Application of Porphyrins in Antibacterial Photodynamic Therapy
Abstract
:1. Introduction
1.1. Antibacterial Photodynamic Therapy (aPDT)
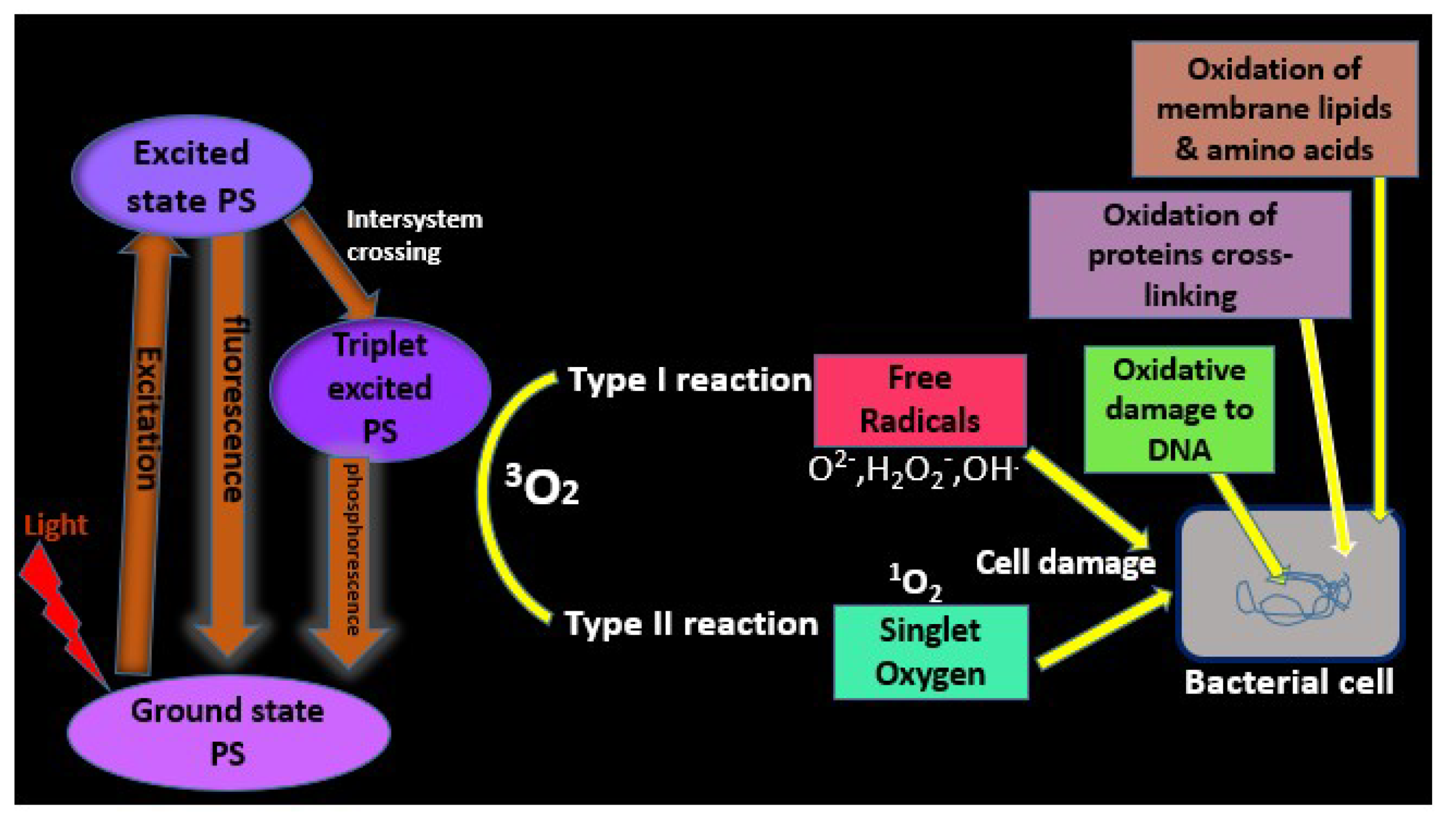
1.1.1. Mechanism of Porphyrin Photosensitization: Photophysical and Photochemical Processes
1.1.2. Type I Reaction
1.1.3. Type II Reaction
1.2. What Are Porphyrins?
1.2.1. Porphyrins as Colors of Life
1.2.2. Classification of Porphyrins
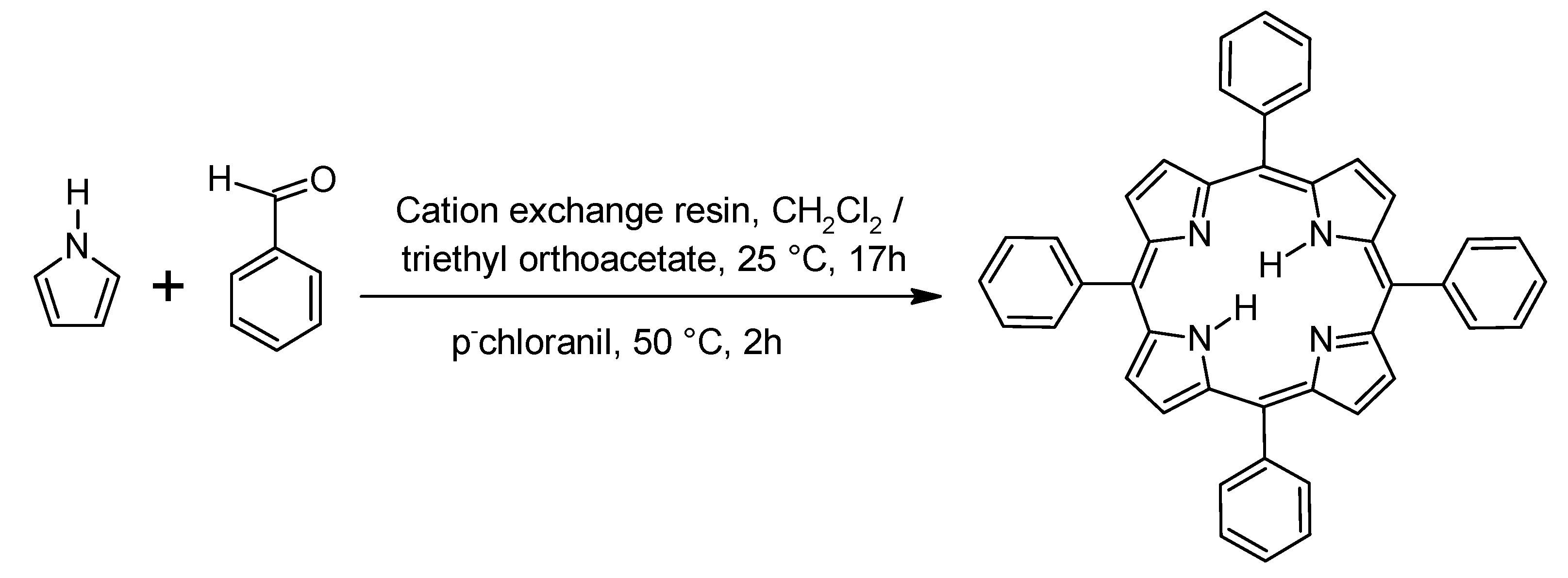
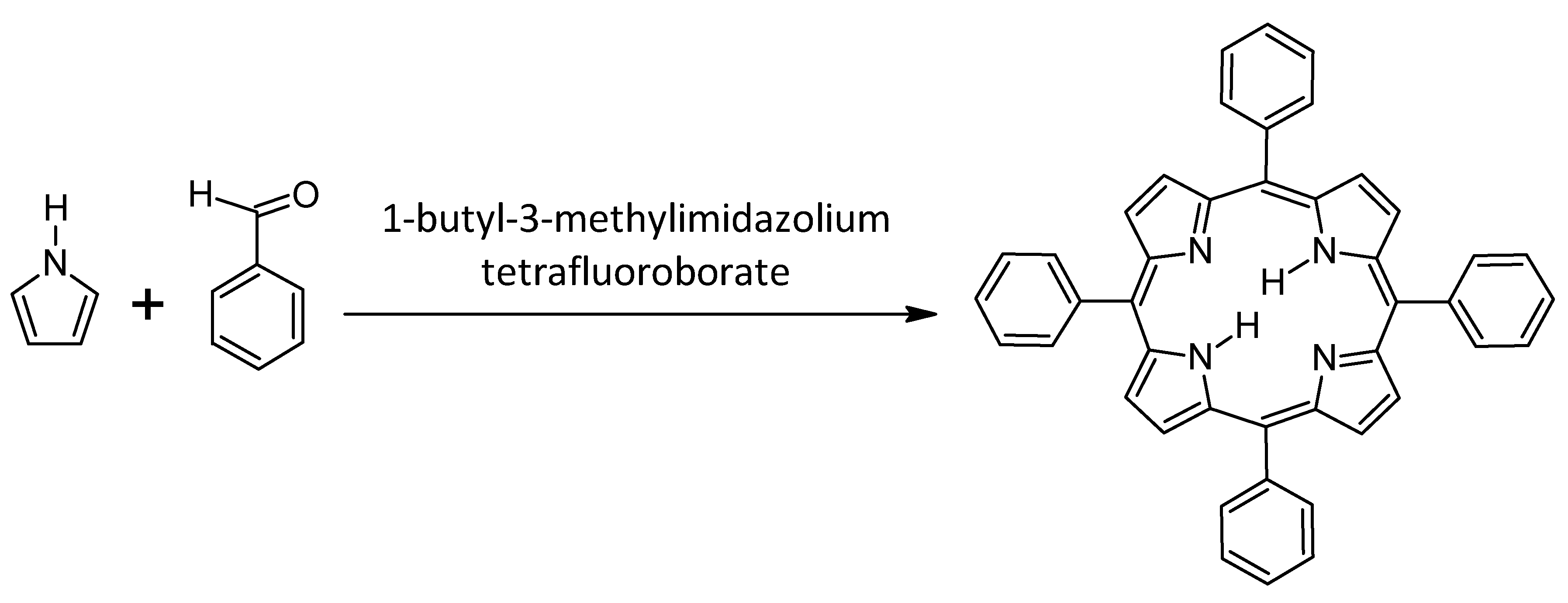
1.2.3. General Synthesis of Porphyrins
Rothemund’s Method
Adler–Longo’s Method
Lindsey’s Method
Other Synthetic Methods
Microwave-Assisted Synthesis
Solventless Reactions
Ionic Liquids as Alternative Solvents
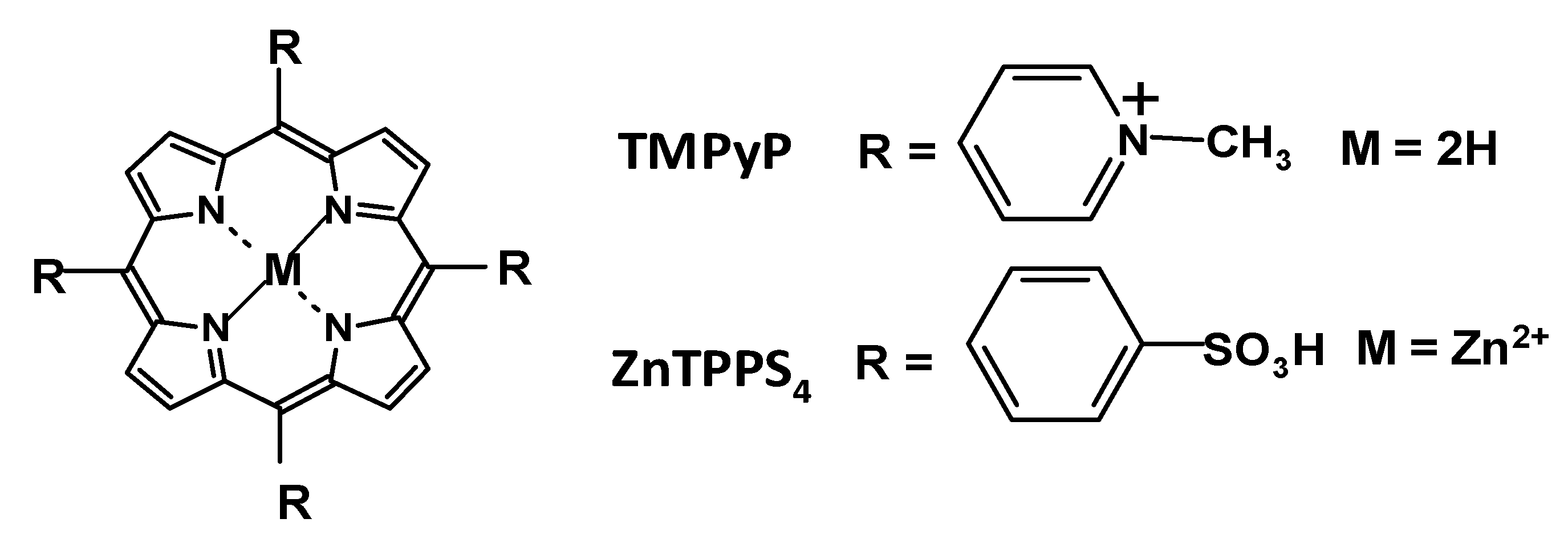
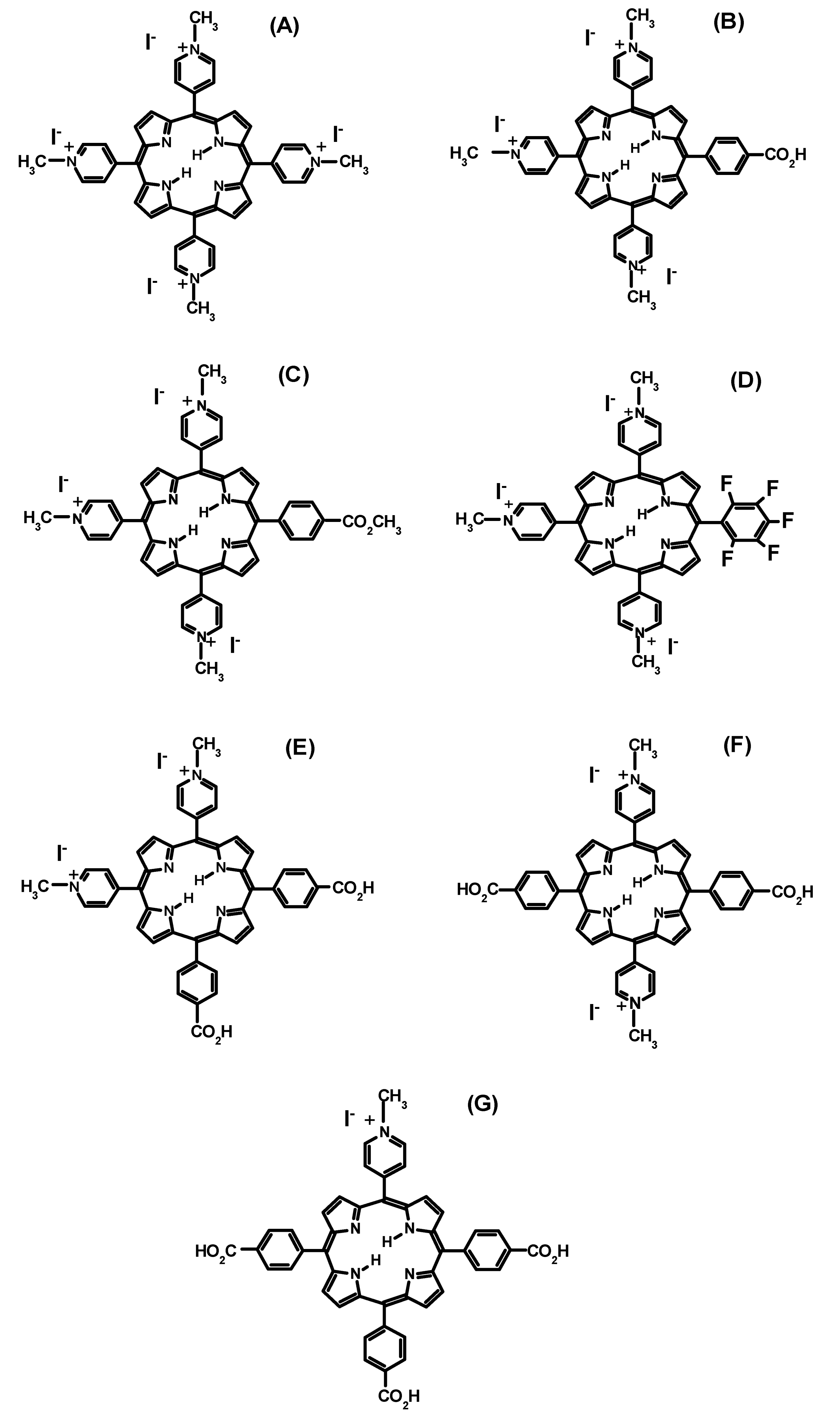
1.3. Unique Properties of Porphyrins as Drugs for aPDT
- Porphyrins have relatively low toxicity in vitro and in vivo and can be functionalized to be water soluble or water insoluble [58].
- They can be cleared in a reasonable time from the body and rapidly from the skin to avoid photosensitive reaction [32].
- Porphyrins can also possess competent amphiphilicity and ability for numerous chemical modifications [59].
- They have high quantum yield (above 0.70) for 1O2 generation and high one-photon absorption coefficient (≈500,000 M−1cm−1) [60].
2. Bacteria
2.1. Differences in Membrane Structure Between Gram-(+) and Gram-(−) Bacteria
2.1.1. Mode of Porphyrin Action on Bacterial Cell
- The first is that the PS settles outside the cell, generating reactive oxygen species in solution, which can diffuse into the cells of the target organism and react to induce cellular damage.
- The second mechanism is that the PS binds to or becomes localized at the cell membrane (by hydrophobic or coulombic interactions)—upon light absorption, the PS transfers energy (e.g., an electron, hydrogen atom etc.) to target biomolecules within the cell, resulting in ROS production that cause cell damage. Anionic porhphyrins follow this mechanism of photosensitization.
- The third possibility is that the PS penetrates the interior of the cell and becomes associated with an intracellular target, possibly a protein (inducing enzymatic damage) or the nucleus (inducing genetic damage). Cationic porphyrins that bind strongly to the polyanionic macromolecules such as DNA are good examples of this type of phototoxic agent [79].
2.1.2. Mechanisms of Porphyrin Photodynamic Inactivation of Bacteria
Functional Damage
Morphological Changes
Cell Membrane Damage
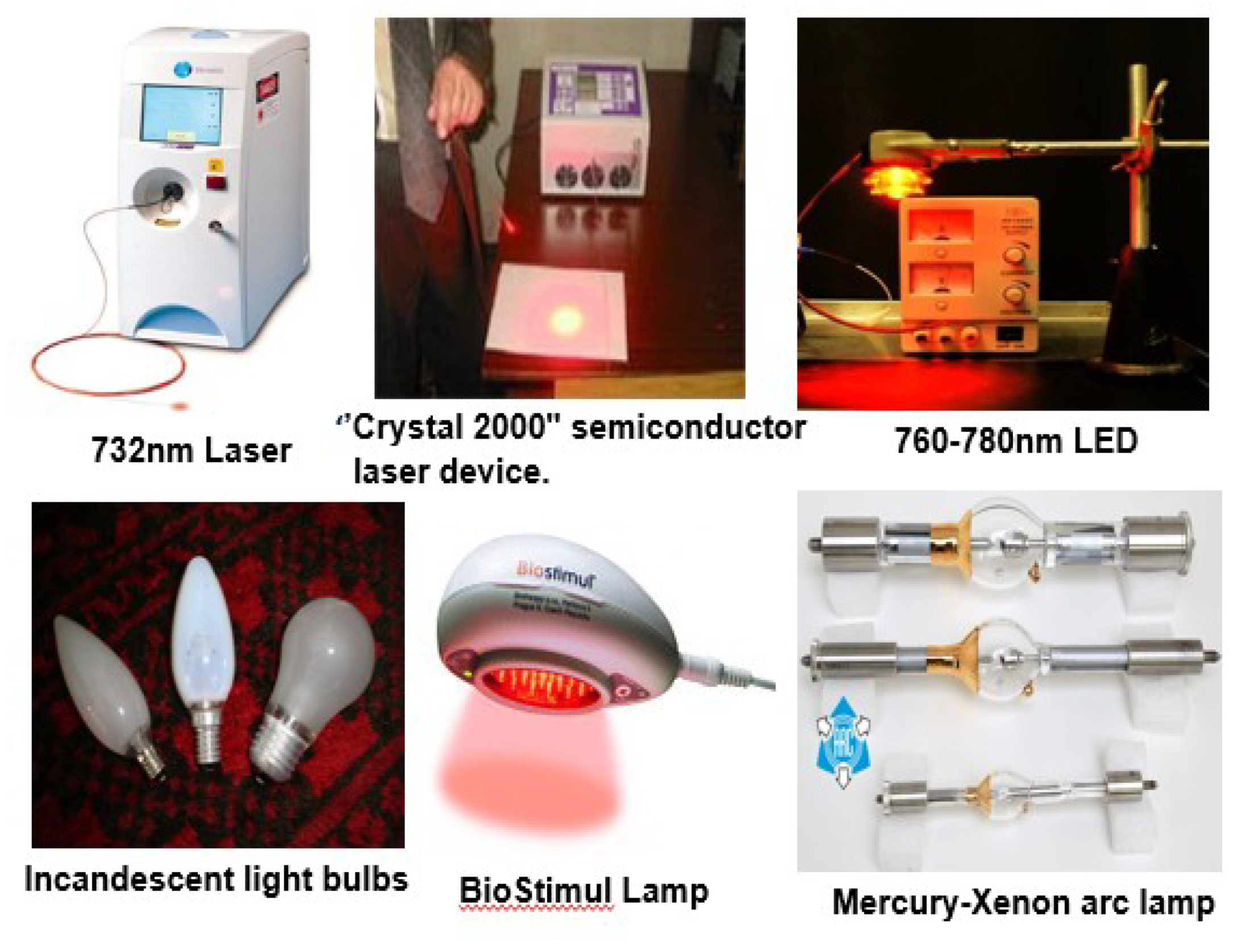
3. Choice of Light Source
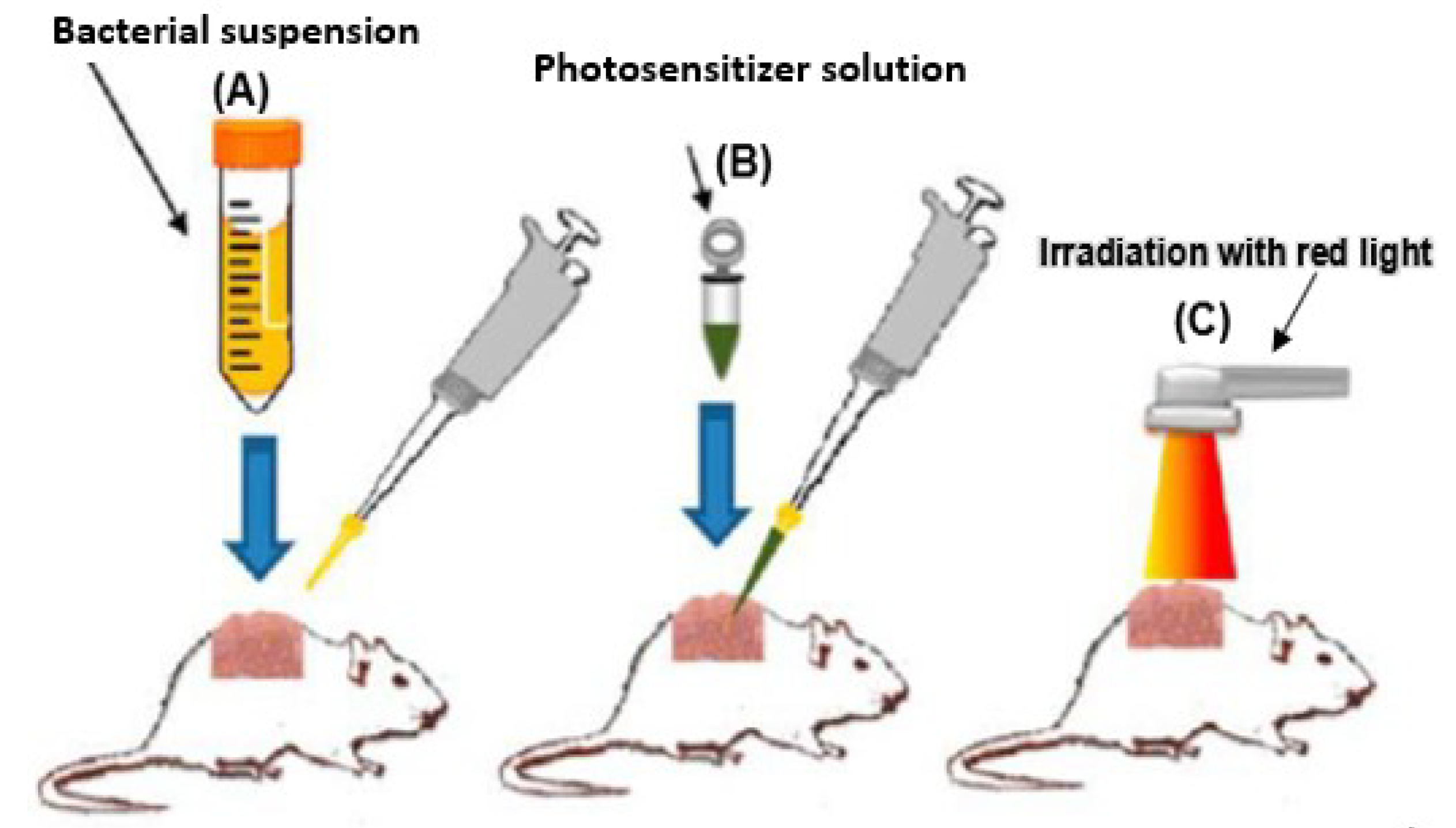
4. Antibacterial Photodynamic Effects of Porphyrins
5. Significance of aPDT
6. Clinical Applications of aPDT
6.1. Treatment of Wound Infections
6.2. Treatment of Acne
6.3. Periondontal Diseases
6.4. Treatment of Environmental Waters
7. Other Applications of Porphyrins
8. Side-Effects and Drawbacks of aPDT
9. Future Perspective and Directions of aPDT
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Almeida, A.; Cunha, A.; Faustino, M.A.F.; Tomé, A.C.; Neves, M.G.P.M.S. Porphyrins as Antimicrobial Photosensitizing Agents. In Photodynamic Inactivation of Microbial Pathogens: Medical and Environmental Applications; Hamblin, M.R., Jori, G., Eds.; RSC Publishing: Cambridge, UK, 2011; Volume 11, pp. 83–160. ISBN 978-1-84973-144-7. [Google Scholar]
- Maisch, T.; Hackbarth, S.; Regensburger, J.; Felgenträger, A.; Bäumler, W.; Landthaler, M.; Röder, B. Photodynamic inactivation of multi resistant bacteria (PIB)–a new approach to treat superficial infections in the 21st century. J. Dtsch. Dermatologischen Ges. 2011, 9, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Carrel, M.; Perencevich, E.N.; David, M.Z. USA 300 methicillin-resistant Staphylococcus aureus, United States, 2000–2013. Emerg Infect. Dis. 2015, 21, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Pollack, A. Rising Threat of Infections Unfazed by Antibiotics, New York Times. Available online: http://www.biocence.com/download/raging_antibiotic.pdf (accessed on 11 November 2018).
- Songca, S.P.; Oluwafemi, O.S. Photodynamic therapy: A new light for the developing world. Afr. J. Biotechnol. 2013, 12, 3590–3599. [Google Scholar] [CrossRef]
- Obiero, C.W.; Seale, A.C.; Berkley, J.A. Empiric Treatment of Neonatal Sepsis in Developing Countries. Paediatr. Infect. Dis. J. 2015, 34, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Whitelaw, A. Hospital Acquired Infections. Available online: http://m.news24.com/health24/Medical/Diseases/Hospital-Acquired-Infections-20120721 (accessed on 11 November 2018).
- Wise, R.J. The urgent need for new antibacterial agents. J. Antimicrob. Chemother. 2011, 66, 1939–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Tegos, G.P.; Hamblin, M.R. Disruptive innovations, new anti-infectives in the age of resistance. Curr. Opin. Pharmacol. 2013, 13, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial photodynamic therapy: Overview of a promising approach to fight antibiotic-resistant bacterial infections. J. Clin. Trans. Res. 2015, 1, 140–167. [Google Scholar] [CrossRef]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photo antimicrobials-are we afraid of the light. Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Garcez, A.S.; Núñez, S.C. Bacterial reduction in root canals using antimicrobial photodynamic therapy. In Lasers in Dentistry: Guide for Clinical Practice; de Freitas, P.M., Simões, A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 131–138. ISBN 978-1-118-27502-3. [Google Scholar]
- Chitsazi, M.T.; Shirmohammadi, A.; Pourabbas, R.; Abolfazli, N.; Farhoudi, I.; Azar, B.D.; Farhadi, F. Clinical and Microbiological Effects of Photodynamic Therapy Associated with Non-surgical Treatment in Aggressive Periodontitis. J. Dent. Res. Dent. Clin Dent. Prospect. 2014, 8, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.L.; Novaes, A.B., Jr.; Grisi, M.F.; Taba, M., Jr.; Souza, S.L.; Palioto, D.B.; de Oliveira, P.G.; Casati, M.Z.; Casarin, R.C.; Messora, M.R. Antimicrobial Photodynamic Therapy as an Adjunct to Non-Surgical Treatment of Aggressive Periodontitis: A Split-Mouth Randomized Controlled Trial. J. Periodontol. 2015, 86, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.G.; de Lima, M.A.; Okamoto, T.; Milanezi, L.A.; Júnior, E.C.G.; Fernandes, L.A.; de Almeida, J.M.; Theodoro, L.H. Effect of photodynamic therapy on the healing of cutaneous third-degree-burn: Histological study in rats. Lasers Med. Sci. 2010, 25, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Faustino, M.A.; Tomé, J.P.; Neves, M.G.; Tomé, A.C.; Cavaleiro, J.A.; Cunha, Â.; Gomes, N.C.; Almeida, A. Photodynamic antimicrobial chemotherapy in aquaculture: Photoinactivation studies of Vibrio fischeri. PLoS ONE 2011, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, Z.-Q.; Jin, H.; Sun, S.; Liu, T.-J.; Wang, X.; Fan, H.-J.; Hou, S.-K.; Ding, H. Photodynamic antimicrobial chemotherapy with the novel amino acid-porphyrin conjugate 4I: In vitro and in vivo studies. PLoS ONE 2017, 12, e0176529–e0176541. [Google Scholar] [CrossRef] [PubMed]
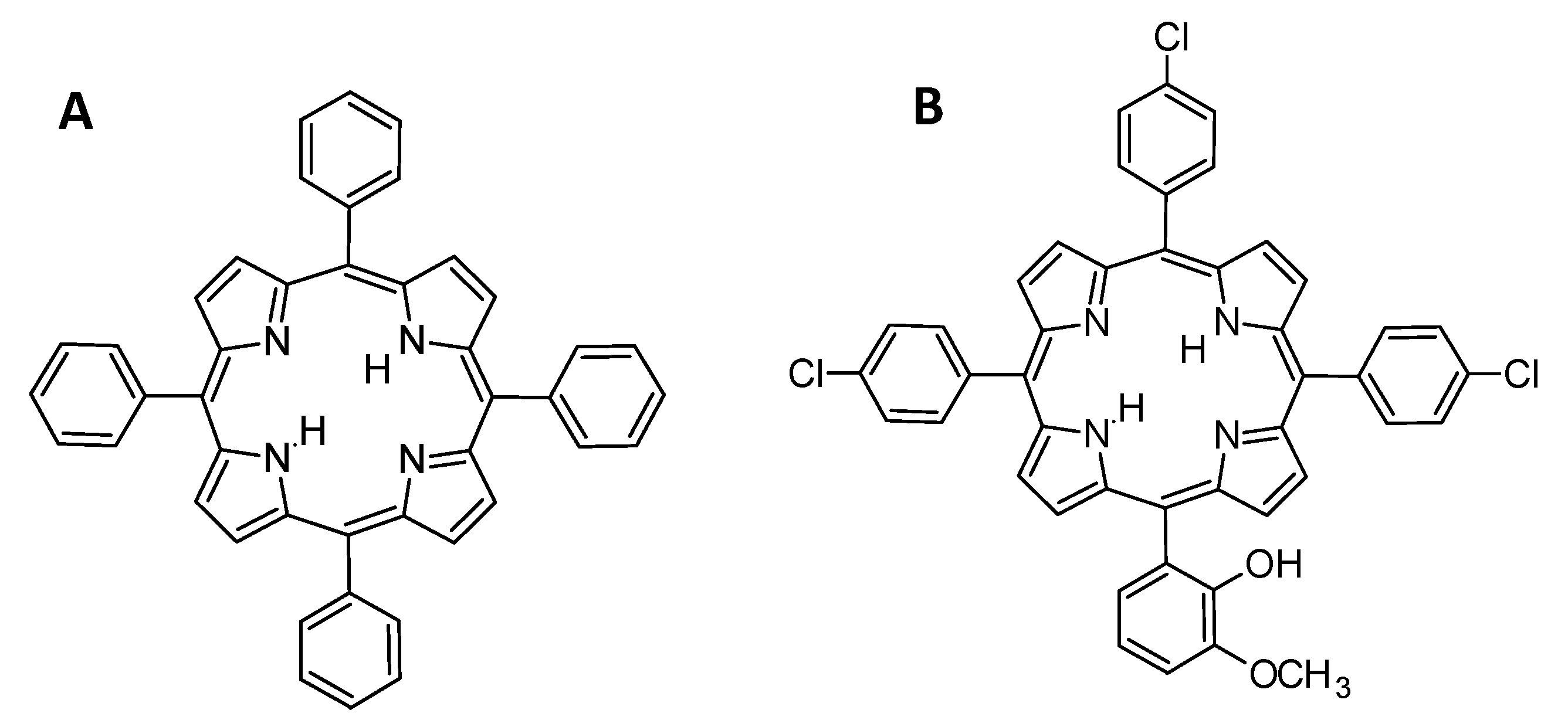
- Arredondo-Espinoza, E.; López-Cortina, S.; Balderas-Rentería, I. Synthesis and Photodynamic Activity of 5, 10, 15-Tris (p-chlorophenyl)-20-(2- hydroxy-3-methoxyphenyl)-21H, 23H-porphyrin. J. Mex. Chem. Soc. 2014, 58, 369–373. [Google Scholar]
- Cieplik, F.; Pummer, A.; Regensburger, J.; Hiller, K.A.; Späth, A.; Tabenski, L.; Buchalla, W.; Maisch, T. The impact of absorbed photons on antimicrobial photodynamic efficacy. Front. Microbiol. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Hashimoto, M.C.; Prates, R.A.; Kato, I.T.; Núñez, S.C.; Courrol, L.C.; Ribeiro, M.S. Antimicrobial photodynamic therapy on drug-resistant Pseudomonas aeruginosa-induced infection. An in vivo study. Photochem. Photobiol. 2012, 88, 590–595. [Google Scholar] [CrossRef]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef]
- Nitzan, Y.; Gutterman, M.; Malik, Z.; Ehrenberg, B. Inactivation of gram-negative bacteria by photosensitized porphyrin. Photochem. Photobiol. 1992, 55, 89–96. [Google Scholar] [CrossRef]
- Jori, G.; Fabris, C.; Soncin, M.; Ferro, S.; Coppellotti, O.; Dei, D.; Fantetti, L.; Chiti, G.; Roncucci, G. Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications. Lasers Surg. Med. 2006, 38, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Malatesti, N.; Munitic, I.; Jurak, I. Porphyrin-based cationic amphiphilic photosensitisers as potential anticancer, antimicrobial and immunosuppressive agents. Biophys. Rev. 2017, 9, 149–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calzavara-Pinton, P.G.; Szeimies, R.M.; Ortel, B. Photodynamic Therapy and Fluorescence Diagnosis in Dermatology; Elsevier: Amsterdam, The Netherlands, 2001; ISBN 9780080538846. [Google Scholar]
- MacRobert, A.J.; Bown, S.G.; Phillips, D. What are the ideal photoproperties for a sensitizer? Ciba Found. Symp. 1989, 14, 4–12. [Google Scholar]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynmic therapy. Part one -photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Omar, G.S.M. Killing of Organisms Responsible for Wound Infections Using a Light-Activated Antimicrobial Agent. Ph.D. Thesis, University College, London, UK, 2010. [Google Scholar]
- Mojzisova, H.; Bonneau, S.; Brault, D. Structural and physico-chemical determinants of the interactions of macrocyclic photosensitizers with cells. Eur. Biophys. J. 2007, 36, 943–953. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, I.J.; Dougherty, J. Basic principles of photodynamic therapy. J. Porphyr. Phthalocyanines 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Luksiene, Z. Photodynamic therapy: Mechanism of action and ways to improve the efficiency of treatment. Medicina 2003, 39, 1137–1150. [Google Scholar] [PubMed]
- Alves, E.; Faustino, M.A.; Tomé, J.P.; Neves, M.G.; Tomé, A.C.; Cavaleiro, J.A.; Cunha, Â.; Gomes, N.C.; Almeida, A. Nucleic acid changes during photodynamic inactivation of bacteria by cationic porphyrins. Bioorg. Med. Chem. 2013, 21, 4311–4318. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M.; Crossley, K.B. Photosensitising agents—Circumventing resistance and breaking down biofilms: A review. Int. Biodeterior. Biodegrad. 2004, 53, 119–126. [Google Scholar] [CrossRef]
- Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G.A. The role of oxygen in the visible-light inactivation of Staphylococcus aureus. J. Photochem. Photobiol. 2008, 92, 180–184. [Google Scholar] [CrossRef]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, A.; Tomé, J.P.C.; Almeida, A. An insight on bacterial cellular targets of photodynamic inactivation. Future Med. Chem. 2014, 6, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kwon, O.J.; Park, J.W. Inactivation of catalase and superoxide dismutase by singlet oxygen derived from photoactivated dye. Biochimie 2001, 83, 437–444. [Google Scholar] [CrossRef]
- Planas, O.; Boix-Garriga, E.; Rodríguez-Amigo, B.; Torra, J.; Bresolí-Obach, R.; Flors, C.; Viappiani, C.; Agut, M.; Ruiz-González, R.; Nonell, S. Chapter 9: Newest approaches to singlet oxygen photosensitisation in Biological Media. In Photochemistry; Fasani, E., Albini, A., Eds.; Royal Society of Chemistry: Cambridge, UK, 2015; Volume 42, pp. 233–278. ISBN 978-1-84973-956-6. [Google Scholar]
- Milgrom, L. The Colours of Life: An Introduction to the Chemistry of Porphyrins and Related Compounds; Oxford University Press: New York, NY, USA, 1997; pp. 66–99. ISBN1 0198553803. ISBN2 9780198553809. [Google Scholar]
- Dolphin, D. The Porphyrins, Volumes 1–7; Academic Press: New York, NY, USA, 1978; pp. 740–810. ISBN 0-12-220107-8. [Google Scholar]
- Wijesekera, T.P.; Dolphin, D. Some preparations and properties of porphyrins. In Methods in Porphyrin Photosensitization; Springer: Boston, MA, USA, 1985; pp. 229–266. ISBN 978-1-4612-9276-0. [Google Scholar]
- Geier, R. Available online: http://www.colgate.edu/facultysearch/facultydirectory/ggeier (accessed on 11 November 2018).
- Connor, A.E.O.; Gallagher, W.M.; Byrne, A.T. Porphyrin and Non-porphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef]
- Schuitmaker, J.J.; Baas, P.; van Leengoed, H.L.; van der Meulen, F.W.; Star, W.M.; van Zandwijk, N. Photodynamic therapy: A promising new modality for the treatment of cancer. J. Photochem. Photobiol. B Biol. 1996, 34, 3–12. [Google Scholar] [CrossRef]
- Mccarthy, J.R.; Bhaumik, J.; Merbouh, N.; Weissleder, R. High-yielding syntheses of hydrophilic conjugatable chlorins and bacteriochlorins. Org. Biomol. Chem. 2009, 7, 3430–3436. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, E.D.; Dolphin, D.; Brückner, C. Porphyrin-based photosensitizers for use in photodynamic therapy. Tetrahedron 1998, 54, 4151–4202. [Google Scholar] [CrossRef]
- Embleton, M.L.; Nair, S.P.; Heywood, W.; Menon, D.C.; Cookson, B.D.; Wilson, M. Development of a novel targeting system for lethal photosensitization of antibiotic-resistant strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 3690–3696. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, J.S. Synthesis of meso-substituted porphyrins. In The Porphyrin Handbook; Kadish, M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2000; ISBN 0-12-393200-9. [Google Scholar]
- Rothemund, P.; Menotti, A.R. Porphyrin Studies. IV. The Synthesis of α,β,γ,δ-Tetraphenylporphine. J. Am. Chem Soc. 1941, 63, 267–270. [Google Scholar] [CrossRef]
- Adler, A.D.; Longo, F.R.; Finarelli, J.D.; Goldmacher, J.; Assour, J.; Korsakoff, L. A simplified synthesis for meso-tetraphenylporphin. J. Org. Chem. 1967, 32, 476. [Google Scholar] [CrossRef]
- Boens, B.; Faugeras, P.A.; Vergnaud, J.; Lucas, R.; Teste, K.; Zerrouki, R. Iodine-catalyzed one-pot synthesis of unsymmetrical meso-substituted porphyrins. Tetrahedron 2010, 66, 1994–1996. [Google Scholar] [CrossRef]
- Kappe, C.O.; Stadler, A.; Dalinger, D. Microwaves in Organic and Medicinal Chemistry, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2012; ISBN1 3527331859. ISBN2 9783527331857. [Google Scholar]
- Pinto, S.M.; Henriques, C.A.; Tomé, V.A.; Vinagreiro, C.S.; Calvete, M.J.; Dąbrowski, J.M.; Piñeiro, M.; Arnaut, L.G.; Pereira, M.M. Synthesis of meso-substituted porphyrins using sustainable chemical processes. J. Porphyr. Phthalocyanines 2016, 20, 45–60. [Google Scholar] [CrossRef]
- Naik, R.; Joshi, P.; Kaiwar Deshpande, R.K. Facile synthesis of mesosubstituted dipyrromethanes and porphyrins using cation exchange resin. Tetrahedron 2003, 59, 2207–2213. [Google Scholar] [CrossRef]
- Nia, S.; Gong, X.; Drain, C.M.; Jurow, M.; Rizvi, W.; Qureshy, M.J. Solvent-free synthesis of meso-tetraarylporphyrins in air: Product, diversity and yield optimization. J. Porphyr. Phthalocyanines 2010, 14, 621–629. [Google Scholar] [CrossRef]
- Babu, M.M.; Amaravathi, M.L.; Giribabu, L.G.; Chandramouli, G. Synthesis of meso-substituted porphyrins in room temperature ionic liquid. J. Chem Res. Synop. 2008, 11, 666–668. [Google Scholar] [CrossRef]
- Mondal, D.; Bera, S. Porphyrins and phthalocyanines: Promising molecules for light-triggered antibacterial nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 1–14. [Google Scholar] [CrossRef]
- Lammer, A.D.; Cook, M.E.; Sessler, J.L. Synthesis and anti-cancer activities of a water-soluble gold (III) porphyrin. J. Porphyr. Phthalocyanines 2015, 19, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its Applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef]
- Nyman, E.S.; Hynninen, P.H. Research advances in the use of tetrapyrrolic photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B 2004, 73, 1–28. [Google Scholar] [CrossRef]
- Singh, S.; Aggarwal, A.; Bhupathiraju, N.D.K.; Arianna, G.; Tiwari, K.; Drain, C.M. Glycosylated porphyrins, phthalocyanines, and other porphyrinoids for diagnostics and therapeutics. Chem. Rev. 2015, 115, 10261–10306. [Google Scholar] [CrossRef]
- Stojiljkovic, I.; Evavold, B.D.; Kumar, V. Antimicrobial properties of porphyrins. Expert Opin. Invest. Drugs 2001, 10, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Ahmad, A.; Mehboob, R. Nosocomial infections and their control strategies. Asia-Pac. J. Trop. Biomed. 2015, 5, 509–514. [Google Scholar] [CrossRef] [Green Version]
- Bruckner, M.Z. Gram staining. Microbial Life Educational Resources. 2016. Available online: http://serc.carleton.edu/microbelife/research_methods/microscopy/gramstain.html (accessed on 11 November 2018).
- Banfi, S.; Caruso, E.; Buccafurni, L.; Battini, V.; Zazzaron, S.; Barbieri, P.; Orlandi, V. Antibacterial activity of tetraaryl-porphyrin photosensitizers: An in vitro study on Gram negative and Gram-positive bacteria. J. Photochem. Photobiol. B 2006, 85, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, C.S.; Karunakaran, S.C.; Paul, A.K.; Kussovski, V.; Mantareva, V.; Ramaiah, D.; Selvaraj, L.; Angelov, I.; Avramov, L.; Nandakumar, K.; et al. Antimicrobial photodynamic efficiency of novel cationic porphyrins towards periodontal gram- positive and Gram (−) pathogenic bacteria. Photochem. Photobiol. 2014, 90, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Bertoloni, G.; Rossi, F.; Valduga, G.; Jori, G.; Vanlier, J. Photosensitizing activity of water-soluble and lipid-soluble phthalocyanines on Escherichia coli. FEMS Microbiol. Lett. 1990, 71, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Malik, Z.; Ladan, H.; Nitzan, Y. Photodynamic inactivation of gram-negative bacteria—Problems and possible solutions. J. Photochem. Photobiol. B 1992, 14, 262–266. [Google Scholar] [CrossRef]
- Rovaldi, C.R.; Pievsky, A.; Sole, N.A.; Friden, P.M.; Rothstein, D.M.; Spacciapoli, P. Photoactive porphyrin derivative with broad-spectrum activity against oral pathogens in vitro. Antimicrob. Agents Chemother. 2000, 44, 3364–3367. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; O’Donnell, D.A.; Murthy, N.; Rajagopalan, K.; Michaud, N.; Sherwood, M.E.; Hasan, T. Polycationic photosensitizer conjugates: Effects of chain length and Gram classification on the photodynamic inactivation of bacteria. J. Antimicrob. Chemother. 2002, 49, 941–951. [Google Scholar] [CrossRef]
- Verma, S.; Sallum, U.W.; Athar, H.; Rosenblum, L.; Foley, J.W.; Hasan, T. Antimicrobial photodynamic efficacy of side-chain functionalized benzo[a]phenothiazinium dyes. Photochem. Photobiol. 2009, 85, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Grinhol, M.; Szramka, B.; Olender, K.A.; Graczyk, A. Bactericidal effect of photodynamic therapy against methicillin-resistant Staphylococcus aureus strain with the use of various porphyrin photosensitizers. Acta Biochim. Pol. 2007, 54, 665–670. [Google Scholar]
- Embleton, M.L.; Nair, S.P.; Cookson, B.D.; Wilson, M. Antibody-directed photodynamic therapy of methicillin-resistant Staphylococcus aureus. Microb. Drug Resist. 2004, 10, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Zahra, T.; Contag, C.H.; McManus, A.T.; Hasan, T. Optical monitoring and treatment of potentially lethal wound infections in vivo. J. Infect. Dis. 2003, 187, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Huang, Y.Y.; Hamblin, M.R. Photodynamic therapy for localized infections-state of the art. Photodiagn. Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.A.; Marley, K.A. Oxidative mechanisms of phototoxicity. In Environmental Oxidants; Nriagu, J.O., Simmons, M.S., Eds.; John Wiley & Sons: New York, NY, USA, 1994; pp. 269–318. ISBN1 0471579289. ISBN2 9780471579281. [Google Scholar]
- Gonzalez-Delgado, J.A.; Kennedy, P.J.; Ferreira, M.; Tome, J.P.C.; Samento, B. Use of Photosensitizers in Semisolid Formulations for Microbial Photodynamic Inactivation. J. Med. Chem. 2016, 59, 4428–4442. [Google Scholar] [CrossRef] [PubMed]
- Valduga, G.; Bertoloni, G.; Reddi, E.; Jori, G. Effect of extracellularly generated singlet oxygen on gram-positive and gram-negative bacteria. J. Photochem. Photobiol. B 1993, 21, 81–86. [Google Scholar] [CrossRef]
- Bhatti, M.; Nair, S.P.; Macrobert, A.J.; Henderson, B.; Shepherd, P.; Cridland, J.; Wilson, M. Identification of photolabile outer membrane proteins of Porphyromonas gingivalis. Curr. Microbiol. 2001, 43, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Ragàs, X.; He, X.; Agut, M.; Roxo-Rosa, M.; Gonsalves, A.R.; Serra, A.C.; Nonell, S. Singlet Oxygen in Antimicrobial Photodynamic Therapy: Photosensitizer-Dependent Production and Decay in E. coli. Molecules 2013, 18, 2712–2725. [Google Scholar] [CrossRef]
- Jori, G.; Roncucci, G. Photodynamic Therapy in Microbial Infections. Adv. Clin. Exper. Med. 2006, 15, 421–426. [Google Scholar]
- Salton, M.R.J. Structure and function of bacterial cell membranes. Ann. Rev. Microbiol. 1967, 21, 417–442. [Google Scholar] [CrossRef]
- Gábor, K.; Szocs, K.; Maillard, P.; Csik, G. Photobiological activity of exogenous and endogenous porphyrin derivatives in Escherichia coli and Enterococcus hirae cells. Radiat. Environ. Biophys. 2001, 40, 145–151. [Google Scholar] [CrossRef]
- Hoque, J.; Akkapeddi, P.; Yadav, V.; Manjunath, G.B.; Uppu, D.S.; Konai, M.M.; Yarlagadda, V.; Sanyal, K.; Haldar, J. Broad spectrum antibacterial and antifungal polymeric paint materials: Synthesis, structure–activity relationship, and membrane-active mode of action. ACS Appl. Mater. Interfaces. 2015, 7, 1804–1815. [Google Scholar] [CrossRef] [PubMed]
- Egyeki, M.; Turóczy, G.; Majer, Z.; Tóth, K.; Fekete, A.; Maillard, P.; Csık, G. Photosensitized inactivation of T7 phage as surrogate of non-enveloped DNA viruses: Efficiency and mechanism of action. Biochim. Biophys. Acta 2003, 1624, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Winckler, K.D. Special section: Focus on anti-microbial photodynamic therapy (PDT). J. Photochem. Photobiol. B 2007, 86, 43–44. [Google Scholar] [CrossRef] [PubMed]
- Niemz, N.H. Laser-tissue interactions. Fundamentals and Applications, 3rd ed.; Springer: New York, NY, USA, 2007; pp. 19–25. ISBN1 3540721916. ISBN2 9783540721918. [Google Scholar]
- Brancaleon, L.; Moseley, H. Laser and non-laser light sources for photodynamic therapy. Lasers Med. Sci. 2002, 17, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Calin, M.A.; Parasca, S.V. Light sources for photodynamic inactivation of bacteria. Lasers Med. Sci. 2009, 24, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Patterson, M.S. The physics, biophysics and technology of photodynamic therapy. Phys. Med. Biol. 2008, 53, R61–R109. [Google Scholar] [CrossRef] [PubMed]
- Mitton, D.; Ackroyd, R. A brief overview of photodynamic therapy in Europe. Photodiagn. Photodyn. Ther. 2008, 5, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Caminos, D.A.; Spesia, M.B.; Durantini, E.N. Photodynamic inactivation of Escherichia coli by novel meso-substituted porphyrins by 4-(3-N,N,N-trimethy-lammoniumpropoxy)phenyl and 4-(trifluoromethyl)phenyl groups. Photochem. Photobiol. Sci. 2006, 5, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Thakuri, P.S.; Joshi, R.; Basnet, S.; Pandey, S.; Taujale, S.D.; Mishra, N. Antibacterial photodynamic therapy on Staphylococcus aureus and Pseudomonas aeruginosa in-vitro. Nepal Med. Coll. J. 2011, 13, 281–284. [Google Scholar]
- Luksiene, Z. New approach to inactivation of harmful and pathogenic microorganisms by photosensitization. Food Technol. Biotechnol. 2005, 43, 411–418. [Google Scholar]
- Orenstein, A.; Klein, D.; Kopolovic, J.; Winkler, E.; Malik, Z.; Keller, N.; Nitzan, Y. The use of porphyrins for eradication of Staphylococcus aureus in burn wound infections. FEMS Immunol. Med. Microbiol. 1997, 19, 307–314. [Google Scholar] [CrossRef]
- Zoltan, T.; Vargas, F.; Rivas, C.; López, V.; Perez, J.; Biasutto, A. Synthesis, Photochemical and Photoinduced Antibacterial Activity Studies of meso-Tetra(pyren-1-yl)porphyrin and its Ni, Cu and Zn Complexes. Sci. Pharm. 2010, 78, 767–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavares, A.; Dias, S.R.; Carvalho, C.M.; Faustino, M.A.; Tomé, J.P.; Neves, M.G.; Tomé, A.C.; Cavaleiro, J.A.; Cunha, Â.; Gomes, N.C.; et al. Mechanisms of photoinactivation of Gram-negative recombinant bioluminescent bacteria by cationic porphyrins. Photochem. Photobiol. Sci. 2011, 10, 1659–1669. [Google Scholar] [CrossRef]
- Maish, T.; Bosl, C.; Szeimies, R.M.; Love, B.; Abels, C. Determination of the antimicrobial efficacy of a new porphyrin-based photosensitizer against MRSA ex vivo. Photochem. Photobiol. Sci. 2007, 6, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Taub, A.F. Photodynamic therapy: Other uses. Dermatol Clin. 2007, 25, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, Y.; Ashkenazi, H. Photoinactivation of Deinococcus radiodurans: An Unusual Gram-Positive Microorganism. Photochem. Photobiol. 1999, 69, 505–510. [Google Scholar] [CrossRef]
- Nitzan, Y.; Ashkenazi, H. Photoinactivation of Acinetobacter baumannii and Escherichia coli B by a Cationic Hydrophilic Porphyrin at Various Light Wavelengths. Curr. Microbiol. 2001, 42, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T.; Szeimies, R.M.; Lehn, N.; Abels, C. Antibacterial photodynamic therapy. A new treatment for bacterial skin diseases? Hautarzt 2005, 56, 1048–1055. [Google Scholar] [CrossRef]
- Hanakova, A.; Bogdanova, K.; Tomankova, K.; Pizova, K.; Malohlava, J.; Binder, S.; Bajgar, R.; Langova, K.; Kolar, M.; Mosinger, J.; et al. The application of antimicrobial photodynamic therapy on S. aureus and E. coli using porphyrin photosensitizers bound to cyclodextrin. Microbiol. Res. 2014, 16, 163–170. [Google Scholar] [CrossRef]
- Ruiz-González, R.; Montserrat, A.; Reddi, E.; Nonell, S. A Comparative Study on Two Cationic Porphycenes: Photophysical and Antimicrobial Photoinactivation Evaluation. Int. J. Mol. Sci. 2015, 16, 27072–27086. [Google Scholar] [CrossRef] [Green Version]
- Wikene, K.O.; Bruzell, E.; Tønnesen, H.H. Improved antibacterial phototoxicity of a neutral porphyrin in natural deep eutectic solvents. J. Photochem. Photobiol. B 2015, 148, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Fayyaza, F.; Rassab, M.; Rabbania, M. Microwave-assisted synthesis of 5,10,15,20-tetrakis(4-nitrophenyl)porphyrin and zinc derivative and study of their bacterial photoinactivation. Iran. Chem. Commum. 2015, 4, 175–185. [Google Scholar]
- Lambrechts, S.A.G.; Demidova, T.N.; Aalders, M.C.G.; Hasan, T.; Hamblin, M.R. Photodynamic therapy for Staphylococcus aureus infected burn wounds in mice. Photochem. Photobiol. Sci. 2005, 4, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Dia, T.; Tegos, G.P.; Zhiyentayev, T.; Mylonakis, E.; Hamblin, M.R. Photodynamic therapy for methicillin-resistant Staphylococcus aureus infection in a mouse skin abrasion model. Lasers Surg. Med. 2010, 42, 38–44. [Google Scholar] [CrossRef]
- Tavares, A.; Carvalho, C.; Faustino, M.A.; Neves, M.G.; Tomé, J.P.; Tomé, A.C.; Cavaleiro, J.A.; Cunha, Â.; Gomes, N.; Alves, E.; et al. Antimicrobial photodynamic therapy: Study of bacterial recovery viability and potential development of resistance after treatment. Mar. Drugs 2010, 8, 91–105. [Google Scholar] [CrossRef]
- Costa, L.; Tomé, J.P.; Neves, M.G.; Tomé, A.C.; Cavaleiro, J.A.; Faustino, M.A.; Cunha, Â.; Gomes, N.C.; Almeida, A. Evaluation of resistance development and viability recovery by T4-like bacteriophages after repeated cycles of aPDT. Antivir Res. 2011, 91, 278–282. [Google Scholar] [CrossRef]
- Wardlaw, J.L.; Sullivan, T.J.; Lux, C.N.; Austin, F.W. Photodynamic therapy against common bacteria causing wound and skin infections. Vet. J. 2011, 192, 374–377. [Google Scholar] [CrossRef]
- O’Riordan, K.; Akilov, O.E.; Hasan, T. The potential for photodynamic therapy in the treatment of localized infections. Photodiagn. Photodyn. Ther. 2005, 2, 247–262. [Google Scholar] [CrossRef]
- Songca, S.P. Photodynamic Therapy for the Developing World. In Professorial Inaugural Lecture; Walter Sisulu University: Mthatha, South Africa, 2010; p. 22. [Google Scholar]
- Babilas, P.; Schreml, S.; Landthaler, M.; Szeimies, R.M. Photodynamic therapy in dermatology: State-of-the-art. Photodermatol. Photoimmunol. Photomed. 2010, 26, 118–132. [Google Scholar] [CrossRef]
- Gold, M.H. Photodynamic Therapy in Dermatology; Springer: New York, NY, USA, 2011; p. 181. ISBN1 1441912983. ISBN2 9781441912985. [Google Scholar]
- Wan, M.T.; Lin, J.Y. Current evidence and applications of photodynamic therapy in dermatology. Clin. Cosmet. Investig. Dermatol. 2014, 7, 145–163. [Google Scholar] [CrossRef]
- Gursoy, H.; Ozcakir-Tomruk, C.; Tanalp, J.; Yilmaz, S. Photodynamic therapy in dentistry: A literature review. Clin. Oral Investig. 2013, 17, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Aoki, A.; Romanos, G.; Schwarz, F.; Miron, R.J.; Cosgarea, R. Is Photodynamic Therapy an Effective Treatment for Periodontal and Peri-Implant Infections? Dent. Clin. North Am. 2015, 59, 831–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.Y.; Dai, T.; Hamblin, M.R. Photodynamic therapy for infections: Clinical applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperandio, F.F.; Huang, Y.Y.; Hamblin, M.R. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Patent. Anti-infect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef]
- Robson, M.C.; Mannari, R.J.; Smith, P.D.; Payne, W.G. Maintenance of wound bacterial balance. Am. J. Surg. 1999, 178, 399–402. [Google Scholar] [CrossRef]
- Grinholc, M.; Rapacka-Zdonczyk, A.; Rybak, B.; Szabados, F.; Bielawski, K.P. Multi- resistant strains are as susceptible to photodynamic inactivation as their naïve counterparts: Protoporphyrin IX-mediated photoinactivation reveals differences between methicillin-resistant and methicillin-sensitive Staphylococcus aureus strains. Photomed Laser Surg. 2014, 32, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Fayyaz, F.; Rahimi, R.; Rassa, M.; Yaghoobi, R.H. Photodynamic Antimicrobial Chemotherapy, A Pathway for Photo-Inactivation of Bacteria by Porphyrin Compounds. In Proceedings of the 1st International Electronic Conference on Molecular Science: Cell Signaling, Survival and Growth. Sciforum Electronic Conference Series 1, Basel, Switzerland, 15–22 October 2015; p. b003. [Google Scholar] [CrossRef]
- Barra, F.; Roscetto, E.; Soriano, A.A.; Vollaro, A.; Postiglione, I.; Pierantoni, G.M.; Palumbo, G.; Catania, M.R. Photodynamic and Antibiotic Therapy in Combination to Fight Biofilms and Resistant Surface Bacterial Infections. Int. J. Mol. Sci. 2015, 16, 20417–20430. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeu, M.; Rocha, S.; Cunha, A.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Effect of Photodynamic Therapy on the Virulence Factors of Staphylococcus aureus. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bojar, R.A.; Holland, K.T. Acne and Propionibacterium acne. Clin. Dermatol. 2004, 22, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Farrar, M.D.; Ingham, E. Acne: Inflammation. Clin. Dermatol. 2004, 22, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Meffert, H.; Gaunitz, K.; Gutewort, T.; Amlong, U.J. Aknetherapie mit sichtbarem Licht. Verkürzung der Bestrahlungszeit durch Verwendung eines Hochdruckstrahlers vom Blaulichttyp. Dermatol. Monatsschr. 1990, 176, 597–603. [Google Scholar] [PubMed]
- Kawada, A.; Aragane, Y.; Kameyama, H.; Sangen, Y.; Tezuka, T. Acne phototherapy with a high-intensity, enhanced, narrow-band, blue light source: An open study and in vitro investigation. J. Dermatol. Sci. 2002, 30, 129–135. [Google Scholar] [CrossRef]
- Ashkenazi, H.; Malik, Z.; Harth, Y.; Nitzan, Y. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol. Med. Microbiol. 2003, 35, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.; Loewenstein, M.; Borelli, C.; Jacob, K.; Vogeser, M.; Burgdorf, W.H.C.; Plewig, G. Induction of a chemoattractive proinflammatory cytokine response after stimulation of keratinocytes with Propionibacterium acnes and coproporphyrin III. Br. J. Dermatol. 2005, 153, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M.; Smalley, H.; Flint, C. The use of photosensitisers in acne treatment. J. Photochem. Photobiol. B 2011, 105, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M. Susceptibility of oral bacteria biofilms to antimicrobial agents. J. Med. Microbiol. 1996, 44, 79–87. [Google Scholar] [CrossRef]
- Garcia, V.G.; Júnior, G.; Clementino, E.; Fernandes, L.A.; Bosco, A.F.; Nagata, H.; José, M.; Casatti, C.A.; Ervolino, E.; Theodoro, L.H. Adjunctive Antimicrobial Photodynamic Treatment of Experimentally Induced Periodontitis in Rats with Ovariectomy. J. Periodontol. 2013, 84, 556–565. [Google Scholar] [CrossRef]
- Takasaki, A.A.; Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Wang, C.Y.; Koshy, G.; Romanos, G.; Ishikawa, I.; Izumi, Y. Application of antimicrobial photodynamic therapy in periodontal and peri-implant diseases. Periodontol. 2000 2009, 5, 1–32. [Google Scholar] [CrossRef]
- Cieplik, F.; Pummer, A.; Leibl, C.; Regensburger, J.; Schmalz, G.; Buchalla, W.; Hiller, K.A.; Maisch, T. Photodynamic inactivation of root canal bacteria by light activation through human dental hard and simulated surrounding tissue. Front. Microbiol. 2016, 7, 929. [Google Scholar] [CrossRef]
- Jemli, M.; Alouini, Z.; Sabbahi, S.; Gueddari, M. Destruction of fecal bacteria in wastewater by three photosensitizers. J. Environ. Monit. 2002, 4, 511–516. [Google Scholar] [CrossRef]
- Bonnett, R.; Krysteva, M.A.; Lalov, I.G.; Artarsky, S.V. Water disinfection using photosensitizers immobilized on chitosan. Water Res. 2006, 40, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.; Gomes, A.T.; Fernandes, S.C.; Prata, A.C.; Almeida, M.A.; Cunha, M.A.; Tomé, J.P.; Faustino, M.A.; Neves, M.G.; Tomé, A.C.; et al. Photoinactivation of bacteria in wastewater by porphyrins: Bacterial β-galactosidase activity and leucine-uptake as methods to monitor the process. J. Photochem. Photobiol. B 2007, 88, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Costa, L.; Carvalho, C.M.; Tomé, J.P.; Faustino, M.A.; Neves, M.G.; Tomé, A.C.; Cavaleiro, J.A.; Cunha, Â.; Almeida, A. Charge effect on the photoinactivation of Gram-negative and Gram-positive bacteria by cationic meso-substituted porphyrins. BMC Microbiol. 2009, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Alves, E.; Carvalho, C.M.; Tomé, J.P.; Faustino, M.A.; Neves, M.G.; Tomé, A.C.; Cavaleiro, J.A.; Cunha, Â.; Almeida, A. Sewage bacteriophage photoinactivation by cationic porphyrins: A study of charge effect. Photochem. Photobiol. Sci. 2008, 7, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Almeida, A.; Carvalho, C.M.B.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â. Porphyrin derivatives as photosensitizers for the inactivation of Bacillus cereus endospores. J. Appl. Microbiol. 2009, 106, 1986–1995. [Google Scholar] [CrossRef]
- Alves, E.; Carvalho, C.M.; Tomé, J.P.; Faustino, M.A.; Neves, M.G.; Tomé, A.C.; Cavaleiro, J.A.; Cunha, Â.; Mendo, S.; Almeida, A. Photodynamic inactivation of recombinant bioluminescent Escherichia coli by cationic porphyrins under artificial and solar irradiation. J. Ind. Microbiol. Biotechnol. 2008, 35, 1447–1454. [Google Scholar] [CrossRef]
- Almeida, J.; Tomé, J.P.; Neves, M.G.; Tomé, A.C.; Cavaleiro, J.A.; Cunha, Â.; Costa, L.; Faustino, M.A.; Almeida, A. Photodynamic inactivation of multidrug-resistant bacteria in hospital wastewaters: Influence of residual antibiotics. Photochem. Photobiol. Sci. 2014, 13, 626–633. [Google Scholar] [CrossRef]
- Carvalho, C.M.; Alves, E.; Costa, L.; Tomé, J.P.; Faustino, M.A.; Neves, M.G.; Tomé, A.C.; Cavaleiro, J.A.; Almeida, A.; Cunha, A.; et al. Functional cationic nanomagnet-porphyrin hybrids for the photoinactivation of microorganisms. ACS Nano 2010, 4, 7133–7140. [Google Scholar] [CrossRef]
- Alves, E.; Rodrigues, J.M.; Faustino, M.A.; Neves, M.G.; Cavaleiro, J.A.; Lin, Z.; Cunha, Â.; Nadais, M.H.; Tomé, J.P.; Almeida, A. A new insight on nanomagneteporphyrin hybrids for photodynamic inactivation of microorganisms. Dyes Pigment. 2014, 110, 80–88. [Google Scholar] [CrossRef]
- McCormick, B.P.P.; Pansa, M.F.; Sanabria, L.N.M.; Carvalho, C.M.; Faustino, M.A.F.; Neves, M.G.P.; Cavaleiro, J.A.; Vittar, N.B.R.; Rivarola, V.A. Cationic porphyrin derivatives for application in photodynamic therapy of cancer. Laser Phys. 2014, 24, 045603. [Google Scholar] [CrossRef]
- Chen, J.J.; Gao, L.J.; Liu, T.J. Photodynamic therapy with a novel porphyrin-based photosensitizer against human gastric cancer. Oncol. Lett. 2016, 11, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Broughton, L.J.; Giuntini, F.; Savoie, H.; Bryden, F.; Boyle, R.W.; Maraveyas, A.; Madden, L.A. Duramycin-porphyrin conjugates for targeting of tumour cells using photodynamic therapy. J. Photochem. Photobiol. B 2016, 163, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Neri, C.R.; Prado, A.P.J.M.; Ribeiro, A.O.; Serra, O.V.A.; Iamamoto, Y. Determination of the photodynamic activity of porphyrins: Potential photosensitizers for treatment of age-related macular degeneration. Mater. Sci. 2002, 20, 69–75. [Google Scholar]
- Das, R.A.; Romano, A.; Chiosi, F.; Menzione, M.; Rinaldi, M. Combined treatment modalities for age related macular degeneration. Curr. Drug Targets 2011, 12, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Higashino, T.; Imahori, H. Porphyrins as excellent dyes for dye-sensitized solar cells: Recent developments and insights. Dalton Trans. 2015, 44, 448–463. [Google Scholar] [CrossRef] [PubMed]
- Krishna, J.V.; Mrinalini, M.; Prasanthkumar, S.; Giribabu, L. Recent Advances on Porphyrin Dyes for Dye-Sensitized Solar Cells. In Dye-Sensitized Solar Cells; Academic Press: New York, NY, USA, 2019; pp. 231–284. [Google Scholar] [CrossRef]
- Sun, D.; Tham, F.S.; Reed, C.A.; Chaker, L.; Burgess, M.; Boyd, P.D. Porphyrin- fullerene host- guest chemistry. J. Am. Chem. Soc. 2000, 122, 10704–10705. [Google Scholar] [CrossRef]
- Vinodh, M.; Alipour, F.H.; Mohamod, A.A.; Al-Azemi, T.F. Molecular assemblies of porphyrins and macrocyclic receptors: Recent developments in their synthesis and applications. Molecules 2012, 17, 11763–11799. [Google Scholar] [CrossRef]
- Ali, M.F.; Perzanowski, H.; Bukhari, A.; Al-Haji, A.A. Nickel and vanadyl porphyrins in Saudi Arabian crude oils. Energy Fuels 1993, 7, 179–184. [Google Scholar] [CrossRef]
- Sundararaman, P.; Raedeke, L.D. Vanadyl porphyrins in exploration: Maturity indicators for source rocks and oils. Appl. Geochem. 1993, 8, 245–254. [Google Scholar] [CrossRef]
- Reddy, V.N.; Rekha Rani, K.; Chandana, G.; Sehrawat, S. Photodynamic therapy. Indian J. Dent. Adv. 2009, 1, 46–50. [Google Scholar]
- Huang, Z.; Xu, H.; Meyers, A.D.; Musani, A.I.; Wang, L.; Tagg, R.; Barqawi, A.B.; Chen, Y.K. Photodynamic therapy for treatment of solid tumors—potential and technical challenges. Technol. Cancer Res. Treat. 2008, 7, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Fowley, C.; Nomikou, N.; McHale, A.P.; McCaughan, B.; Callan, J.F. Extending the tissue penetration capability of conventional photosensitisers: A carbon quantum dot–protoporphyrin IX conjugate for use in two-photon excited photodynamic therapy. Chem. Commun. 2013, 49, 8934–8936. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, L.B.; Boyle, R.W. Photodynamic therapy: Novel third-generation photosensitizers one step closer? Br. J. Pharmacol. 2008, 154, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Fei, B.; Halig, L.V.; Qin, X.; Hu, Z.; Xu, H.; Wang, Y.A.; Chen, Z.; Kim, S.; Shin, D.M.; et al. Targeted Iron-Oxide Nanoparticle for Photodynamic Therapy and Imaging of Head and Neck Cancer. ACS Nano 2014, 8, 6620–6632. [Google Scholar] [CrossRef] [PubMed]
- Mathews, M.S.; Angell-Petersen, E.; Sanchez, R.; Sun, C.H.; Vo, V.; Hirschberg, H.; Madsen, S.J. The effects of ultra-low fluence rate single and repetitive photodynamic therapy on glioma spheroids. Lasers Surg. Med. 2009, 41, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Craig, R.A.; McCoy, C.P.; Gorman, S.P.; Jones, D.S. Photosensitisers—The progression from photodynamic therapy to anti-infective surfaces. Expert Opin. Drug Deliv. 2015, 12, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Moghissi, K. PDT: The plight. Photodiagn. Photodyn. Ther. 2007, 4, 223–277. [Google Scholar] [CrossRef]













© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amos-Tautua, B.M.; Songca, S.P.; Oluwafemi, O.S. Application of Porphyrins in Antibacterial Photodynamic Therapy. Molecules 2019, 24, 2456. https://doi.org/10.3390/molecules24132456
Amos-Tautua BM, Songca SP, Oluwafemi OS. Application of Porphyrins in Antibacterial Photodynamic Therapy. Molecules. 2019; 24(13):2456. https://doi.org/10.3390/molecules24132456
Chicago/Turabian StyleAmos-Tautua, Bamidele M., Sandile P. Songca, and Oluwatobi S. Oluwafemi. 2019. "Application of Porphyrins in Antibacterial Photodynamic Therapy" Molecules 24, no. 13: 2456. https://doi.org/10.3390/molecules24132456





