The Cytotoxic Properties of Some Tricyclic 1,3-Dithiolium Flavonoids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
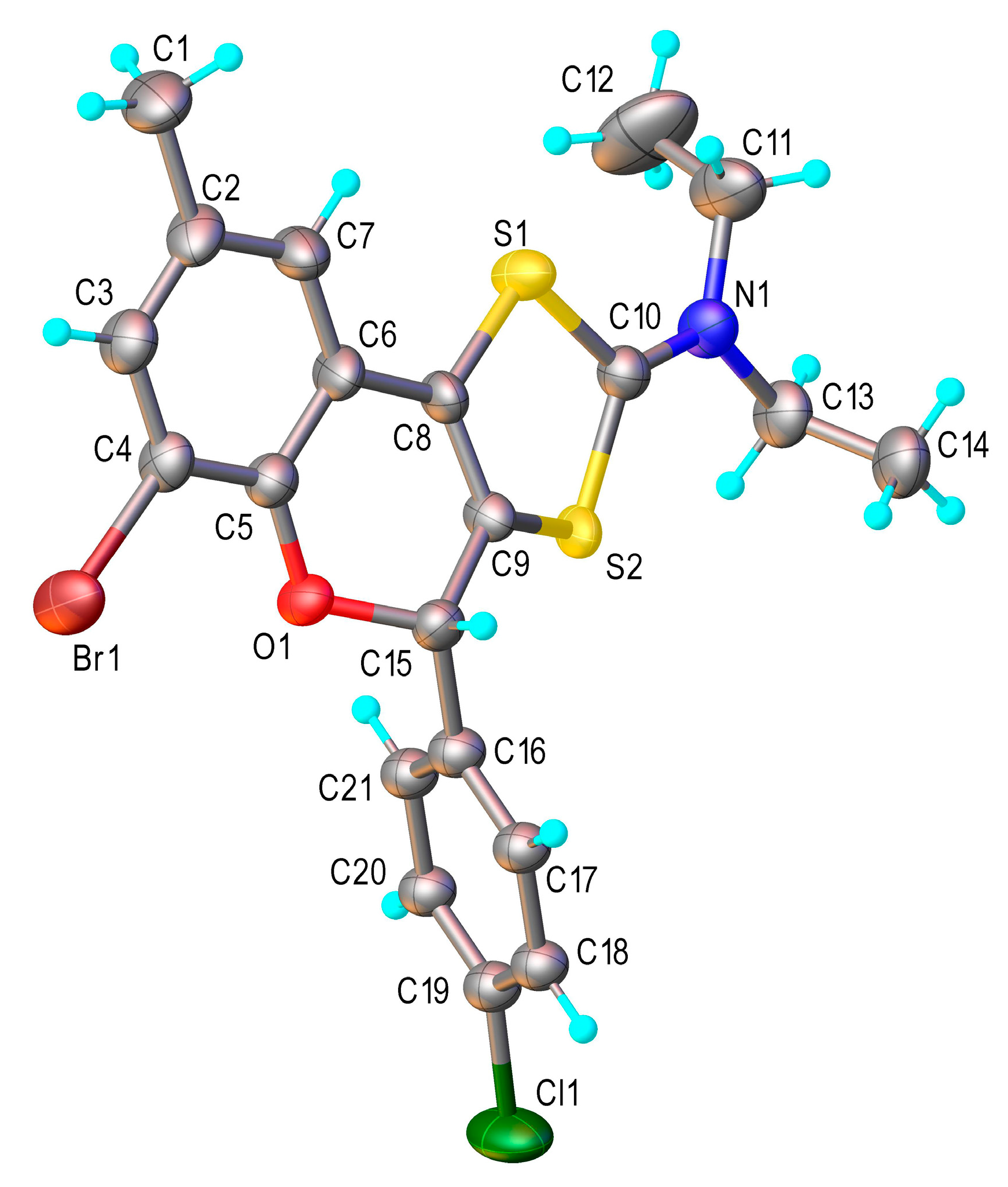
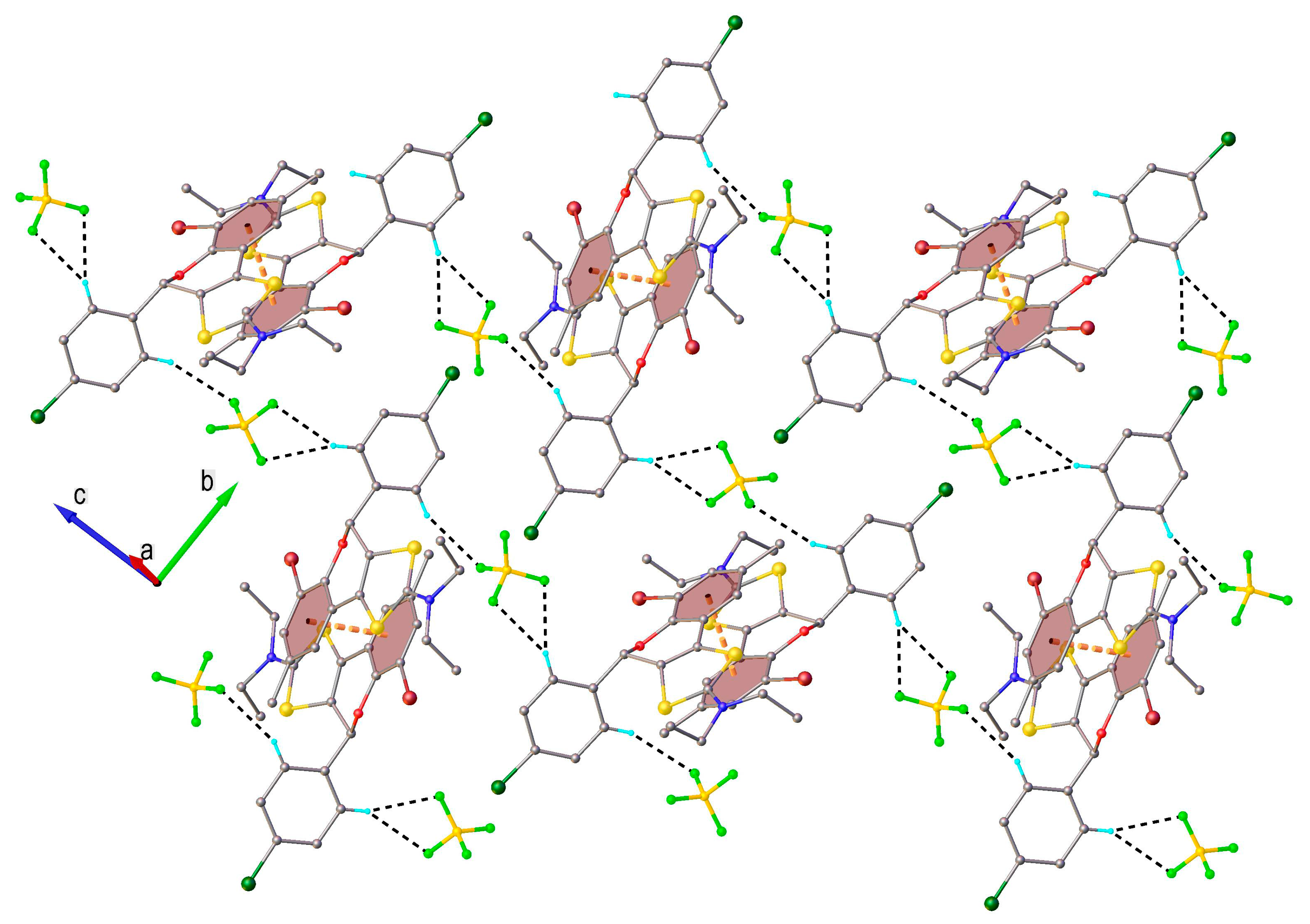
2.2. X-Ray Crystallography
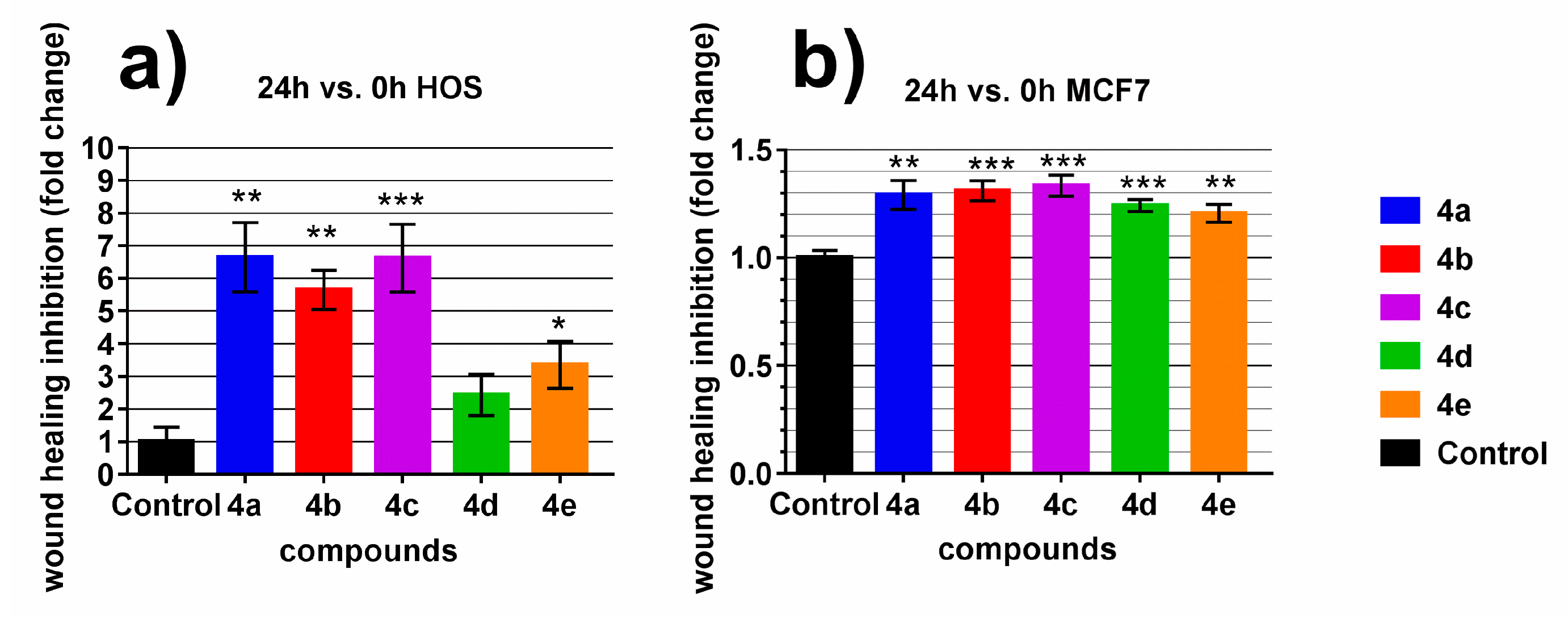
2.3. Wound Scratch Assay
2.4. MTS Assay
2.5. Live/Dead Staining
3. Experimental
3.1. Chemistry
3.1.1. General Procedure for 8-bromo-2-(4-chlorophenyl)-6-methyl-4-oxochroman-3-yl N,N-diethyldithiocarbamate (3b)
3.1.2. 6-Bromo-2-(4-chlorophenyl)-8-methyl-4-oxochroman-3-yl N,N-diethyldithiocarbamate (3c)
3.1.3. 6,8-Dibromo-2-(4-chlorophenyl)-7-methyl-4-oxochroman-3-yl N,N-diethyldithiocarbamate (3d)
3.1.4. 2-(4-Chlorophenyl)-6,8-diiodo-4-oxochroman-3-yl N,N-diethyldithiocarbamate (3e)
3.1.5. General Procedure for 2-N,N-diethylamino-6-bromo-4-(4-chlorophenyl)-8-methyl-4H-1,3-dithiol [4,5-c]chromen-2-ylium tetrafluoroborate (4b)
3.1.6. 2-N,N-Diethylamino-8-bromo-4-(4-chlorophenyl)-6-methyl-4H-1,3-dithiol[4,5-c]chromen-2-ylium tetrafluoroborate (4c)
3.1.7. 2-N,N-Diethylamino-6,8-dibromo-4-(4-chlorophenyl)-7-methyl-4H-1,3-dithiol[4,5-c]chromen-2-ylium tetrafluoroborate (4d)
3.1.8. 2-N,N-Diethylamino-4-(4-chlorophenyl)-6,8-diiodo-4H-1,3-dithiol[4,5-c]chromen-2-ylium tetrafluoroborate (4e)
3.2. X-Ray Structure Determination
Crystal Data for Compound 4b
4. Materials and Methods
4.1. General Information
4.2. Cell Culture
4.3. Wound Scratch Assay
4.4. MTS Assay
4.5. Live/Dead Staining
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- French, G.L. The continuing crisis in antibiotic resistance. Int. J. Antimicrob. Agents 2010, 36, S3–S7. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for emerging pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Fan, W.; Qian, P.; Zhang, D.; Wei, Y.; Chen, H.; Li, X. Apigenin Restricts FMDV Infection and Inhibits Viral IRES Driven Translational Activity. Viruses 2015, 7, 1613–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edziri, H.; Mastouri, M.; Mahjoub, M.A.; Mighri, Z.; Mahjoub, A.; Verschaeve, L. Antibacterial, Antifungal and Cytotoxic Activities of Two Flavonoids from Retama raetam Flowers. Molecules 2012, 17, 7284–7293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angeloni, C.; Hrelia, S. Quercetin Reduces Inflammatory Responses in LPS-Stimulated Cardiomyoblasts. Oxid. Med. Cell Longev. 2012, 8, 837104. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.D.; Shiao, C.K.; Lee, Y.C.; Shih, Y.W. Apigenin, a dietary flavonoid, inhibits proliferation of human bladder cancer T-24 cells via blocking cell cycle progression and inducing apoptosis. Cancer Cell Int. 2015, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Ruel, G.; Couillard, C. Evidences of the cardioprotective potential of fruits: The case of cranberries. Mol. Nutr. Food Res. 2007, 51, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Arsalandeh, F.; Khodagholi, F.; Ahmadian, S.; Foolad, F.; Kamsorkh, F.M.; Farimani, M.M.; Shaerzadeh, F. Prevention of recognition memory loss and moderation of mitochondrial dynamic tendency toward fusion by flavone derivatives in Aβ-injected rats: A comparison between two flavonoids with different polarity. Nutr. Neurosci. 2019, 22, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.L.; Yang, M.; Huang, L.; Li, J.L. Antibacterial and antioxidant flavonoid derivatives from the fruits of Metaplexis japonica. Food Chem. 2019, 289, 308–312. [Google Scholar] [CrossRef]
- Park, K.D.; Cho, S.J. Synthesis and antimicrobial activities of 3-O-alkyl analogues of (+)-catechin: Improvement of stability and proposed action mechanism. Eur. J. Med. Chem. 2010, 45, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Q.; Shi, L.; Li, Q.S.; Liu, P.G.; Luo, Y.; Zhao, J.; Zhu, H.L. Synthesis of C(7) modified chrysin derivatives designing to inhibit β-ketoacyl-acyl carrier protein synthase III (FabH) as antibiotics. Bioorg. Med. Chem. 2009, 17, 6264–6269. [Google Scholar] [CrossRef] [PubMed]
- Sarbu, L.G.; Bahrin, L.G.; Babii, C.; Stefan, M.; Birsa, M.L. Synthetic flavonoids with antimicrobial activity: A review. J. Appl. Microbiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Bahrin, L.G.; Apostu, M.O.; Birsa, L.M.; Stefan, M. The antibacterial properties of sulfur containing flavonoids. Bioorg. Med. Chem. Lett. 2014, 24, 2315–2318. [Google Scholar] [CrossRef] [PubMed]
- Babii, C.; Bahrin, L.G.; Neagu, A.N.; Gostin, I.; Mihasan, M.; Birsa, L.M.; Stefan, M. Antibacterial activity and proposed action mechanism of a new class of synthetic tricyclic flavonoids. J. Appl. Microbiol. 2016, 120, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Bahrin, L.G.; Sarbu, L.G.; Hopf, H.; Jones, P.G.; Babii, C.; Stefan, M.; Birsa, M.L. The influence of halogen substituents on the biological properties of sulfur-containing flavonoids. Bioorg. Med. Chem. 2016, 24, 3166–3173. [Google Scholar] [CrossRef] [PubMed]
- Bahrin, L.G.; Hopf, H.; Jones, P.G.; Sarbu, L.G.; Babii, C.; Mihai, A.C.; Stefan, M.; Birsa, M.L. Antibacterial structure–activity relationship studies of several tricyclic sulfur-containing flavonoids. Beilstein J. Org. Chem. 2016, 12, 1065–1071. [Google Scholar] [CrossRef]
- Babii, C.; Mihalache, G.; Bahrin, L.G.; Neagu, A.N.; Gostin, I.; Mihai, C.T.; Sarbu, L.G.; Birsa, L.M.; Stefan, M. A novel synthetic flavonoid with potent antibacterial properties: In vitro activity and proposed mode of action. PLoS ONE 2018, 13, e0194898. [Google Scholar] [CrossRef]
- Bahrin, L.G.; Jones, P.G.; Hopf, H. Tricyclic flavonoids with 1, 3-dithiolium substructure. Beilstein J. Org. Chem. 2012, 8, 1999–2003. [Google Scholar] [CrossRef]
- Birsa, M.L. Synthesis of some new substituted flavanones and related 4-chromanones by a novel synthetic method. Synth. Commun. 2002, 32, 115–118. [Google Scholar] [CrossRef]
- CCDC 1919638 Contains the Supplementary Crystallographic Data for Compound 4b; The Cambridge Crystallographic Data Centre: Cambridge, UK; Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 30 May 2019).
- Goanta, M.; Ciobanu, A.S.; Birsa, A.; Asaftei, I.V.; Birsa, M.L. Synthesis of 4-(3-Bromo-2-Hydroxy-5-Methylphenyl)-2-Dialkylamino-1, 3-Dithiolium Chlorides. Acta Chem. Iasi 2009, 17, 35–48. [Google Scholar]
- Birsa, M.L. Dithiolium derivatives. I. An. St. Univ. “Al. I. Cuza” Iasi 1998, 6, 57–64. [Google Scholar]
- Birsa, M.L. Dithiolium derivatives. II. An. St. Univ. “Al. I. Cuza” Iasi 1999, 7, 341–347. [Google Scholar]
- Sarbu, L.G.; Birsa, M.L. Synthesis of Iodine Containing Mesoionic 2-(1, 3-Dithiolium) phenolates. Acta Chem. Iasi 2011, 19, 125–135. [Google Scholar]
- CrysAlis RED; Version 1.171.36.32; Oxford Diffraction Ltd.: Abingdon, UK, 2003.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar]
- Gebäck, T.; Schulz, M.M.P.; Koumoutsakos, P.; Detmar, M. A novel and simple software tool for automated analysis of monolayer wound healing assays. Biotechniques 2008, 46, 265–274. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Br1–C4 | 1.895(5) | C8–C9 | 1.351(10) |
| S1–C8 | 1.730(5) | C10–S2 | 1.738(6) |
| S1–C10 | 1.719(5) | C11–C12 | 1.501(11) |
| O1–C5 | 1.376(6) | C13–C14 | 1.510(8) |
| O1–C15 | 1.443(7) | Cl1–C19 | 1.723(4) |
| O1–C15X | 1.443(10) | C9–C15 | 1.529(9) |
| N1–C10 | 1.301(6) | C15–C16 | 1.509(7) |
| N1–C11 | 1.478(8) | C17–C16 | 1.39 |
| N1–C13 | 1.485(7) | C17–C18 | 1.39 |
| C1–C2 | 1.518(8) | C16–C21 | 1.39 |
| C2–C3 | 1.384(8) | C21–C20 | 1.39 |
| C2–C7 | 1.388(7) | C20–C19 | 1.39 |
| C3–C4 | 1.380(8) | C19–C18 | 1.39 |
| C4–C5 | 1.377(7) | F1–B1 | 1.356(9) |
| C5–C6 | 1.410(7) | F2–B1 | 1.304(9) |
| C6–C7 | 1.375(8) | F3–B1 | 1.369(8) |
| C6–C8 | 1.457(7) | F4–B1 | 1.348(8) |
| C10–S1–C8 | 95.0(2) | N1–C10–S1 | 122.0(4) |
| C5–O1–C15 | 118.2(4) | N1–C10–S2 | 121.6(4) |
| C10–N1–C11 | 120.3(5) | N1–C11–C12 | 112.1(7) |
| C10–N1–C13 | 121.4(5) | N1–C13–C14 | 112.1(5) |
| C11–N1–C13 | 118.3(4) | O1–C15–C9 | 107.2(6) |
| C3–C2–C1 | 122.3(5) | O1–C15–C16 | 111.2(5) |
| C3–C2–C7 | 117.8(5) | C16–C15–C9 | 112.0(6) |
| C7–C2–C1 | 119.9(6) | C16–C17–C18 | 120 |
| C4–C3–C2 | 121.1(5) | C17–C16–C15 | 119.5(4) |
| C3–C4–Br1 | 118.7(4) | C17–C16–C21 | 120 |
| C5–C4–Br1 | 119.9(4) | C21–C16–C15 | 120.5(4) |
| C5–C4–C3 | 121.4(5) | C20–C21–C16 | 120 |
| O1–C5–C4 | 120.9(5) | C21–C20–C19 | 120 |
| O1–C5–C6 | 121.2(4) | C20–C19–Cl1 | 120.7(3) |
| C4–C5–C6 | 117.9(5) | C18–C19–Cl1 | 119.2(3) |
| C5–C6–C8 | 115.5(5) | C18–C19–C20 | 120 |
| C7–C6–C5 | 120.2(5) | C19–C18–C17 | 120 |
| C7–C6–C8 | 124.2(5) | F1–B1–F3 | 107.5(7) |
| C6–C7–C2 | 121.6(5) | F2–B1–F1 | 104.3(8) |
| C6–C8–S1 | 121.5(4) | F2–B1–F3 | 109.7(6) |
| C9–C8–S1 | 117.9(4) | F2–B1–F4 | 111.9(7) |
| C9–C8–C6 | 120.4(5) | F4–B1–F1 | 107.1(7) |
| S1–C10–S2 | 116.4(3) | F4–B1–F3 | 115.6(6) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarbu, L.G.; Shova, S.; Peptanariu, D.; Sandu, I.A.; Birsa, L.M.; Bahrin, L.G. The Cytotoxic Properties of Some Tricyclic 1,3-Dithiolium Flavonoids. Molecules 2019, 24, 2459. https://doi.org/10.3390/molecules24132459
Sarbu LG, Shova S, Peptanariu D, Sandu IA, Birsa LM, Bahrin LG. The Cytotoxic Properties of Some Tricyclic 1,3-Dithiolium Flavonoids. Molecules. 2019; 24(13):2459. https://doi.org/10.3390/molecules24132459
Chicago/Turabian StyleSarbu, Laura G., Sergiu Shova, Dragos Peptanariu, Isabela A. Sandu, Lucian M. Birsa, and Lucian G. Bahrin. 2019. "The Cytotoxic Properties of Some Tricyclic 1,3-Dithiolium Flavonoids" Molecules 24, no. 13: 2459. https://doi.org/10.3390/molecules24132459





