives Group. Molecules 2019, 24, 2321. https://doi.org/10.3390/molecules24122321
Popiół J, Gunia-Krzyżak A, Piska K, Żelaszczyk D, Koczurkiewicz P, Słoczyńska K, Wójcik-Pszczoła K, Krupa A, Kryczyk-Poprawa A, Żesławska E, et al. Discovery of Novel UV-Filters with Favorable Safety Profiles in the 5-Arylideneimidazolidine-2,4-dione Derivatives Group. Molecules. 2019; 24(12):2321. https://doi.org/10.3390/molecules24122321
Chicago/Turabian StylePopiół, Justyna, Agnieszka Gunia-Krzyżak, Kamil Piska, Dorota Żelaszczyk, Paulina Koczurkiewicz, Karolina Słoczyńska, Katarzyna Wójcik-Pszczoła, Anna Krupa, Agata Kryczyk-Poprawa, Ewa Żesławska, and et al. 2019. "Discovery of Novel UV-Filters with Favorable Safety Profiles in the 5-Arylideneimidazolidine-2,4-dione Derivatives Group" Molecules 24, no. 12: 2321. https://doi.org/10.3390/molecules24122321
APA StylePopiół, J., Gunia-Krzyżak, A., Piska, K., Żelaszczyk, D., Koczurkiewicz, P., Słoczyńska, K., Wójcik-Pszczoła, K., Krupa, A., Kryczyk-Poprawa, A., Żesławska, E., Nitek, W., Żmudzki, P., Marona, H., & Pękala, E. (2019). Discovery of Novel UV-Filters with Favorable Safety Profiles in the 5-Arylideneimidazolidine-2,4-dione Derivatives Group. Molecules, 24(12), 2321. https://doi.org/10.3390/molecules24122321






