Toward Rational Design of Novel Anti-Cancer Drugs Based on Targeting, Solubility, and Bioavailability Exemplified by 1,3,4-Thiadiazole Derivatives Synthesized Under Solvent-Free Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
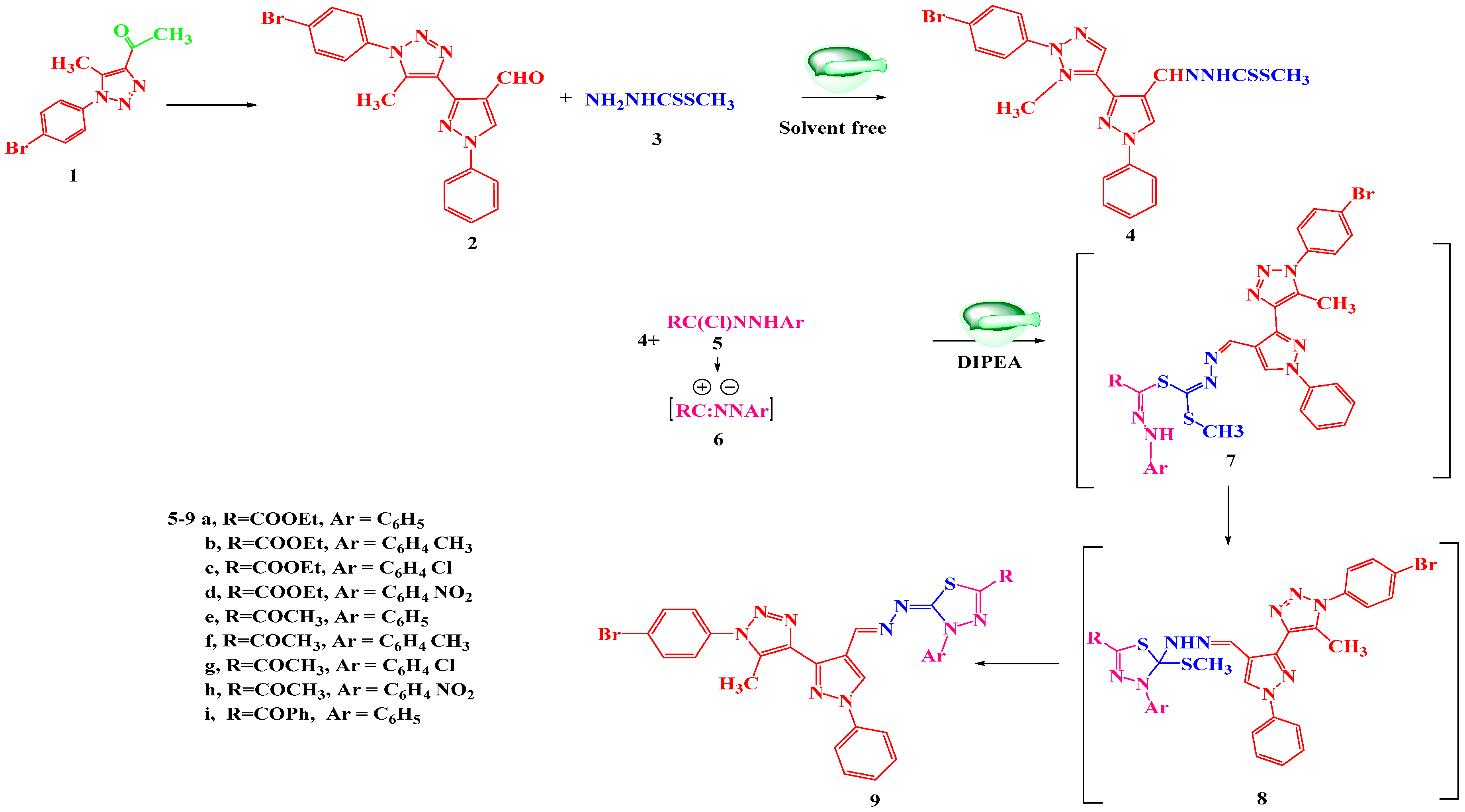
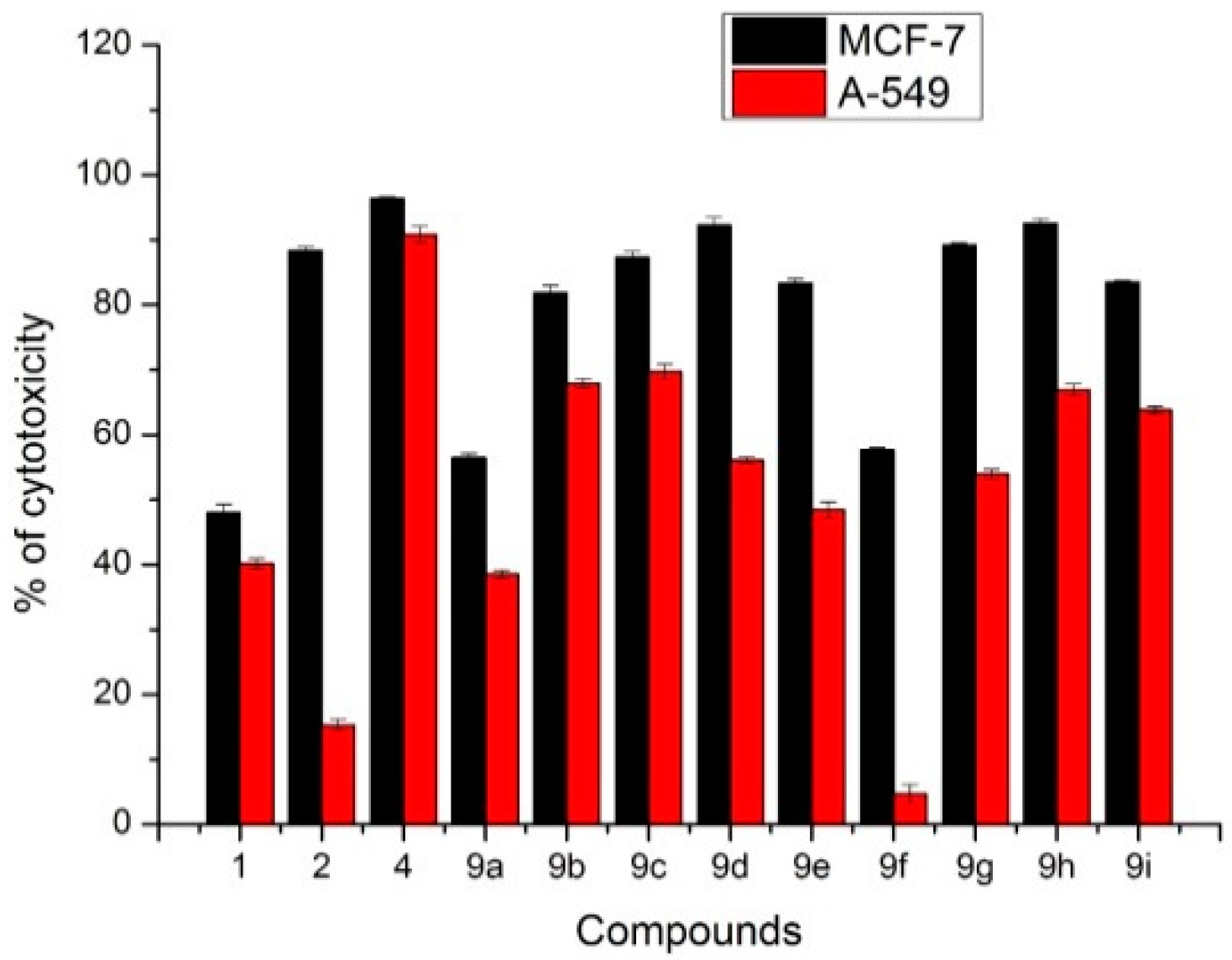
2.2. Anti-Cancer Activity
2.3. Microspheres Encapsulation
2.4. Morphology and Size of Microspheres
2.5. Thermal Gravimetric Analysis (TGA)
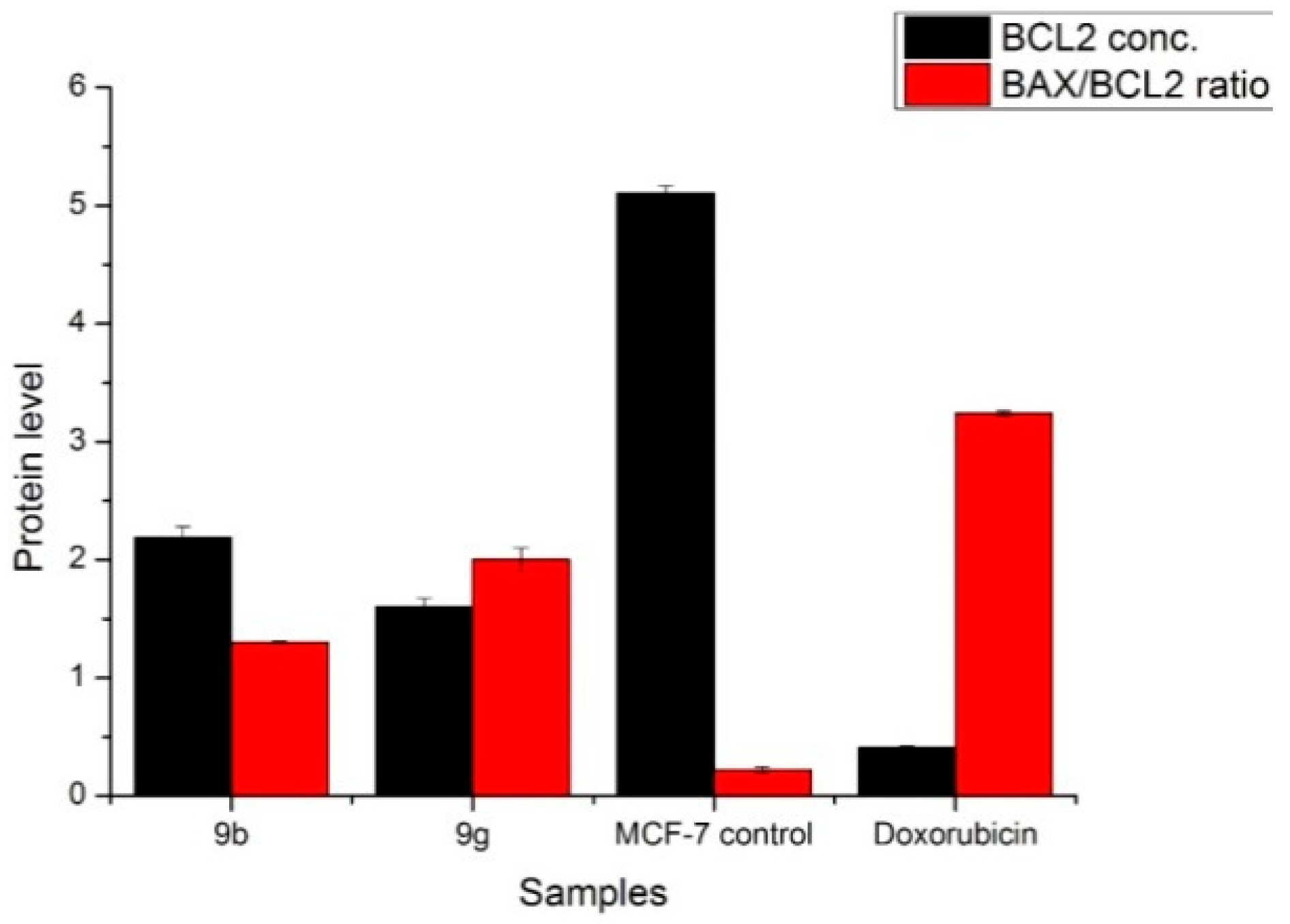
2.6. Antitumor Activity of M9b and M9g over Breast Cancer Cell Line (MCF-7)
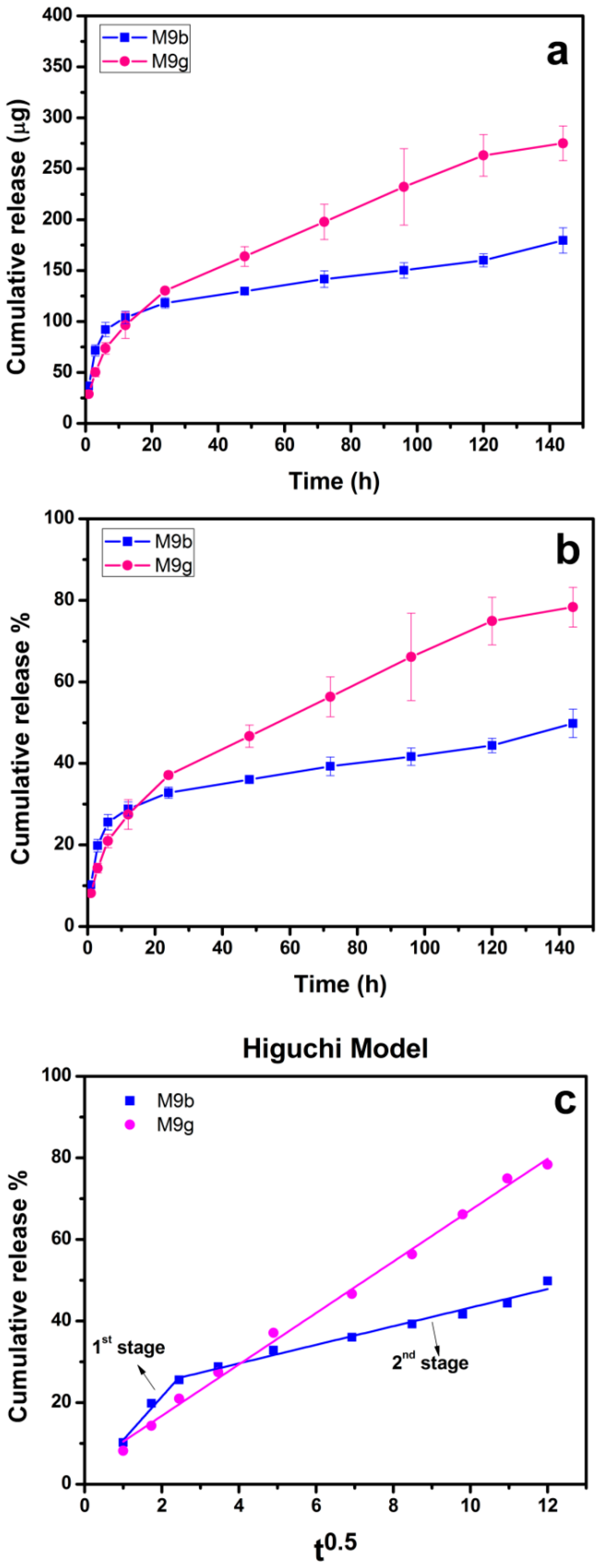
2.7. Drug Release Profile and Kinetics
3. Experimental
3.1. Chemistry
Experimental Instrumentation
3.2. Synthesis
3.2.1. Methyl 2-((3-(1-(4-bromophenyl)-5-methyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)hydrazine-1-carbodithioate (4)
3.2.2. General Procedures for Synthesis of 9a–i
3.3. Anti-Cancer Activity
3.3.1. Cell Lines
3.3.2. Cell Culture
3.3.3. Cell Viability Assay
3.3.4. Determination of IC50 Values
3.3.5. Measurement of DNA Fragmentation using DPA Assay
3.3.6. Human CASP7 (Caspase 7) Estimation
3.3.7. Measurement of BCl-2 Levels
3.3.8. Measurement of Bax Levels
3.4. Encapsulation in Na-alginate Microspheres
3.5. In Vitro Release Test
3.6. Drug Releasing Mechanism
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Vogus, D.R.; Krishnan, V.; Mitragotri, S. A review on engineering polymer drug conjugates to improve combination chemotherapy. Curr. Opin. Colloid Interface Sci. 2017, 31, 75–85. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Yang, M.-Y.; Sun, Z.-H.; Tan, C.-X.; Weng, J.-Q.; Wu, H.-K.; Liu, X.-H. Synthesis and antifungal activity of 1,3,4-thiadiazole derivatives containing pyridine group. Lett. Drug Des. Discov. 2014, 11, 1107–1111. [Google Scholar] [CrossRef]
- Yan, S.-L.; Yang, M.-Y.; Sun, Z.-H.; Min, L.-J.; Tan, C.-X.; Weng, J.-Q.; Wu, H.-K.; Liu, X.-H. Synthesis and antifungal activity of 1,2,3-thiadiazole derivatives containing 1,3,4-thiadiazole moiety. Lett. Drug Des. Discov. 2014, 11, 940–943. [Google Scholar] [CrossRef]
- Tong, J.-Y.; Sun, N.-B.; Wu, H.-K. Synthesis, crystal structure and biological activity of N-(5-(O-tolyl)-1,3,4-thiadiazol-2-yl) cyclopropanecarboxamide. J. Chem. Soc. Pak. 2013, 35, 1351–1355. [Google Scholar]
- Yang, M.-Y.; Zhao, W.; Sun, Z.-H.; Tan, C.-X.; Weng, J.-Q.; Liu, X.-H. Synthesis and biological activity of acylthiourea derivatives contain 1,2,3-thiadiazole and 1,3,4-thiadiazole. Lett. Drug Des. Discov. 2015, 12, 314–318. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Da, Y. Synthesis of 2-(5-(2-chlorophenyl)-2-furoylamido)-5-aryloxymethyl-1,3,4-thiadiazoles under microwave irradiation. Synth. Commun. 2001, 31, 1829–1836. [Google Scholar] [CrossRef]
- Liu, X.-H.; Shi, Y.-X.; Ma, Y.; Zhang, C.-Y.; Dong, W.-L.; Pan, L.; Wang, B.-L.; Li, B.-J.; Li, Z.-M. Synthesis, antifungal activities and 3D-QSAR study of N-(5-substituted-1,3,4-thiadiazol-2-yl) cyclopropanecarboxamides. Eur. J. Med. Chem. 2009, 44, 2782–2786. [Google Scholar] [CrossRef]
- Ahmad, T.; Singh, A.K.; Jaiswal, N.; Singh, D. Synthesis and Pharmacological Activity of 1,3,4-Thiadiazole Derivatives. ChemInform 2012, 43, 30. [Google Scholar] [CrossRef]
- Abdelhamid, A.O.; El-Idreesy, T.T.; Abdelriheem, N.A.; Dawoud, H.R.M. Green One-Pot Solvent-Free Synthesis of Pyrazolo[1,5-a]pyrimidines, Azolo[3,4-d]pyridiazines, and Thieno[2,3-b]pyridines Containing Triazole Moiety. J. Heterocycl. Chem. 2016, 53, 710–718. [Google Scholar] [CrossRef]
- Rashdan, H.R.M.; Gomha, S.M.; El-Gendey, M.S.; El-Hashash, M.A.; Soliman, A.M.M. Eco-friendly one-pot synthesis of some new pyrazolo[1,2-b]phthalazinediones with antiproliferative efficacy on human hepatic cancer cell lines. Green Chem. Lett. Rev. 2018, 11, 264–274. [Google Scholar] [CrossRef]
- Brockunier, L.L.; Parmee, E.R.; Ok, H.O.; Candelore, M.R.; Cascieri, M.A.; Colwell, L.F., Jr.; Deng, L.; Feeney, W.P.; Forrest, M.J.; Hom, G.J. Human β3-adrenergic receptor agonists containing 1,2,3-triazole-substituted benzenesulfonamides. Bioorganic Med. Chem. Lett. 2000, 10, 2111–2114. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Gao, Y.; Hou, Y.-L.; Zhang, C.; Yu, S.-J.; Bian, Q.; Li, Z.-M.; Zhao, W.-G. Design, synthesis, and fungicidal evaluation of a series of novel 5-methyl-1H-1,2,3-trizole-4-carboxyl amide and ester analogues. Eur. J. Med. Chem. 2014, 86, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Brik, A.; Muldoon, J.; Lin, Y.C.; Elder, J.H.; Goodsell, D.S.; Olson, A.J.; Fokin, V.V.; Sharpless, K.B.; Wong, C.H. Rapid diversity-oriented synthesis in microtiter plates for in situ screening of HIV protease inhibitors. ChemBioChem 2003, 4, 1246–1248. [Google Scholar] [CrossRef] [PubMed]
- Whiting, M.; Muldoon, J.; Lin, Y.C.; Silverman, S.M.; Lindstrom, W.; Olson, A.J.; Kolb, H.C.; Finn, M.; Sharpless, K.B.; Elder, J.H. Inhibitors of HIV-1 protease by using in situ click chemistry. Angew. Chem. Int. Ed. 2006, 45, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Biorn, A.C.; Cocklin, S.; Madani, N.; Si, Z.; Ivanovic, T.; Samanen, J.; Van Ryk, D.I.; Pantophlet, R.; Burton, D.R.; Freire, E. Mode of action for linear peptide inhibitors of HIV-1 gp120 interactions. Biochemistry 2004, 43, 1928–1938. [Google Scholar] [CrossRef] [PubMed]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase:[1,2,3]-triazoles by regiospecific copper (I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Shah, D.B.; Ramanathan, M. Glycogen synthase kinase-3: A potential target for drug discovery in the treatment of neurodegenerative disorders. Curr. Enzym. Inhib. 2017, 13, 107–128. [Google Scholar] [CrossRef]
- Olesen, P.H.; Sørensen, A.R.; Ursø, B.; Kurtzhals, P.; Bowler, A.N.; Ehrbar, U.; Hansen, B.F. Synthesis and in vitro characterization of 1-(4-aminofurazan-3-yl)-5-dialkylaminomethyl-1H-[1,2,3]triazole-4-carboxylic acid derivatives. A new class of selective GSK-3 inhibitors. J. Med. Chem. 2003, 46, 3333–3341. [Google Scholar] [CrossRef]
- Krasiński, A.; Radić, Z.; Manetsch, R.; Raushel, J.; Taylor, P.; Sharpless, K.B.; Kolb, H.C. In situ selection of lead compounds by click chemistry: Target-guided optimization of acetylcholinesterase inhibitors. J. Am. Chem. Soc. 2005, 127, 6686–6692. [Google Scholar] [CrossRef]
- Mocharla, V.P.; Colasson, B.; Lee, L.V.; Röper, S.; Sharpless, K.B.; Wong, C.H.; Kolb, H.C. In situ click chemistry: Enzyme-generated inhibitors of carbonic anhydrase II. Angew. Chem. 2005, 117, 118–122. [Google Scholar] [CrossRef]
- Syed, M.A.; Ramappa, A.K.; Alegaon, S. Synthesis and evaluation of antitubercular and anti fungal activity of some novel 6-(4-substituted aryl)-2-(3,5-dimethyl-1H-pyrazol-1-yl) imidazo[2,1-b][1,3,4] thiadiazole derivatives. Asian J. Pharm. Clin. Res. 2013, 6, 47–51. [Google Scholar]
- Caballé, C.; Urdaneta, E.; Marzo, F.; Larralde, J.; Santidrián, S. Inhibition of in vitro intestinal absorption of D-galactose by cefroxadine, cefatrizine and cefaloglycin. Indian J. Pharmacol. 2003, 35, 163–167. [Google Scholar]
- Totobenazara, J.; Burke, A.J. New click-chemistry methods for 1,2,3-triazoles synthesis: Recent advances and applications. Tetrahedron Lett. 2015, 56, 2853–2859. [Google Scholar] [CrossRef]
- Krasiński, A.; Fokin, V.V.; Sharpless, K.B. Direct synthesis of 1,5-disubstituted-4-magnesio-1,2,3-triazoles, revisited. Org. Lett. 2004, 6, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Toda, F. Solvent-free organic synthesis. Chem. Rev. 2000, 100, 1025–1074. [Google Scholar] [CrossRef] [PubMed]
- Gedye, R.; Smith, F.; Westaway, K.; Ali, H.; Baldisera, L.; Laberge, L.; Rousell, J. The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett. 1986, 27, 279–282. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef]
- Shiraishi, S.; Imai, T.; Iwaoka, D.; Otagiri, M. Improvement of absorption rate of indomethacin and reduction of stomach irritation by alginate dispersions. J. Pharm. Pharmacol. 1991, 43, 615–620. [Google Scholar] [CrossRef]
- Shiraishi, S.; Arahira, M.; Imai, T.; Otagiri, M. Enhancement of dissolution rates of several drugs by low-molecular chitosan and alginate. Chem. Pharm. Bull. 1990, 38, 185–187. [Google Scholar] [CrossRef]
- Mondal, N.; Pal, T.K.; Ghosal, S.K. Development and validation of RP-HPLC method to determine letrozole in different pharmaceutical formulations and its application to studies of drug release from nanoparticles. Acta Pol. Pharm. 2009, 66, 11–17. [Google Scholar] [PubMed]
- Dey, S.K.; Mandal, B.; Bhowmik, M.; Ghosh, L.K. Development and in vitro evaluation of Letrozole loaded biodegradable nanoparticles for breast cancer therapy. Braz. J. Pharm. Sci. 2009, 45, 585–591. [Google Scholar] [CrossRef]
- Siddiqa, A.J.; Chaudhury, K.; Adhikari, B. Letrozole dispersed on poly (vinyl alcohol) anchored maleic anhydride grafted low density polyethylene: A controlled drug delivery system for treatment of breast cancer. Colloids Surf. B Biointerfaces 2014, 116, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Alemrayat, B.; Elhissi, A.; Younes, H.M. Preparation and characterization of letrozole-loaded poly(d,l-lactide) nanoparticles for drug delivery in breast cancer therapy. Pharm. Dev. Technol. 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rashdan, H.R.; Roaiah, H.M.; Muhammad, Z.A.; Wietrzyk, J.; Milczarek, M.; Soliman, A.M. Design, efficient synthesis, mechanism of reaction and antiproliferative activity against cancer and normal cell lines of a novel class of fused pyrimidine derivatives. Acta Polooiae Pharm. Drug Res. 2018, 75, 679–688. [Google Scholar]
- Klayman, D.L.; Bartosevich, J.F.; Griffin, T.S.; Mason, C.J.; Scovill, J.P. 2-Acetylpyridine thiosemicarbazones. 1. A new class of potential antimalarial agents. J. Med. Chem. 1979, 22, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.O.; Fahmi, A.A.; Ali, A.B. Reactions with hydrazonoyl halides 66: Synthesis of some new 1,3,4-thiadiazoles, triazolino[4,3-a]pyrimidines and isoxazolo[3,4-d]pyridazines containing coumarin moiety. Eur. J. Chem. 2011, 2, 544–551. [Google Scholar] [CrossRef]
- Abdelhamid, A.O.; Mohamed, G.S. Reactions with hydrazonoyl halides XXIV [1]: Synthesis of some new unsymmetrical azines and dihydro-1,3,4-thiadiazoles. Phosphorus Sulfur Silicon Relat. Elem. 1999, 152, 115–128. [Google Scholar] [CrossRef]
- Abdelhamid, A.O.; Abdel-Riheem, N.A.; Emam, H.A. Reactions with Hydrazonoyl Halides. Part XXV. Synthesis of Some New 2,3-Dihydro-1,3,4-thiadiazoles and 5-Arylazothiazoles. J. Chem. Res. Synop. 1999, 9, 532–533. [Google Scholar] [CrossRef]
- Thabrew, M.I.; HUGHES, R.D.; MCFARLANE, I.G. Screening of hepatoprotective plant components using a HepG2 cell cytotoxicity assay. J. Pharm. Pharmacol. 1997, 49, 1132–1135. [Google Scholar] [CrossRef]
- Selim, M.S.; Amer, S.K.; Mohamed, S.S.; Mounier, M.M.; Rifaat, H.M. Production and characterisation of exopolysaccharide from Streptomyces carpaticus isolated from marine sediments in Egypt and its effect on breast and colon cell lines. J. Genet. Eng. Biotechnol. 2018, 16, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Cohen, J.J.; Duke, R. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J. Immunol. 1984, 132, 38–42. [Google Scholar] [PubMed]
- Burton, K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem. J. 1956, 62, 315. [Google Scholar] [CrossRef] [PubMed]
- Denault, J.-B.; Salvesen, G.S. Human caspase-7 activity and regulation by its N-terminal peptide. J. Biol. Chem. 2003, 278, 34042–34050. [Google Scholar] [CrossRef] [PubMed]
- Barbareschi, M.; Caffo, O.; Veronese, S.; Leek, R.D.; Fina, P.; Fox, S.; Bonzanini, M.; Girlando, S.; Morelli, L.; Eccher, C. Bcl-2 and p53 expression in node-negative breast carcinoma: A study with long-term follow-up. Hum. Pathol. 1996, 27, 1149–1155. [Google Scholar] [CrossRef]
- Onur, R.; Semerciöz, A.; Orhan, I.; Yekeler, H. The effects of melatonin and the antioxidant defence system on apoptosis regulator proteins (Bax and Bcl-2) in experimentally induced varicocele. Urol. Res. 2004, 32, 204–208. [Google Scholar] [CrossRef]
- Poncelet, D.; Lencki, R.; Beaulieu, C.; Halle, J.; Neufeld, R.; Fournier, A. Production of alginate beads by emulsification/internal gelation. I. Methodology. Appl. Microbiol. Biotechnol. 1992, 38, 39–45. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

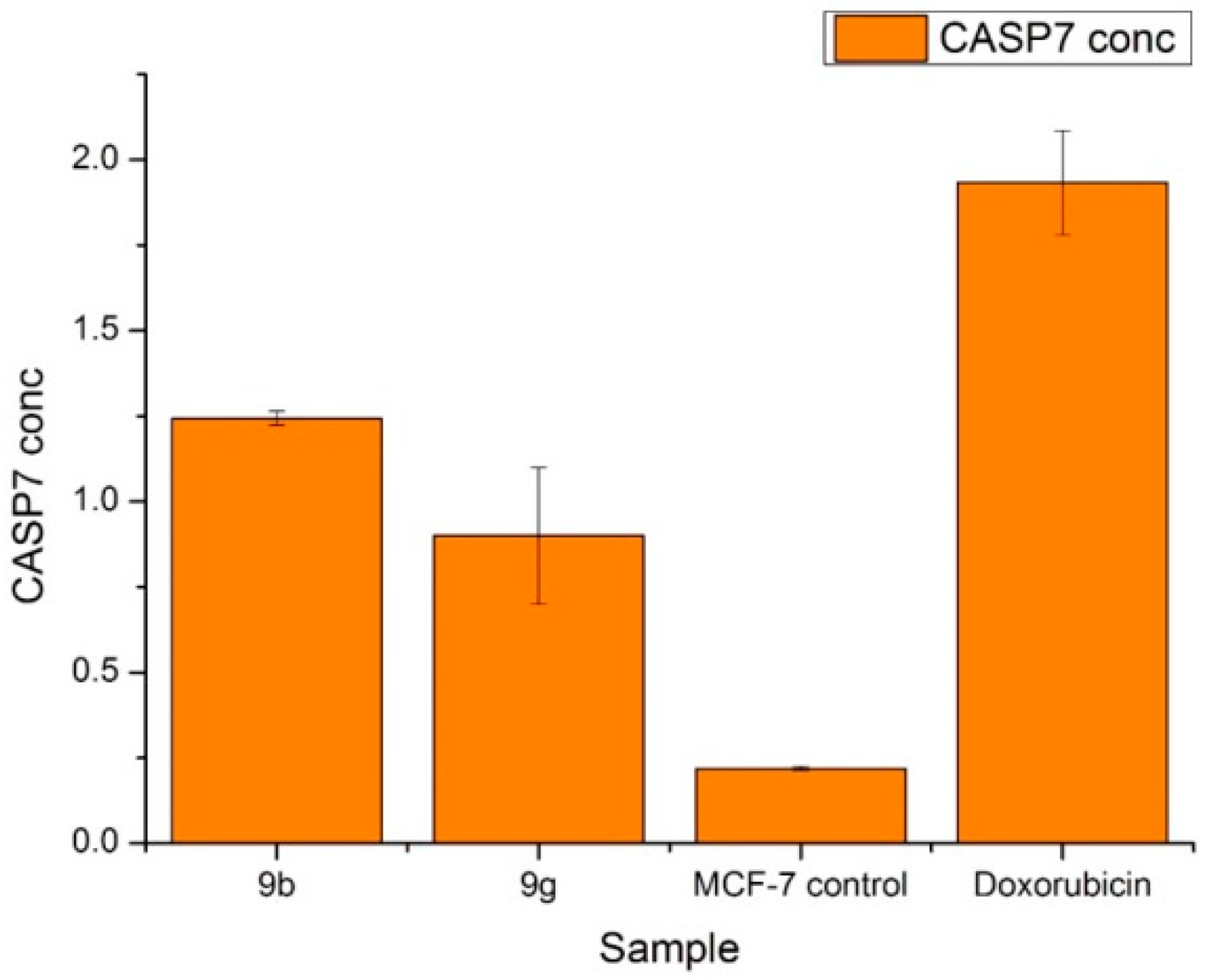


| BAX CONC. | |
|---|---|
| 9b | 160.5 |
| 9g | 241.7 |
| cont. | 40.743 |
| Doxorubicin | 259.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashdan, H.R.M.; Farag, M.M.; El-Gendey, M.S.; Mounier, M.M. Toward Rational Design of Novel Anti-Cancer Drugs Based on Targeting, Solubility, and Bioavailability Exemplified by 1,3,4-Thiadiazole Derivatives Synthesized Under Solvent-Free Conditions. Molecules 2019, 24, 2371. https://doi.org/10.3390/molecules24132371
Rashdan HRM, Farag MM, El-Gendey MS, Mounier MM. Toward Rational Design of Novel Anti-Cancer Drugs Based on Targeting, Solubility, and Bioavailability Exemplified by 1,3,4-Thiadiazole Derivatives Synthesized Under Solvent-Free Conditions. Molecules. 2019; 24(13):2371. https://doi.org/10.3390/molecules24132371
Chicago/Turabian StyleRashdan, Huda R.M., Mohammad M. Farag, Marwa S. El-Gendey, and Marwa M. Mounier. 2019. "Toward Rational Design of Novel Anti-Cancer Drugs Based on Targeting, Solubility, and Bioavailability Exemplified by 1,3,4-Thiadiazole Derivatives Synthesized Under Solvent-Free Conditions" Molecules 24, no. 13: 2371. https://doi.org/10.3390/molecules24132371





