Development of a High-Quality ELISA Method for Dinotefuran Based on a Novel and Newly-Designed Antigen
Abstract
1. Introduction
2. Results and Discussion
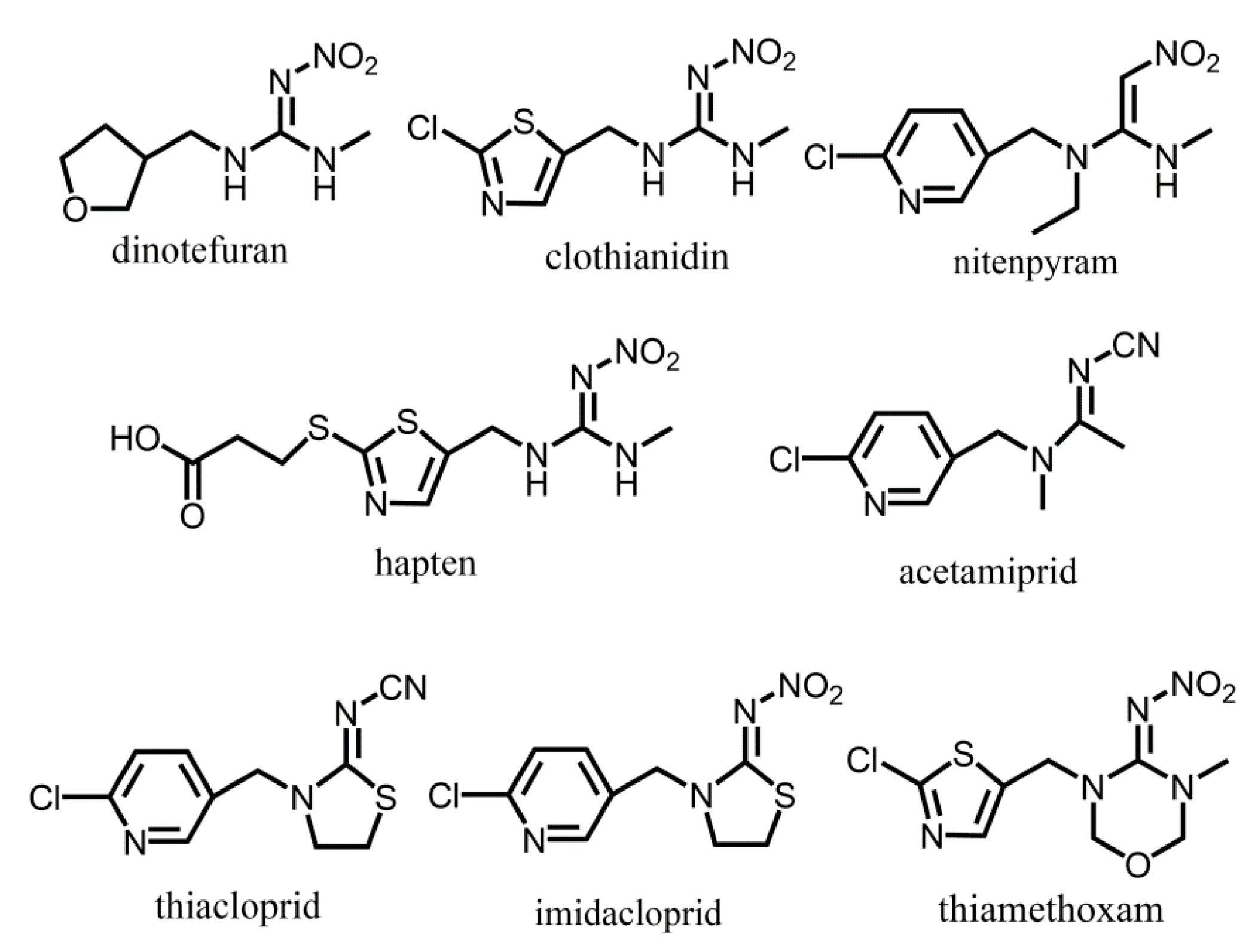
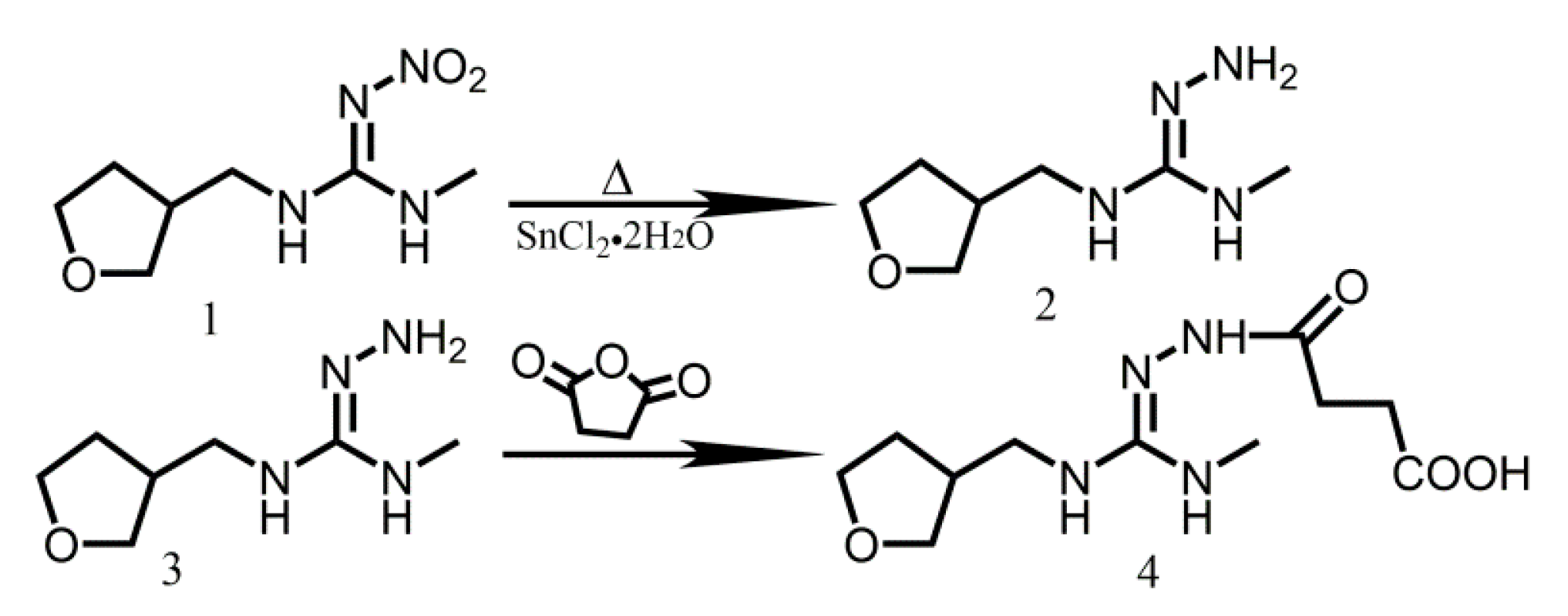
2.1. Design and Synthesis of Haptens and Antigens
2.2. Positive Monoclonal Hybridomas from the Semisolid Medium Screening
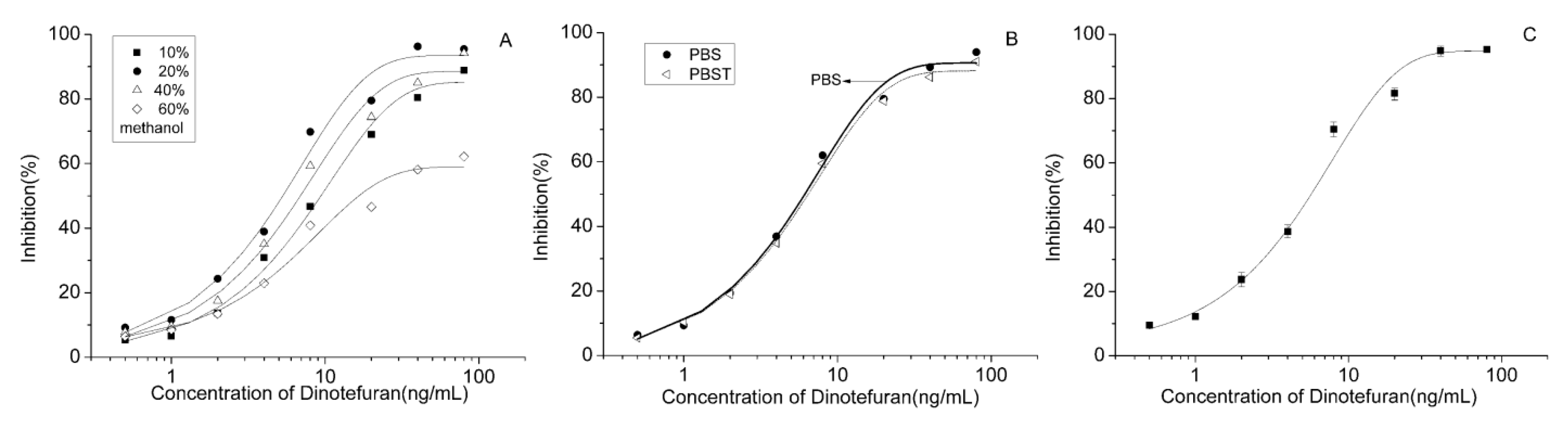
2.3. Affinity, Sensitivity, and Specificity of the Antibody
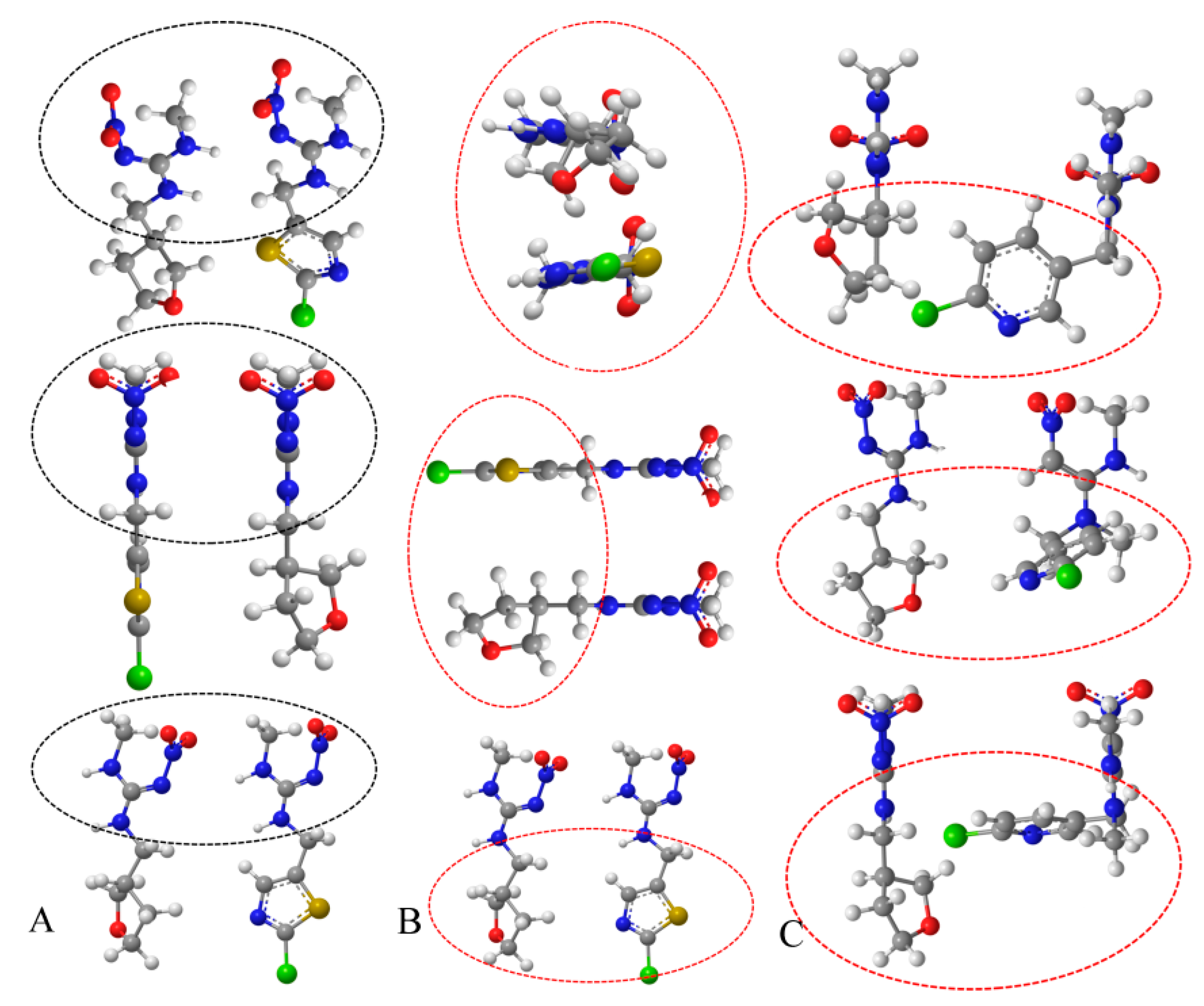
2.4. The Analysis of the Coupling Site of the Hapten
2.5. Establishment of an Optimized CI-ELISA
2.6. Analysis of Spiked Samples
3. Conclusions
4. Methods and Materials
4.1. General Experimental Reagents and Instruments
4.2. Synthesis of Hapten
4.3. Preparation of Antigen (Hapten-Protein Conjugates)
4.4. Immunization
4.5. Cell Fusion and Cloning
4.6. Non-Competitive ELISA and Competitive Indirect ELISA
4.7. Affinity, Sensitivity, and Specificity
4.8. Optimization of the CI-ELISA
4.9. Analysis of Spiked Samples
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, T.; Wang, X.; Xu, J.; You, X.; Chen, D.; Wang, F.; Li, Y. Biochemical and genetic toxicity of dinotefuran on earthworms (Eisenia fetida). Chemosphere 2017, 176, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, C.K. Neonicotinoid contamination of global surface waters and associatedrisk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Hem, L.; El-Aty, A.A.; Park, J.H.; Shim, J.H. Determination of dinotefuran in pepper using liquid chromatography: contribution to safety evaluation. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 765–768. [Google Scholar] [CrossRef]
- Rahman, M.M.; El-Aty, A.A.; Choi, J.H.; Kim, S.W.; Shin, S.C.; Shim, J.H. Consequences of the matrix effect on recovery of dinotefuran and its metabolites in green tea during tandem mass spectrometry analysis. Food Chem. 2015, 168, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Dubetz, C.; Palace, V.P. Neonicotinoids in the Canadian aquatic environment: a literature review on current use products with a focus on fate, exposure, and biological effects. Sci. Total. Environ. 2014, 505, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.; Koutroubas, S. Farmers’ exposure to pesticides: toxicity types and ways of prevention. Toxins 2016, 4, 1. [Google Scholar] [CrossRef]
- Tanner, G.; Czerwenka, C. LC-MS/MS analysis of neonicotinoid insecticides in honey: Methodology and residue findings in Austrian honeys. J. Agric. Food Chem. 2011, 59, 12271–12277. [Google Scholar] [CrossRef]
- Knopp, D. Immunoassay development for environmental analysis. Anal. Bioanal. Chem. 2006, 385, 425–427. [Google Scholar] [CrossRef]
- Watanabe, E.; Baba, K.; Miyake, S. Analytical evaluation of enzyme-linked immunosorbent assay for neonicotinoid dinotefuran for potential application to quick and simple screening method in rice samples. Talanta 2011, 84, 1107–1111. [Google Scholar] [CrossRef]
- Uchigashima, M.; Watanabe, E.; Ito, S.; Iwasa, S.; Miyake, S. Development of Immunoassay Based on Monoclonal Antibody Reacted with the Neonicotinoid Insecticides Clothianidin and Dinotefuran. Sensors 2012, 12, 15858–15872. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Turrillas, F.A.; Mercader, J.V.; Agulló, C.; Abad-Somovilla, A.; Abad-Fuentesa, A. A class-selective immunoassay for simultaneous analysis of anilinopyrimidine fungicides using a rationally designed hapten. Analyst 2017, 142, 3975–3985. [Google Scholar] [CrossRef] [PubMed]
- Bronshtein, A.; Chuang, J.C.; Van Emon, J.M.; Altstein, M. Development of a Multianalyte Enzyme-Linked Immunosorbent Assay for Permethrin and Aroclors and Its Implementation for Analysis of Soil/Sediment and House Dust Extracts. J. Agric. Food Chem. 2012, 60, 4235–4242. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Pennington, J.E.; Kubler, A.M.; Conscience, J.F. A simple, single-step technique for selecting and cloning hybridomas for the production of monoclonal antibodies. J. Immunol. Methods 1982, 50, 161–171. [Google Scholar] [CrossRef]
- Abad, A.; Moreno, M.J.; Montoya, A. Hapten synthesis and production of monoclonal antibodies to the N-methylcarbamate pesticide methiocarb. J. Agric. Food Chem. 1998, 46, 2417–2426. [Google Scholar] [CrossRef]
- Gui, W.J.; Jin, R.Y.; Chen, Z.L.; Cheng, J.L.; Zhu, G.N. Hapten synthesis for enzyme-linked immunoassay of the insecticide triazophos. Anal. Biochem. 2006, 357, 9–14. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Ferreira, J.M.S.; Talamini, V.; Facco, J.D.F.; Rizzetti, T.M.; Prestes, O.D. Determination of pesticides in coconut (Cocos nucifera Linn.) water and pulp using modified QuEChERS and LC-MS/MS. Food Chem. 2016, 213, 616–624. [Google Scholar] [CrossRef]
- Machado, I.; Gérez, N.; Pistón, M.; Heinzen, H.; Cesio, M.V. Determination of pesticide residues in globe artichoke leaves and fruits by GC-MS and LC-MS/MS using the same QuEChERS procedure. Food Chem. 2017, 227, 227–236. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, Q.; Zhang, W.; Shirley, J.G.; Li, P.W. Development of a Monoclonal Antibody-Based Enzyme Immunoassay for the Pyrethroid Insecticide Deltamethrin. J. Agric. Food Chem. 2010, 58, 8189–8195. [Google Scholar] [CrossRef]
- Erlanger, B.F. Principles and methods for the preparation of drug protein conjugates for immunological studies. Pharmacol. Rev. 1973, 25, 271–280. [Google Scholar]
- Tijssen, P. Practice and Theory of Enzyme Immunoassay; Elsevier: Amsterdam, The Netherlands, 1985. [Google Scholar]
- Garrett, S.D.; Appleford, D.J.A.; Wyatt, G.M.; Lee, H.A.; Morgan, M.R.A. Production of a recombinant anti-parathion antibody (ScFv): stability in methanolic food extracts and comparison to an anti-parathion monoclonal antibody. J. Agric. Food Chem. 1997, 45, 4183–4189. [Google Scholar] [CrossRef]
- Yu, K.O.A.; Im, J.S.; Illarionov, P.A.; Ndonye, R.M.; Howell, A.R.; Besra, G.S.; Porcelli, S.A. Production and characterization of monoclonal antibodies against complexes of the NKT cell ligand R-galactosylceramide bound to mouse CD1d. J. Immunol. Methods 2007, 323, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Abubaker, S.; He, X.; Sun, G.; Strickland, P.T.; Groopman, J.D. Development of aflatoxin B1-lysine adduct monoclonal antibody for human exposure studies. Appl. Environ. Microbiol. 2001, 67, 2712–2717. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.N.; Wu, G.; Wu, H.M. Synthesis and identification of the antigens for the organophpsphorus insecticide chlorpyrifos. Sci. Agric. Sin. 2003, 36, 657–662. [Google Scholar]
- Shan, G.M.; Stoutamire, D.W.; Wengatz, I.; Gee, S.J.; Hammock, B.D. Development of an immunoassay for the pyrethroid insecticide esfenvalerate. J. Agric. Food Chem. 1999, 47, 2145–2155. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Antibody | Titer | IC50 (mg/L) |
|---|---|---|
| 2D6 | 160,000 | 29.0 ± 0.23 |
| 2F10 | 128,000 | 24.1 ± 0.12 |
| 3C7 | 64,000 | 6.17 ± 0.05 |
| Analyte | Structure | CR(%) | ||
|---|---|---|---|---|
| 2D6 | 2F10 | 3C7 | ||
| Dinotefuran | 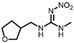 | 100 | ||
| Acetamiprid | 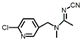 | 4.90 | 4.37 | 1.44 |
| Nitenpyram | 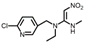 | 5.67 | 5.06 | 1.13 |
| Diuron | 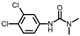 | 0.17 | 0.20 | 0.14 |
| Thiamethoxam | 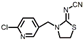 | <0.096 | <0.096 | <0.048 |
| Clothianidin | 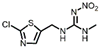 | 0.11 | 0.20 | <0.096 |
| Tetrahydrofuran |  | 9.1 | 6.7 | 6.1 |
| Spiked Concentration (ng/mL) | Average Recovery (n = 3) | RSD (%) | ||
|---|---|---|---|---|
| ELISA | LC | ELISA | LC | |
| 1 | 91.02 | 90.06 | 4.31 | 1.59 |
| 5 | 98.45 | 92.76 | 4.08 | 1.88 |
| 10 | 87.49 | 96.96 | 4.75 | 4.43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Liu, J.; Luo, J. Development of a High-Quality ELISA Method for Dinotefuran Based on a Novel and Newly-Designed Antigen. Molecules 2019, 24, 2426. https://doi.org/10.3390/molecules24132426
Zhao F, Liu J, Luo J. Development of a High-Quality ELISA Method for Dinotefuran Based on a Novel and Newly-Designed Antigen. Molecules. 2019; 24(13):2426. https://doi.org/10.3390/molecules24132426
Chicago/Turabian StyleZhao, Fangfang, Jingkun Liu, and Jinhui Luo. 2019. "Development of a High-Quality ELISA Method for Dinotefuran Based on a Novel and Newly-Designed Antigen" Molecules 24, no. 13: 2426. https://doi.org/10.3390/molecules24132426
APA StyleZhao, F., Liu, J., & Luo, J. (2019). Development of a High-Quality ELISA Method for Dinotefuran Based on a Novel and Newly-Designed Antigen. Molecules, 24(13), 2426. https://doi.org/10.3390/molecules24132426




