Apoptosis Induction Pathway in Human Colorectal Cancer Cell Line SW480 Exposed to Cereal Phenolic Extracts
Abstract
:1. Introduction
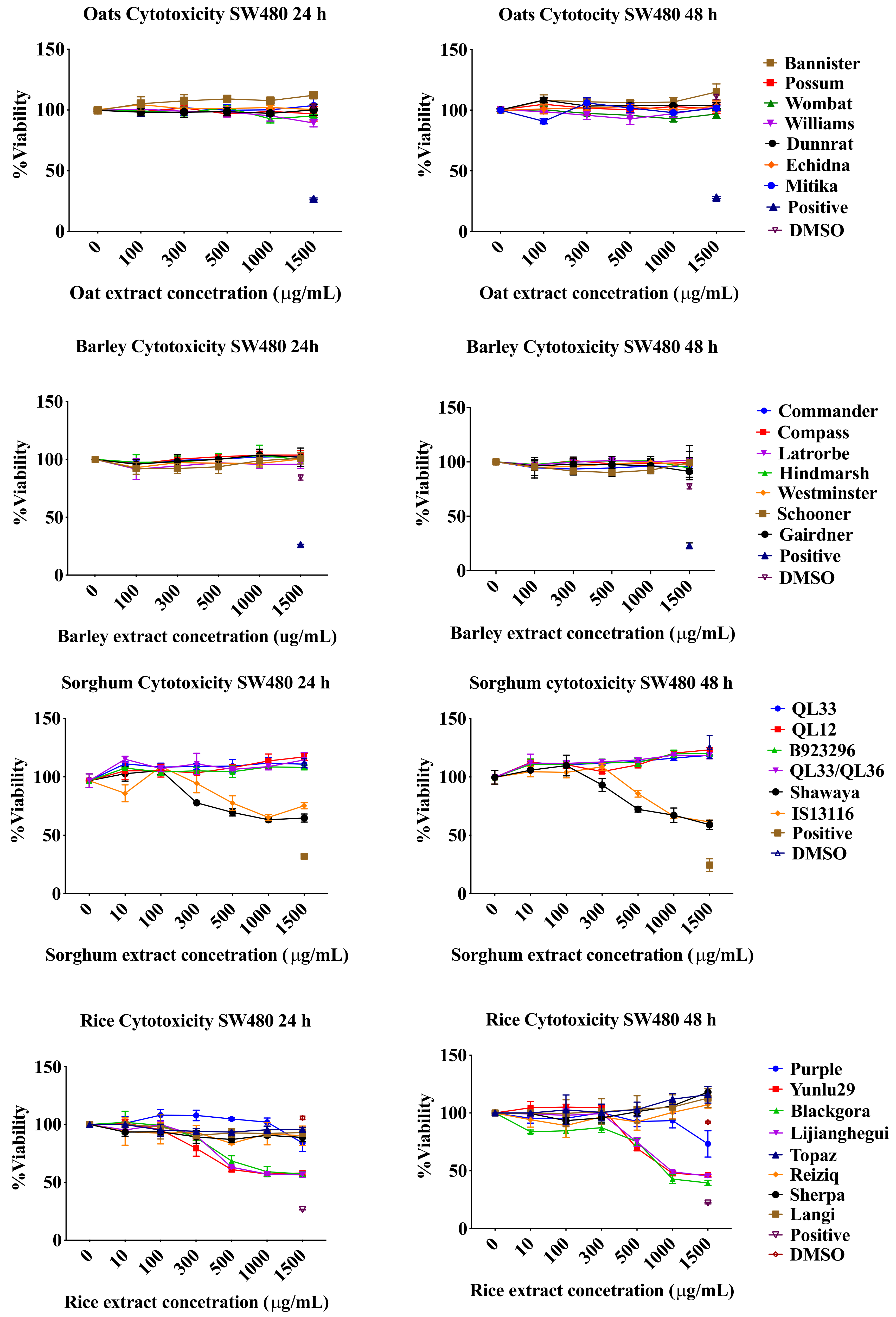
2. Results
2.1. Resazurin Assay
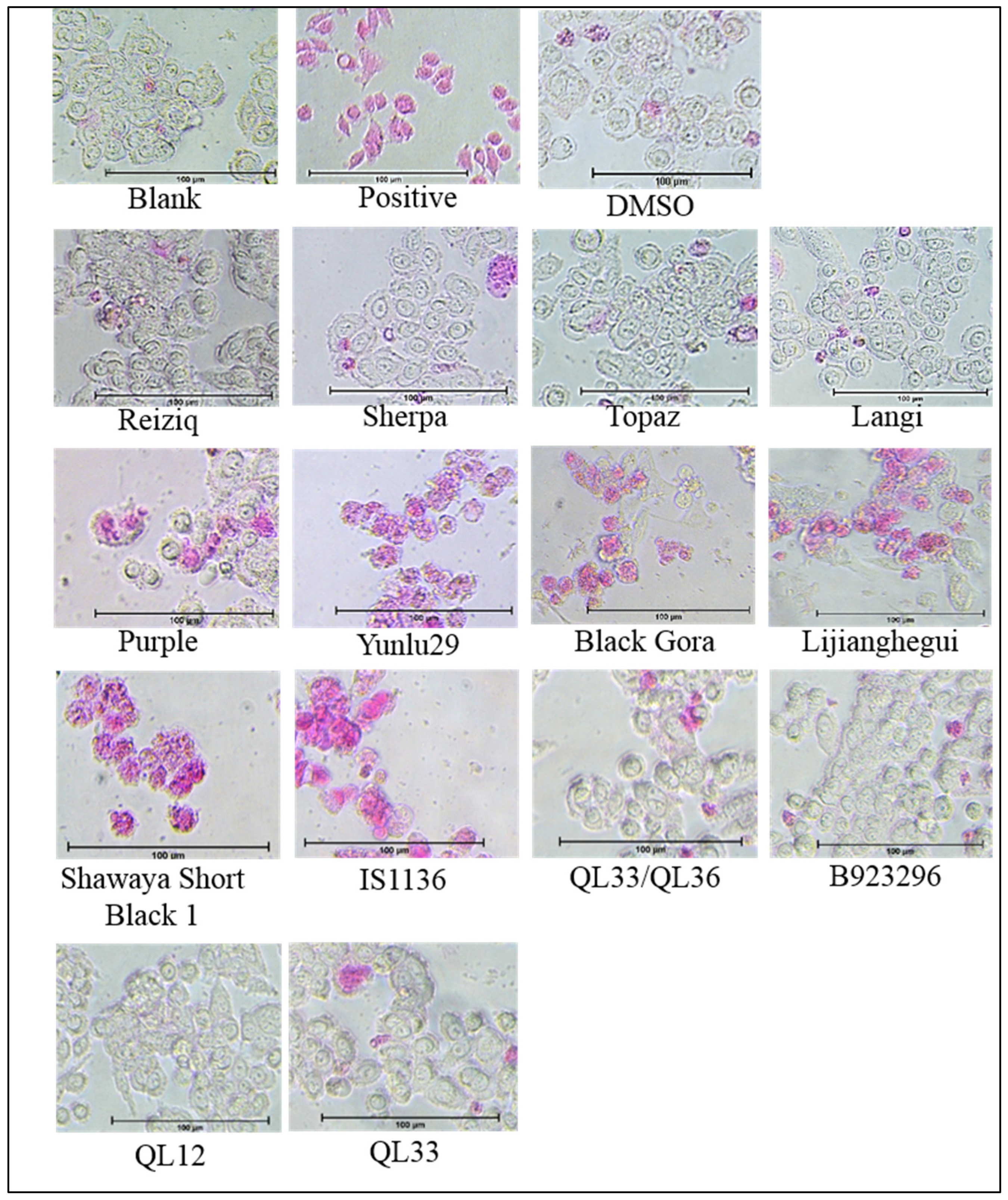
2.2. Apoptosis Detection and Morphology
2.3. Annexin Analysis
2.4. Caspase and p53 Detection Assays
3. Discussion
3.1. Anti-Proliferative Effect of Cereal Phenolic Extracts
3.2. Mechanism of Apoptotic Effect
4. Materials and Methods
4.1. Materials
4.2. Preparation of Extracts
4.3. Cell Culture
4.4. Resazurin Cytotoxicity Assay
4.5. APOPercentage™ Assays
4.6. Muse Annexin Assay
4.7. Muse Multiple Caspase Assay
4.8. Muse Caspases 3/7 Assay
4.9. p53 Expression in SW480 Cells
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Health Organisation. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available online: https://www.iarc.fr/news-events/latest-world-cancer-statistics-globocan-2012-estimated-cancer-incidence-mortality-and-prevalence-worldwide-in-2012/ (accessed on 20 August 2018).
- WHO. Cancer. Available online: http://www.who.int/mediacentre/factsheets/fs297/en (accessed on 11 April 2017).
- Hui, C.; Bin, Y.; Xiaoping, Y.; Long, Y.; Chunye, C.; Mantian, M.; Wenhua, L. Anticancer activities of an anthocyanin-rich extract from black rice against breast cancer cells in vitro and in vivo. Nutr. Cancer 2010, 62, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Wongjaikam, S.; Summart, R.; Chewonarin, T. Apoptosis induction in colon cancer cell lines and alteration of aberrant crypt foci in rat colon by purple rice (Oryza sativa L. var. glutinosa) extracts. Nutr. Cancer 2014, 66, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Masisi, K.; Beta, T.; Moghadasian, M.H. Antioxidant properties of diverse cereal grains: A review on in vitro and in vivo studies. Food Chem. 2016, 196, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Malaguti, M.; Dinelli, G.; Leoncini, E.; Bregola, V.; Bosi, S.; Cicero, A.F.G.; Hrelia, S. Bioactive Peptides in Cereals and Legumes: Agronomical, Biochemical and Clinical Aspects. Int. J. Mol. Sci. 2014, 15, 21120–21135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awika, J.M.; Yang, L.Y.; Browning, J.D.; Faraj, A. Comparative antioxidant, antiproliferative and phase II enzyme inducing potential of sorghum (Sorghum bicolor) varieties. LWT Food Sci. Technol. 2009, 42, 1041–1046. [Google Scholar] [CrossRef]
- Sousa, A.; Araujo, P.; Azevedo, J.; Cruz, L.; Fernandes, I.; Mateus, N.; de Freitas, V. Antioxidant and antiproliferative properties of 3-deoxyanthocyanidins. Food Chem. 2016, 192, 142–148. [Google Scholar] [CrossRef]
- Wu, G.; Johnson, K.S.; Bornman, J.F.; Bennett, S.J.; Clarke, M.W.; Singh, V.; Fang, Z. Growth temperature and genotype both play important roles in sorghum grain phenolic composition. Sci. Rep. 2016, 1–10. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Wang, R.; Luo, X.; Li, Y.; Li, J.; Li, Y.; Chen, Z. Phenolic contents, cellular antioxidant activity and antiproliferative capacity of different varieties of oats. Food Chem. 2018, 239, 260–267. [Google Scholar] [CrossRef]
- Chen, C.Y.O.; Milbury, P.E.; Collins, F.W.; Blumberg, J.B. Avenanthramides are bioavailable and have antioxidant activity in humans after acute consumption of an enriched mixture from oats. J. Nutr. 2007, 137, 1375–1382. [Google Scholar] [CrossRef]
- Guo, W.; Nie, L.; Wu, D.; Wise, M.L.; Collins, F.W.; Meydani, S.N.; Meydani, M. Avenanthramides inhibit proliferation of human colon cancer cell lines in vitro. Nutr. Cancer 2010, 62, 1007–1016. [Google Scholar] [CrossRef]
- Bai, L.; Wang, S. Targeting apoptosis pathways for new cancer therapeutics. Annu. Rev. Med. 2014, 65, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.H. The Role of p53 in Cell Cycle Regulation. Pathol. Res. Pract. 1996, 192, 669–675. [Google Scholar] [CrossRef]
- Fridman, J.S.; Lowe, S.W. Control of apoptosis by p53. Oncogene 2003, 22, 9030. [Google Scholar] [CrossRef] [PubMed]
- Menga, V.; Fares, C.; Troccoli, A.; Cattivelli, L.; Baiano, A. Effects of genotype, location and baking on the phenolic content and some antioxidant properties of cereal species. Int. J. Food Sci. Tech. 2010, 45, 7–16. [Google Scholar] [CrossRef]
- Wu, L.; Huang, Z.H.; Qin, P.Y.; Yao, Y.; Meng, X.J.; Zou, J.Q.; Zhu, K.; Ren, G.X. Chemical Characterization of a Procyanidin-Rich Extract from Sorghum Bran and Its Effect on Oxidative Stress and Tumor Inhibition in Vivo. J. Agr. Food Chem. 2011, 59, 8609–8615. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Santhakumar, A.B.; Chinkwo, K.A.; Blanchard, C.L. Q-TOF LC/MS identification and UHPLC-Online ABTS antioxidant activity guided mapping of barley polyphenols. Food Chem. 2018, 266, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Callcott, E.T.; Santhakumar, A.B.; Chinkwo, K.A.; Vanniasinkam, T.; Luo, J.; Blanchard, C. Profiling polyphenol composition and antioxidant activity in Australian-grown rice using UHPLC-Online ABTS system. J. Cereal Sci. 2018. [Google Scholar] [CrossRef]
- Madhujith, T.; Shahidi, F. Antioxidative and antiproliferative properties of selected barley (Hordeum vulgarae L.) cultivars and their potential for inhibition of low-density lipoprotein (LDL) cholesterol oxidation. J. Agric. Food Chem. 2007, 55, 5018–5024. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, T.; Fu, X.; Abbasi, A.M.; Zheng, B.; Liu, R.H. Phenolics content, antioxidant and antiproliferative activities of dehulled highland barley (Hordeum vulgare L.). J. Funct. Foods 2015, 19, 439–450. [Google Scholar] [CrossRef]
- Choi, J.; Jiang, X.J.; Jeong, J.B.; Lee, S.H. Anticancer activity of protocatechualdehyde in human breast cancer cells. J. Med. Food 2014, 17, 842–848. [Google Scholar] [CrossRef]
- Jeong, J.B.; Lee, S.-H. Anti-cancer activity of protocatechualdehyde through HDAC2-mediated downregulation of cyclin D1 in human colorectal cancer cells. FASEB J. 2013, 27, lb578. [Google Scholar] [CrossRef]
- Scarpa, E.S.; Antonini, E.; Palma, F.; Mari, M.; Ninfali, P. Antiproliferative activity of vitexin-2-O-xyloside and avenanthramides on CaCo-2 and HepG2 cancer cells occurs through apoptosis induction and reduction of pro-survival mechanisms. Eur. J. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.W. Oat phenolics—Avenanthramides, novel substituted n-cinnamoylanthranilate alkaloids from oat groats and hulls. J. Agr. Food Chem. 1989, 37, 60–66. [Google Scholar] [CrossRef]
- Banjerdpongchai, R.; Wudtiwai, B.; Sringarm, K. Cytotoxic and apoptotic-inducing effects of purple rice extracts and chemotherapeutic drugs on human cancer cell lines. Asian Pac. J. Cancer Prev. 2014, 14, 6541–6548. [Google Scholar] [CrossRef] [PubMed]
- Chatthongpisut, R.; Schwartz, S.J.; Yongsawatdigul, J. Antioxidant activities and antiproliferative activity of Thai purple rice cooked by various methods on human colon cancer cells. Food Chem. 2015, 188, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Srisala, S.; Chunhabundit, R.; Kongkachuichai, R.; Jittorntrum, B.; Visetpanit, Y. Effects of bran extracts from Thai molecular breeding rices on growth and apoptosis in human promyelocytic leukemia cells. Thai J. Toxicol. 2009, 24, 81–91. [Google Scholar]
- Forster, G.M.; Raina, K.; Kumar, A.; Kumar, S.; Agarwal, R.; Chen, M.H.; Bauer, J.E.; McClung, A.M.; Ryan, E.P. Rice varietal differences in bioactive bran components for inhibition of colorectal cancer cell growth. Food Chem. 2013, 141, 1545–1552. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Browning, J.D.; Awika, J.M. Sorghum 3-Deoxyanthocyanins possess strong phase II enzyme inducer activity and cancer cell growth inhibition properties. J. Agr. Food Chem. 2009, 57, 1797–1804. [Google Scholar] [CrossRef]
- Suganyadevi, P.; Saravanakumar, K.M.; Mohandas, S. The antiproliferative activity of 3-deoxyanthocyanins extracted from red sorghum (Sorghum bicolor) bran through P53-dependent and Bcl-2 gene expression in breast cancer cell line. Life Sci. 2013, 92, 379–382. [Google Scholar] [CrossRef]
- Rao, S.; Santhakumar, A.B.; Chinkwo, K.A.; Vanniasinkam, T.; Luo, J.; Blanchard, C.L. Chemopreventive Potential of Cereal Polyphenols. Nutr. Cancer 2018, 1–15. [Google Scholar] [CrossRef]
- Devi, P.S.; Kumar, M.S.; Das, S.M. Evaluation of antiproliferative activity of red sorghum bran anthocyanin on a human breast cancer cell line (mcf-7). Int. J. Breast Cancer 2011, 2011, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Darvin, P.; Lim, E.J.; Joung, Y.H.; Hong, D.Y.; Park, E.U.; Park, S.H.; Choi, S.K.; Moon, E.S.; Cho, B.W.; et al. Hwanggeumchal sorghum induces cell cycle arrest, and suppresses tumor growth and metastasis through Jak2/STAT pathways in breast cancer xenografts. PloS ONE 2012, 7, e40531. [Google Scholar] [CrossRef] [PubMed]
- Awika, J.M.; Rooney, L.W.; Waniska, R.D. Properties of 3-Deoxyanthocyanins from Sorghum. J. Agr. Food Chem. 2004, 52, 4388–4394. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Santhakumar, A.B.; Chinkwo, K.A.; Wu, G.; Johnson, S.K.; Blanchard, C.L. Characterization of phenolic compounds and antioxidant activity in sorghum grains. J. Cereal Sci. 2018, 84, 103–111. [Google Scholar] [CrossRef]
- Kögel, D.P.J. Caspase-Independent Cell Death Mechanisms. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA; pp. 2000–2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK6197/ (accessed on 20 August 2018).
- Kong, C.K.L.; Lam, W.S.; Chiu, L.C.M.; Ooi, V.E.C.; Sun, S.S.M.; Wong, Y.S. A rice bran polyphenol, cycloartenyl ferulate, elicits apoptosis in human colorectal adenocarcinoma SW480 and sensitizes metastatic SW620 cells to TRAIL-induced apoptosis. Biochem. Pharm. 2009, 77, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, V.; Ebrahimi, F.; Islam, F.; Vider, J.; Qallandar, O.B.; Pillai, S.; Lu, C.-T.; Lam, A.K.-Y. Tumour suppressor properties of miR-15a and its regulatory effects on BCL2 and SOX2 proteins in colorectal carcinomas. Exp. Cell Res. 2018, 370, 245–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.; Santhakumar, A.B.; Chinkwo, K.A.; Blanchard, C. Identification of phenolic compounds with antioxidant activity in oats using UHPLC-online ABTS and LC QTOF. Cereal Chem. 2019. submitted for publication. [Google Scholar]
- Wu, G.C.; Bornman, J.E.; Bennett, S.J.; Clarke, M.W.; Fang, Z.X.; Johnson, S.K. Individual polyphenolic profiles and antioxidant activity in sorghum grains are influenced by very low and high solar UV radiation and genotype. J. Cereal Sci. 2017, 77, 17–23. [Google Scholar] [CrossRef]
- Ataollahi, F.; Pramanik, S.; Moradi, A.; Dalilottojari, A.; Pingguan-Murphy, B.; Wan Abas, W.A.; Abu Osman, N.A. Endothelial cell responses in terms of adhesion, proliferation, and morphology to stiffness of polydimethylsiloxane elastomer substrates. J. Biomed. Mater. Res. A 2015, 103, 2203–2213. [Google Scholar] [CrossRef]
- Callcott, E.; Santhakumar, A.B.; Strappe, P.; Luo, J.; Blanchard, C.L. Polyphenols from Australian-grown pigmented red and purple rice inhibit adipocyte differentiation. J. Cereal Sci. 2018, 81, 140–146. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, S.; Chinkwo, K.; Santhakumar, A.; Johnson, S.; Blanchard, C. Apoptosis Induction Pathway in Human Colorectal Cancer Cell Line SW480 Exposed to Cereal Phenolic Extracts. Molecules 2019, 24, 2465. https://doi.org/10.3390/molecules24132465
Rao S, Chinkwo K, Santhakumar A, Johnson S, Blanchard C. Apoptosis Induction Pathway in Human Colorectal Cancer Cell Line SW480 Exposed to Cereal Phenolic Extracts. Molecules. 2019; 24(13):2465. https://doi.org/10.3390/molecules24132465
Chicago/Turabian StyleRao, Shiwangni, Kenneth Chinkwo, Abishek Santhakumar, Stuart Johnson, and Christopher Blanchard. 2019. "Apoptosis Induction Pathway in Human Colorectal Cancer Cell Line SW480 Exposed to Cereal Phenolic Extracts" Molecules 24, no. 13: 2465. https://doi.org/10.3390/molecules24132465






