Nardochinoid B Inhibited the Activation of RAW264.7 Macrophages Stimulated by Lipopolysaccharide through Activating the Nrf2/HO-1 Pathway
Abstract
1. Introduction
2. Results
2.1. Anti-Inflammatory Activities of NAB on LPS-Activated RAW264.7 Macrophages
2.1.1. NAB Reduced the Release of NO in LPS-Stimulated RAW264.7 Macrophages
2.1.2. NAB Inhibited the Expression of iNOS Rather Than COX-2 in LPS-Stimulated RAW264.7 Macrophages
2.2. Potential Anti-Inflammatory Mechanisms of NAB on LPS-Induced RAW264.7 Macrophages
2.2.1. NAB Increased the mRNA and Protein Expression Levels of HO-1 in LPS-Stimulated RAW264.7 Cells
2.2.2. NAB Promoted Nrf2 Protein Translocation into the Nucleus in RAW264.7 Macrophages
2.2.3. NAB Suppressed the Production of TNF-α, IL-1β, and IL-6
2.2.4. NAB Failed to Inhibit the Activation of the NF-κB and MAPK Pathways in LPS-Stimulated RAW264.7 Cells
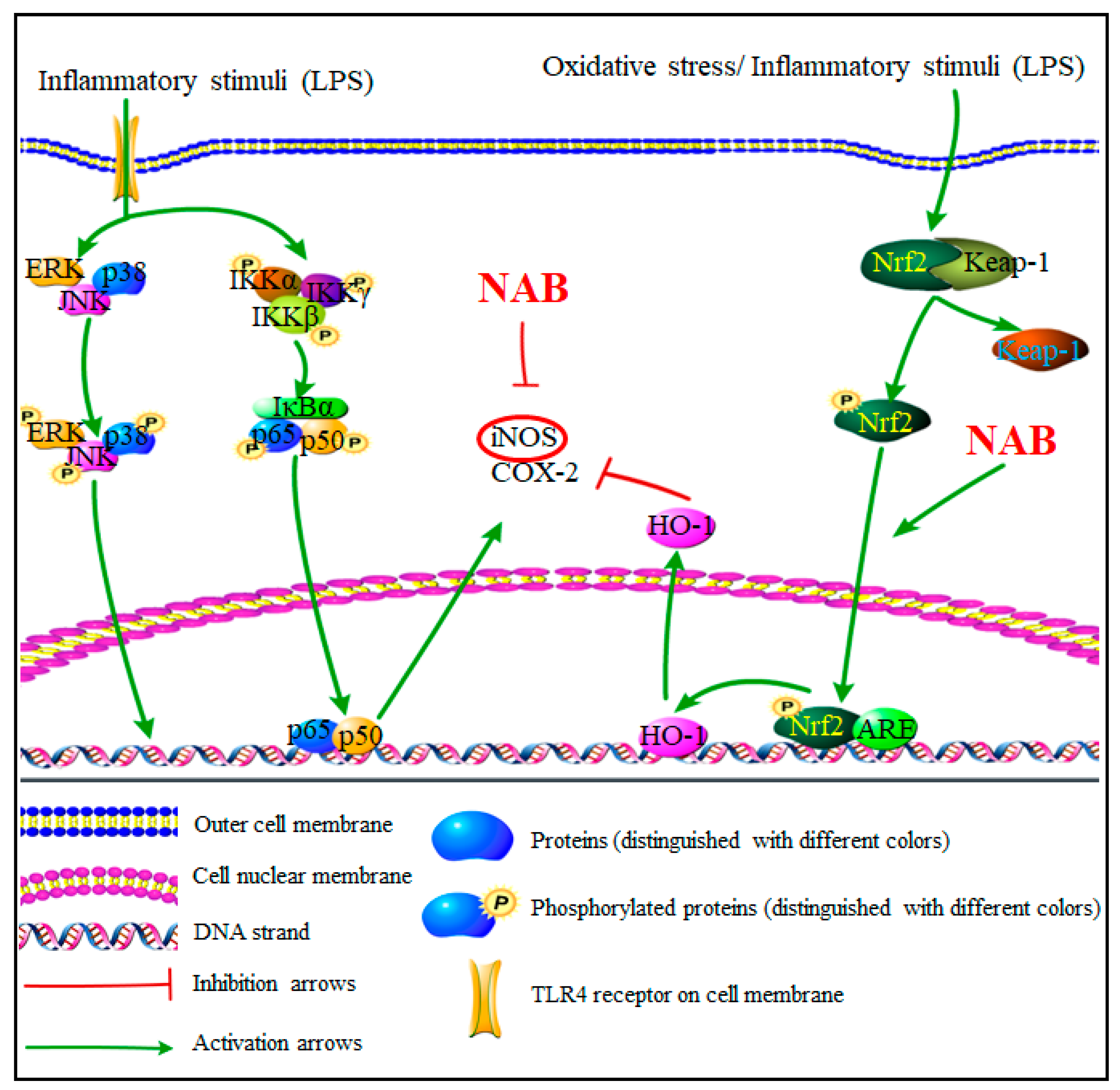
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Determination of NO, PGE2, TNF-α, IL-1β, and IL-6 Production
4.5. Protein Preparation and Western Blot Analysis
4.6. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ashley, N.T.; Weil, Z.M.; Nelson, R.J. Inflammation: Mechanisms, costs, and natural variation. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 385–406. [Google Scholar] [CrossRef]
- Al-Duaij, A.; De Brito, F.; Sedgwick, A.; Scott, D.; Willoughby, D. Susceptibility of cartilage to damage by immunological inflammation. Int. Arch. Allergy Immunol. 1986, 80, 435–437. [Google Scholar] [CrossRef]
- Rao, P.; Knaus, E.E. Evolution of nonsteroidal anti-inflammatory drugs (nsaids): Cyclooxygenase (cox) inhibition and beyond. J. Pharm. Pharm. Sci. 2008, 11, 81s–110s. [Google Scholar] [CrossRef] [PubMed]
- Koeberle, A.; Werz, O. Inhibitors of the microsomal prostaglandin e2 synthase-1 as alternative to non steroidal anti-inflammatory drugs (nsaids)-a critical review. Curr. Med. Chem. 2009, 16, 4274–4296. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G.; Smith, C.W.; Entman, M.L. The inflammatory response in myocardial infarction. Cardiovasc. Res. 2002, 53, 31–47. [Google Scholar] [CrossRef]
- Chamorro, Á. Role of inflammation in stroke and atherothrombosis. Cerebrovasc. Dis. 2004, 17, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dorfmüller, P.; Perros, F.; Balabanian, K.; Humbert, M. Inflammation in pulmonary arterial hypertension. Eur. Respir. J. 2003, 22, 358–363. [Google Scholar] [CrossRef]
- Anker, S.D.; von Haehling, S. Inflammatory mediators in chronic heart failure: An overview. Heart 2004, 90, 464–470. [Google Scholar] [CrossRef]
- Linde, A.; Mosier, D.; Blecha, F.; Melgarejo, T. Innate immunity and inflammation—New frontiers in comparative cardiovascular pathology. Cardiovasc. Res. 2007, 73, 26–36. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Marini, M.; Vittori, E.; Hollemborg, J.; Mattoli, S. Expression of the potent inflammatory cytokines, granulocyte-macrophage-colony-stimulating factor and interleukin-6 and interleukin-8, in bronchial epithelial cells of patients with asthma. J. Allergy Clin. Immunol. 1992, 89, 1001–1009. [Google Scholar] [CrossRef]
- Humes, J.L.; Bonney, R.J.; Pelus, L.; Dahlgren, M.E.; Sadowski, S.J.; Kuehl, F.A.; Davies, P. Macrophages synthesise and release prostaglandins in response to inflammatory stimuli. Nature 1977, 269, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, E.; Moilanen, T.; Knowles, R.; Charles, I.; Kadoya, Y.; Al-Saffar, N.; Revell, P.A.; Moncada, S. Nitric oxide synthase is expressed in human macrophages during foreign body inflammation. Am. J. Pathol. 1997, 150, 881–887. [Google Scholar] [PubMed]
- Miller, B.E.; Krasney, P.A.; Gauvin, D.M.; Holbrook, K.B.; Koonz, D.J.; Abruzzese, R.V.; Miller, R.E.; Pagani, K.A.; Dolle, R.E.; Ator, M.A. Inhibition of mature il-1β production in murine macrophages and a murine model of inflammation by win 67694, an inhibitor of il-1β converting enzyme. J. Immunol. 1995, 154, 1331–1338. [Google Scholar] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Lu, C.-L.; Zhu, Y.-F.; Hu, M.-M.; Wang, D.-M.; Xu, X.-J.; Lu, C.-J.; Zhu, W. Optimization of astilbin extraction from the rhizome of smilax glabra, and evaluation of its anti-inflammatory effect and probable underlying mechanism in lipopolysaccharide-induced raw264. 7 macrophages. Molecules 2015, 20, 625–644. [Google Scholar] [CrossRef]
- Hwang, J.S.; Lee, S.A.; Hong, S.S.; Han, X.H.; Lee, C.; Lee, D.; Lee, C.-K.; Hong, J.T.; Kim, Y.; Lee, M.K. Inhibitory constituents of nardostachys chinensis on nitric oxide production in raw 264.7 macrophages. Bioorg. Med. Chem. Lett. 2012, 22, 706–708. [Google Scholar] [CrossRef]
- Luo, J.-F.; Shen, X.-Y.; Lio, C.K.; Dai, Y.; Cheng, C.-S.; Liu, J.-X.; Yao, Y.-D.; Yu, Y.; Xie, Y.; Luo, P. Activation of nrf2/ho-1 pathway by nardochinoid c inhibits inflammation and oxidative stress in lipopolysaccharide-stimulated macrophages. Front. Pharmacol. 2018, 9, 911. [Google Scholar] [CrossRef]
- Shen, X.Y.; Yu, Y.; Chen, G.D.; Zhou, H.; Luo, J.F.; Zuo, Y.H.; Yao, X.S.; Dai, Y. Six new sesquiterpenoids from nardostachys chinensis batal. Fitoterapia 2017, 119, 75–82. [Google Scholar] [CrossRef]
- Shen, X.-Y.; Qin, D.-P.; Zhou, H.; Luo, J.-F.; Yao, Y.-D.; Lio, C.-K.; Li, H.-B.; Dai, Y.; Yu, Y.; Yao, X.-S. Nardochinoids a–c, three dimeric sesquiterpenoids with specific fused-ring skeletons from nardostachys chinensis. Org. Lett. 2018, 20, 5813–5816. [Google Scholar] [CrossRef]
- Kim, J.; Cha, Y.-N.; Surh, Y.-J. A protective role of nuclear factor-erythroid 2-related factor-2 (nrf2) in inflammatory disorders. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2010, 690, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.; Liu, X.; Macias, A.A.; Baron, R.M.; Perrella, M.A. Heme oxygenase-1–derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J. Clin. Investig. 2008, 118, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Gold, R.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Giovannoni, G.; Selmaj, K.; Tornatore, C.; Sweetser, M.T.; Yang, M.; Sheikh, S.I. Placebo-controlled phase 3 study of oral bg-12 for relapsing multiple sclerosis. N. Engl. J. Med. 2012, 367, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Nivolumab: A review of its use in patients with malignant melanoma. Drugs 2014, 74, 1233–1239. [Google Scholar] [CrossRef]
- Liu, J.; Tang, J.; Zuo, Y.; Yu, Y.; Luo, P.; Yao, X.; Dong, Y.; Wang, P.; Liu, L.; Zhou, H. Stauntoside b inhibits macrophage activation by inhibiting nf-κb and erk mapk signalling. Pharmacol. Res. 2016, 111, 303–315. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kim, S.-J.; Kim, S.S.; Lee, N.H.; Hyun, C.-G. Hypochoeris radicata attenuates lps-induced inflammation by suppressing p38, erk, and jnk phosphorylation in raw 264.7 macrophages. EXCLI J. 2014, 13, 123–136. [Google Scholar]
- Meijvis, S.C.A.; Hardeman, H.; Remmelts, H.H.F.; Heijligenberg, R.; Rijkers, G.T.; van Velzen-Blad, H.; Voorn, G.P.; van de Garde, E.M.W.; Endeman, H.; Grutters, J.C.; et al. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: A randomised, double-blind, placebo-controlled trial. Lancet 2011, 377, 2023–2030. [Google Scholar] [CrossRef]
- Lin, W.; Wu, R.T.; Wu, T.; Khor, T.O.; Wang, H.; Kong, A.N. Sulforaphane suppressed lps-induced inflammation in mouse peritoneal macrophages through nrf2 dependent pathway. Biochem. Pharmacol. 2008, 76, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, L.-O.; Kipp, M.; Lucius, R.; Pufe, T.; Wruck, C.J. Sulforaphane suppresses lps-induced inflammation in primary rat microglia. Inflamm. Res. 2010, 59, 443–450. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef]
- Hui-Yi, L.; Shing-Chuan, S.; Yen-Chou, C. Anti-inflammatory effect of heme oxygenase 1: Glycosylation and nitric oxide inhibition in macrophages. J. Cell. Physiol. 2005, 202, 579–590. [Google Scholar]
- Mcinnes, I.B.; Leung, B.P.; Field, M.; Wei, X.Q.; Huang, F.P.; Sturrock, R.D.; Kinninmonth, A.; Weidner, J.; Mumford, R.; Liew, F.Y. Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J. Exp. Med. 1996, 184, 1519. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Heller, A.; Koch, T.; Schmeck, J.; van Ackern, K. Lipid mediators in inflammatory disorders. Drugs 1998, 55, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, G.A.; Pedersen, A.K.; Patrono, C. Analysis of prostacyclin and thromboxane biosynthesis in cardiovascular disease. Circulation 1983, 67, 1174–1177. [Google Scholar] [CrossRef]
- Bradley, J.R. Tnf-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- DiDonato, J.A.; Mercurio, F.; Karin, M. Nf-kappa b and the link between inflammation and cancer. Immunol. Rev. 2012, 246, 379–400. [Google Scholar] [CrossRef]
- Ghosh, S.; Hayden, M.S. New regulators of nf-κb in inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef]
- Liu, Y.S.; Shepherd, E.G.; Nelin, L.D. Mapk phosphatases—Regulating the immune response. Nat. Rev. Immunol. 2007, 7, 202–212. [Google Scholar] [CrossRef]
- Ghosh, S.; May, M.J.; Kopp, E.B. Nf-kappa b and rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef]
- Rao, K.M.K. Map kinase activation in macrophages. J. Leukoc. Biol. 2001, 69, 3–10. [Google Scholar] [PubMed]
- Sun, L.D.; Wang, F.; Dai, F.; Wang, Y.H.; Lin, D.; Zhou, B. Development and mechanism investigation of a new piperlongumine derivative as a potent anti-inflammatory agent. Biochem. Pharmacol. 2015, 95, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-B.; Han, A.-R.; Park, E.-Y.; Kim, J.-Y.; Cho, W.; Lee, J.; Seo, E.-K.; Lee, K.-T. Inhibition of lps-induced inos, cox-2 and cytokines expression by poncirin through the nf-κb inactivation in raw 264.7 macrophage cells. Biol. Pharm. Bull. 2007, 30, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Bae, G.-S.; Choi, S.-B.; Jo, I.-J.; Kim, D.-G.; Lee, D.-S.; An, R.-B.; Oh, H.; Kim, Y.-C.; Shin, Y.K. Anti-inflammatory effect of desoxo-narchinol-a isolated from nardostachys jatamansi against lipopolysaccharide. Int. Immunopharmacol. 2015, 29, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed]
- Rojo, A.I.; Innamorato, N.G.; Martín-Moreno, A.M.; De Ceballos, M.L.; Yamamoto, M.; Cuadrado, A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental parkinson’s disease. Glia 2010, 58, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Thimmulappa, R.K.; Scollick, C.; Traore, K.; Yates, M.; Trush, M.A.; Liby, K.T.; Sporn, M.B.; Yamamoto, M.; Kensler, T.W.; Biswal, S. Nrf2-dependent protection from lps induced inflammatory response and mortality by cddo-imidazolide. Biochem. Biophys. Res. Commun. 2006, 351, 883–889. [Google Scholar] [CrossRef]
- Koarai, A.; Ichinose, M.; Sugiura, H.; Yamagata, S.; Hattori, T.; Shirato, K. Allergic airway hyperresponsiveness and eosinophil infiltration is reduced by a selective inos inhibitor, 1400w, in mice. Pulm. Pharmacol. Ther. 2000, 13, 267–275. [Google Scholar] [CrossRef]
- Labuda, C.J.; Koblish, M.; Tuthill, P.; Dolle, R.E.; Little, P.J. Antinociceptive activity of the selective inos inhibitor ar-c102222 in rodent models of inflammatory, neuropathic and post-operative pain. Eur. J. Pain 2012, 10, 505. [Google Scholar] [CrossRef]
- Ryter, S.W.; Alam, J.; Choi, A.M. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef]
- Alcaraz, M.; Fernandez, P.; Guillen, M. Anti-inflammatory actions of the heme oxygenase-1 pathway. Curr. Pharm. Des. 2003, 9, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Juan, S.-H.; Shen, S.-C.; Hsu, F.-L.; Chen, Y.-C. Inhibition of lipopolysaccharide-induced nitric oxide production by flavonoids in raw264. 7 macrophages involves heme oxygenase-1. Biochem. Pharmacol. 2003, 66, 1821–1832. [Google Scholar] [CrossRef]
- Finbloom, D.S.; Winestock, K.D. Il-10 induces the tyrosine phosphorylation of tyk2 and jak1 and the differential assembly of stat1 alpha and stat3 complexes in human t cells and monocytes. J. Immunol. 1995, 155, 1079–1090. [Google Scholar] [PubMed]
- Crawley, J.B.; Williams, L.M.; Mander, T.; Brennan, F.M.; Foxwell, B.M. Interleukin-10 stimulation of phosphatidylinositol 3-kinase and p70 s6 kinase is required for the proliferative but not the antiinflammatory effects of the cytokine. J. Biol. Chem. 1996, 271, 16357–16362. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-S.; Chau, L.-Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat. Med. 2002, 8, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Ricchetti, G.A.; Williams, L.M.; Foxwell, B.M. Heme oxygenase 1 expression induced by il-10 requires stat-3 and phosphoinositol-3 kinase and is inhibited by lipopolysaccharide. J. Leukoc. Biol. 2004, 76, 719–726. [Google Scholar] [CrossRef]
- Hwang, D.; Kang, M.-j.; Jo, M.J.; Seo, Y.B.; Park, N.G.; Kim, G.-D. Anti-inflammatory activity of β-thymosin peptide derived from pacific oyster (crassostrea gigas) on no and pge2 production by down-regulating nf-κb in lps-induced raw264. 7 macrophage cells. Mar. Drugs 2019, 17, 129. [Google Scholar] [CrossRef]
- Choo, G.S.; Lim, D.P.; Kim, S.M.; Yoo, E.S.; Kim, S.H.; Kim, C.H.; Woo, J.S.; Kim, H.J.; Jung, J.Y. Anti-inflammatory effects of dendropanax morbifera in lipopolysaccharide-stimulated raw264. 7 macrophages and in an animal model of atopic dermatitis. Mol. Med. Rep. 2019, 19, 2087–2096. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |







| Target Gene | Primer Sequences |
|---|---|
| β-actin_F | 5′-CGGTTCCGATGCCCTGAGGCTCTT-3′ |
| β-actin_R | 5′-CGTCACACTTCATGATGGAATTGA-3′ |
| iNOS_F | 5′-CAGCACAGGAAATGTTTCAGC-3′ |
| iNOS_R | 5′-TAGCCAGCGTACCGGATGA-3′ |
| COX-2_F | 5′-TTTGGTCTGGTGCCTGGTC-3′ |
| COX-2_R | 5′-CTGCTGGTTTGGAATAGTTGCTC-3′ |
| TNF-α_F | 5′-TATGGCTCAGGGTCCAACTC-3′ |
| TNF-α_R | 5′-CTCCCTTTGCAGAACTCAGG-3′ |
| IL-6_F | 5′-GGTGACAACCACGGCCTTCCC-3′ |
| IL-6_R | 5′-AAGCCTCCGACTTGTGAAGTGGT-3′ |
| HO-1_F | 5′-CCCACCAAGTTCAAACAGCTC-3′ |
| HO-1_R | 5′-AGGAAGGCGGTCTTAGCCTC-3′ |
| IL-1β_F | 5′-TTGACGGACCCCAAAAGATG-3′ |
| IL-1β_R | 5′-AGAAGGTGCTCATGTCCTCA-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.-D.; Shen, X.-Y.; Machado, J.; Luo, J.-F.; Dai, Y.; Lio, C.-K.; Yu, Y.; Xie, Y.; Luo, P.; Liu, J.-X.; et al. Nardochinoid B Inhibited the Activation of RAW264.7 Macrophages Stimulated by Lipopolysaccharide through Activating the Nrf2/HO-1 Pathway. Molecules 2019, 24, 2482. https://doi.org/10.3390/molecules24132482
Yao Y-D, Shen X-Y, Machado J, Luo J-F, Dai Y, Lio C-K, Yu Y, Xie Y, Luo P, Liu J-X, et al. Nardochinoid B Inhibited the Activation of RAW264.7 Macrophages Stimulated by Lipopolysaccharide through Activating the Nrf2/HO-1 Pathway. Molecules. 2019; 24(13):2482. https://doi.org/10.3390/molecules24132482
Chicago/Turabian StyleYao, Yun-Da, Xiu-Yu Shen, Jorge Machado, Jin-Fang Luo, Yi Dai, Chon-Kit Lio, Yang Yu, Ying Xie, Pei Luo, Jian-Xin Liu, and et al. 2019. "Nardochinoid B Inhibited the Activation of RAW264.7 Macrophages Stimulated by Lipopolysaccharide through Activating the Nrf2/HO-1 Pathway" Molecules 24, no. 13: 2482. https://doi.org/10.3390/molecules24132482
APA StyleYao, Y.-D., Shen, X.-Y., Machado, J., Luo, J.-F., Dai, Y., Lio, C.-K., Yu, Y., Xie, Y., Luo, P., Liu, J.-X., Yao, X.-S., Liu, Z.-Q., & Zhou, H. (2019). Nardochinoid B Inhibited the Activation of RAW264.7 Macrophages Stimulated by Lipopolysaccharide through Activating the Nrf2/HO-1 Pathway. Molecules, 24(13), 2482. https://doi.org/10.3390/molecules24132482







