Ketogenic Diet-Induced Weight Loss is Associated with an Increase in Vitamin D Levels in Obese Adults
Abstract
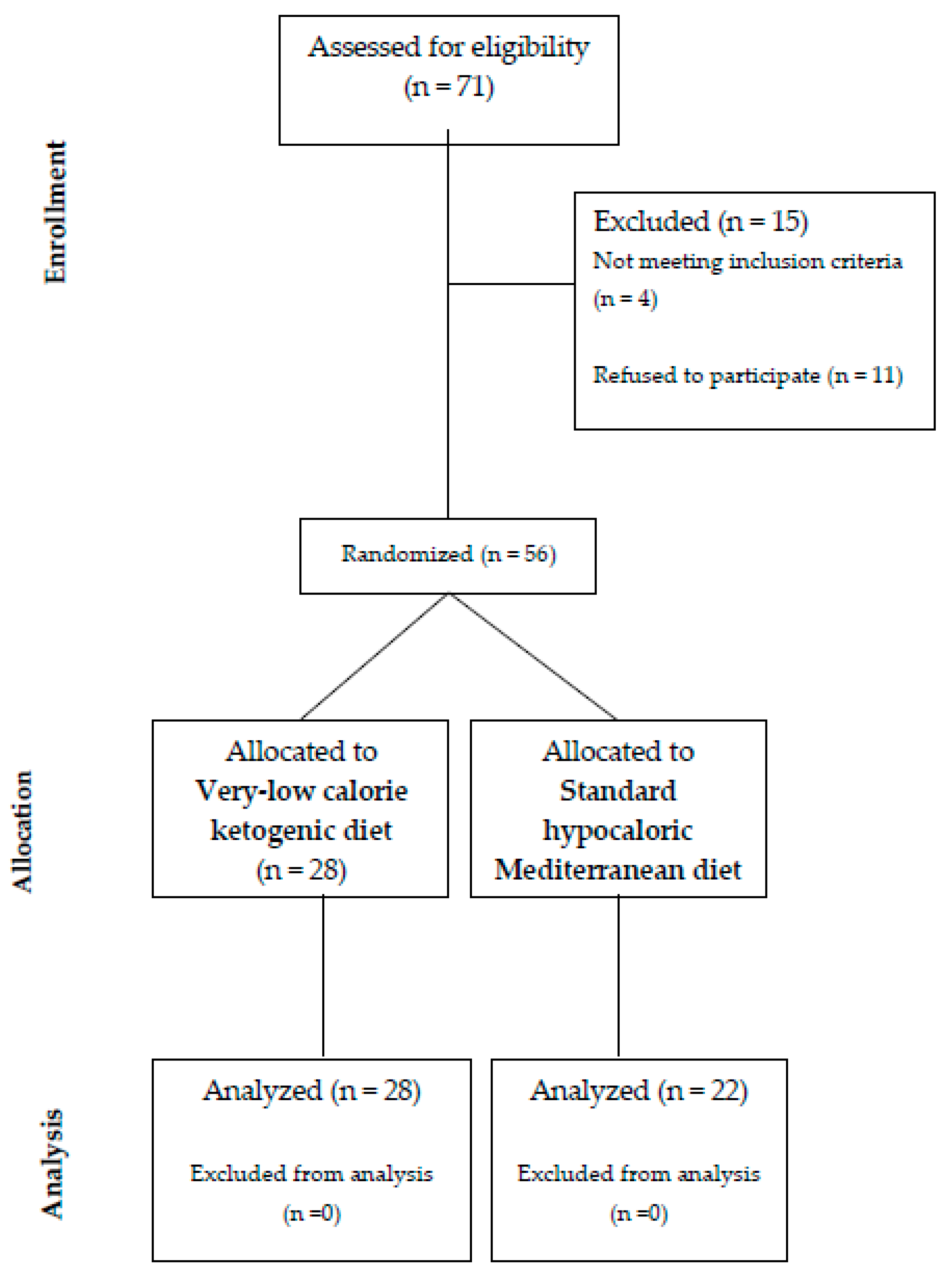
:1. Introduction
2. Results
2.1. Study Population
2.2. Correlational Analysis
2.3. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Anthropometric Measurements
4.3. Laboratory Determinations
4.4. Body Composition Assessment
4.5. Dietary Treatment
4.6. Dietary Adherence
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Reid, I.R.; Bolland, M.J.; Grey, A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet 2014, 383, 146–155. [Google Scholar] [CrossRef]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated action of 1a,25(OH)2vitamin D3: genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.R.; Tripkovic, L.; Hart, K.H.; Lanham-New, S.A. Vitamin D deficiency as a public health issue:using vitamin D2 or vitamin D3 in future fortification strategies. Proc. Nutr. Soc. 2017, 76, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Sorice, G.P.; Ajjan, R.; Mezza, T.; Pilz, S.; Prioletta, A.; Scragg, R.; Volpe, S.L.; Witham, M.D.; Giaccari, A. Can vitamin D deficiency cause diabetes and cardiovascular diseases? Present evidence and future perspectives. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D deficiency and the risk of cardiovascular disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Michos, E.D.; Reis, J.P.; Post, W.S.; Lutsey, P.L.; Gottesman, R.F.; Mosley, T.H.; Sharrett, A.R.; Melamed, M.L. 25-Hydroxyvitamin D deficiency is associated with fatal stroke among whites but not blacks: the NHANES-III linked mortality files. Nutrition 2012, 28, 367–371. [Google Scholar] [CrossRef]
- Giovannucci, E.; Liu, Y.; Hollis, B.W.; Rimm, E.B. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch. Intern. Med. 2008, 168, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Luan, J.; Cooper, A.; Boucher, B.J.; Wareham, N.J. Baseline serum 25-hydroxy vitamin D is predictive of future glycemic status and insulin resistance: The Medical Research Council Ely Prospective Study 1990–2000. Diabetes 2008, 57, 2619–2625. [Google Scholar] [CrossRef]
- Kabadi, S.M.; Lee, B.K.; Liu, L. Joint effects of obesity and vitamin D insufficiency on insulin resistance and type 2 diabetes: results from the NHANES 2001-2006. Diabetes Care 2012, 35, 2048–2054. [Google Scholar] [CrossRef]
- Kayaniyil, S.; Vieth, R.; Retnakaran, R.; Knight, J.A.; Qi, Y.; Gerstein, H.C.; Perkins, B.A.; Harris, S.B.; Zinman, B.; Hanley, A.J. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care 2010, 33, 1379–1381. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Berry, D.J.; Lu, C.; Tikkanen, E.; Pilz, S.; Hiraki, L.T.; Cooper, J.D.; Dastani, Z.; Li, R.; Houston, D.K.; et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloira, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Hyppönen, E.; Boucher, B.J. Adiposity, vitamin D requirements, and clinical implications for obesity-related metabolic abnormalities. Nutr. Rev. 2018, 76, 678–692. [Google Scholar] [CrossRef]
- Walsh, J.S.; Evans, A.L.; Bowles, S.; Naylor, K.E.; Jones, K.S.; Schoenmakers, I.; Jacques, R.M.; Eastell, R. Free 25-hydroxyvitamin D is low in obesity, but there are no adverse associations with bone health. Am. J. Clin. Nutr. 2016, 103, 1465–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, L.; Borrell, L.N. The effect of body mass index on optimal vitamin D status in U.S. adults: the National Health and Nutrition Examination Survey 2001–2006. Ann. Epidemiol. 2013, 23, 409–414. [Google Scholar] [CrossRef]
- Lagunova, Z.; Porojnicu, A.C.; Lindberg, F.; Hexeberg, S.; Moan, J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009, 29, 3713–3720. [Google Scholar] [CrossRef]
- Chandler, P.D.; Wang, L.; Zhang, X.; Sesso, H.D.; Moorthy, M.V.; Obi, O.; Lewis, J.; Prince, R.L.; Dankis, J.S.; Manson, J.E.; et al. Effect of vitamin D supplementation alone or with calcium on adiposity measures: a systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2015, 73, 577–593. [Google Scholar] [CrossRef]
- Walsh, J.S.; Bowels, S.; Evans, A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 389–394. [Google Scholar] [CrossRef]
- Carrelli, A.; Bucovsky, M.; Horst, R.; Cremers, S.; Zhang, C.; Bessler, M.; Schrope, B.; Evanko, J.; Blanco, J.; Silverberg, S.J.; et al. Vitamin D storage in adipose tissue of obese and normal weight women. J. Bone Miner. Res. 2017, 32, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, J. Interest in the ketogenic diet grows for weight loss and type 2 diabetes. JAMA 2018, 19, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Wilder, R. The effects of ketonemia on the course of epilepsy. Mayo Clin. Proc. 1921, 2, 307–308. [Google Scholar]
- Yudkin, J. The low-carbohydrate diet in the treatment of obesity. Postgrad Med. 1972, 51, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Loria, C.M.; Ard, J.D.; Millen, B.E.; Comuzzie, A.G.; Nonas, C.A.; Donato, K.A.; Pi-Sunyer, X.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and The Obesity Society. J. Am. Coll. Cardiol. 2014, 63, 2985–3023. [Google Scholar] [CrossRef]
- Barzel, U.S. Excess dietary protein can adversely affect bone. J. Nutr. 1998, 128, 1051–1053. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Basulado-Hammond, C.; Curtis, R.; Schuller, R. Growth retardation in children with epilepsy on the ketogenic diet: a retrospective chart review. J. Am. Diet. Assoc. 2002, 102, 405–407. [Google Scholar] [CrossRef]
- Bergqvist, C.A.G.; Schall, J.I.; Stallings, V.A.; Zemel, B.S. Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. Am. J. Clin. Nutr. 2008, 88, 1678–1684. [Google Scholar] [CrossRef] [Green Version]
- Colica, C.; Merra, G.; Gasbarrini, A.; De Lorenzo, A.; Cioccoloni, G.; Gualtieri, P.; Perrone, M.A.; Bernardini, S.; Bernardo, V.; Di Renzo, L.; et al. Efficacy and safety of very-low-calorie ketogenic diet: a double blind randomized crossover study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2274–2289. [Google Scholar]
- Sciacqua, A.; Perticone, M.; Grillo, N.; Falbo, T.; Bencardino, G.; Angotti, E.; Arturi, F.; Parlato, G.; Sesti, G.; Perticone, F. Vitamin D and 1-h post-load plasma glucose in hypertensive patients. Cardiovasc. Diabetol. 2014, 13, 48. [Google Scholar] [CrossRef]
- Merra, G.; Gratteri, S.; De Lorenzo, A.; Barrucco, S.; Perrone, M.A.; Avolio, E.; Bernardini, S.; Marchetti, M.; Di Renzo, L. Effects of a very-low-calorie diet on body composition, metabolic state, and genes expression: a randomized double-blind placebo-controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 329–345. [Google Scholar] [PubMed]
- Norton, K.; Olds, T. Anthropometrica; UNSW Press: Sydney, Australia, 1996. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel on Dietetic Products, Nutrition and Allergies, Scientific opinion on the essential composition of total diet replacements for weight control. EFSA J. 2015, 13, 3957. [CrossRef]
- Trichopoulou, A.; Lagiou, P. Healthy traditional Mediterranean diet: an expression of culture, history, and lifestyle. Nutr. Rev. 1997, 55, 383–389. [Google Scholar] [CrossRef] [PubMed]
| All n = 56 | SHMD n = 28 | VLCKD n = 28 | p value | |
|---|---|---|---|---|
| Age, years | 46.8 ± 11.0 | 50.9 ± 13.3 | 42.6 ± 6.6 | 0.090 |
| Sex, M/F | 32/24 | 18/10 | 14/14 | 0.036 |
| Body weight, Kg | 114.1 ± 28.8 | 107.5 ± 18.5 | 113.9 ± 31.0 | 0.307 |
| BMI, Kg/m2 | 39.65 ± 9.7 | 38.8 ± 4.5 | 40.5 ± 10.8 | 0.307 |
| WC, cm | 123.0 ± 18.7 | 126.4 ± 12.2 | 119.1 ± 22.9 | 0.761 |
| HC, cm | 127.2 ± 9.6 | 125.2 ± 7.8 | 129.4 ± 8.3 | 0.098 |
| TBW, l | 44.6 ± 12.8 | 42.6 ± 9.8 | 48.7 ± 14.8 | 0.463 |
| FM, Kg | 49.3 ± 19.9 | 47.0 ± 10.8 | 51.3 ± 26.9 | 0.361 |
| FFM, Kg | 57.6 ± 14.7 | 56.1 ± 12.8 | 59.1 ± 16.9 | 0.647 |
| MM, Kg | 40.8 ± 12.7 | 38.2 ± 11.5 | 43.5 ± 13.9 | 0.354 |
| BMR, Kcal | 1818 ± 298 | 1818 ± 286 | 1820 ± 343 | 0.953 |
| FPG, mg/dL | 112.4 ± 29.9 | 115.3 ± 32.6 | 105.8 ± 25.5 | 0.819 |
| HbA1c, % | 6.3 ± 1.6 | 6.5 ± 1.5 | 6.1 ± 1.4 | 0.013 |
| Fasting insulin, μU/L | 26.9 ± 14.5 | 25.8 ± 15.4 | 28.0 ± 16.7 | 0.509 |
| HOMA | 7.4 ± 0.5 | 7.4 ± 0.9 | 7.3 ± 0.7 | 0.665 |
| eGFR, ml/min/1,73m2 | 104.9 ± 11.0 | 103.7 ± 11.0 | 106.0 ± 12.2 | 0.617 |
| Uric acid, mg/dL | 5.5 ± 1.3 | 5.4 ± 1.3 | 5.6 ± 1.0 | 0.814 |
| Total cholesterol, mg/dL | 184.9 ± 34.1 | 180.7 ± 40.7 | 192.3 ± 27.0 | 0.597 |
| HDL cholesterol, mg/dL | 51.7 ± 13.3 | 51.1 ± 15.4 | 52.2 ± 11.0 | 0.668 |
| LDL cholesterol, mg/dL | 121.4 ± 33.7 | 115.3 ± 43.9 | 126.9 ± 20.5 | 0.408 |
| Triglycerides, mg/dL | 155.0 ± 94.0 | 158.5 ± 62.3 | 151.3 ± 50.0 | 0.706 |
| hsCRP, mg/L | 5.05 ± 3.5 | 5.6 ± 4.3 | 4.5 ± 2.6 | 0.943 |
| SBP, mmHg | 129.6 ± 18.1 | 123.4 ± 16.9 | 132.8 ± 16.3 | 0.129 |
| DBP, mmHg | 79.7 ± 12.3 | 73.5 ± 8.9 | 83.1 ± 11.5 | 0.029 |
| 25(OH)D, ng/ml | 17.8 ± 5.6 | 17.5 ± 6.1 | 18.4 ± 5.9 | 0.567 |
| VLCKD (n = 28)T0 | VLCKD (n = 28) T12 | p Value | |
|---|---|---|---|
| Anthropometric | |||
| Body weight, Kg | 113.9 ± 31.0 | 87.3 ± 22.8 | 0.097 |
| BMI, Kg/m2 | 40.5 ± 10.8 | 33.3 ± 9.72 | 0.212 |
| WC, cm | 119.1 ± 22.9 | 95.0 ± 17.4 | 0.044 |
| HC, cm | 129.4 ± 8.3 | 118.5 ± 7.3 | 0.066 |
| TBW, l | 48.7 ± 14.8 | 55.3 ± 13.9 | 0.035 |
| FM, Kg | 51.3 ± 16.9 | 31.4 ± 8.6 | 0.141 |
| FFM, Kg | 59.1 ± 16.9 | 62.7 ± 15.6 | 0.835 |
| MM, Kg | 43.5 ± 13.9 | 45.5 ± 15.6 | 0.760 |
| BMR, Kcal | 1820 ± 343 | 1646 ± 180 | 0.280 |
| Biochemical | |||
| FPG, mg/dL | 105.8 ± 25.5 | 93.5 ± 13.7 | 0.304 |
| HbA1c, % | 6.1 ± 1.4 | 5.2 ± 0.15 | 0.022 |
| Fasting insulin, μU/L | 28.0 ± 16.7 | 10.9 ± 4.9 | 0.032 |
| HOMA | 7.3 ± 0.7 | 2.6 ± 0.2 | <0.0001 |
| eGFR, ml/min/1,73m2 | 106.0 ± 12.2 | 109.7 ± 11.1 | 0.563 |
| Uric acid, mg/dL | 5.6 ± 1.0 | 4.9 ± 0.8 | 0.257 |
| Total cholesterol, mg/dL | 192.3 ± 27.0 | 172.7 ± 21.0 | 0.158 |
| HDL cholesterol, mg/dL | 52.2 ± 11.0 | 56.5 ± 13.6 | 0.515 |
| LDL cholesterol, mg/dL | 126.9 ± 20.5 | 109.3 ± 29.6 | 0.077 |
| Triglycerides, mg/dL | 151.3 ± 50.0 | 72.3 ± 29.6 | 0.004 |
| hsCRP, mg/L | 4.5 ± 2.6 | 1.8 ± 0.8 | <0.0001 |
| 25(OH)D, ng/mL | 18.4 ± 5.9 | 29.3 ± 6.8 | <0.0001 |
| Hemodynamic | |||
| SBP, mmHg | 132.8 ± 16.3 | 118.8 ± 15.1 | 0.118 |
| DBP, mmHg | 83.1 ± 11.5 | 79.3 ± 4.8 | 0.463 |
| SHMD (n= 28) T0 | SHMD (n= 22) T12 | p Value | |
|---|---|---|---|
| Anthropometric | |||
| Body weight, Kg | 107.5 ± 18.5 | 99.3 ± 15.8 | 0.254 |
| BMI, Kg/m2 | 38.8 ± 4.5 | 36.1 ± 5.7 | 0.321 |
| WC, cm | 126.4 ± 12.2 | 113.0 ± 14.5 | 0.098 |
| HC, cm | 125.2 ± 7.8 | 119.5 ± 5.6 | 0.665 |
| TBW, l | 42.6 ± 9.8 | 42.9 ± 8.9 | 0.754 |
| FM, Kg | 47.0 ± 10.8 | 40.5 ± 8.6 | 0.181 |
| FFM, Kg | 56.1 ± 12.8 | 55.3 ± 11.5 | 0.766 |
| MM, Kg | 38.2 ± 11.5 | 39.7 ± 11.7 | 0.840 |
| BMR, Kcal | 1818 ± 286 | 1730 ± 166 | 0.954 |
| Biochemical | |||
| FPG, mg/dL | 115.3 ± 32.6 | 99.7 ± 11.4 | 0.048 |
| HbA1c, % | 6.5 ± 1.5 | 5.4 ± 0.18 | 0.034 |
| Fasting insulin, μU/L HOMA | 25.8 ± 15.4 7.4 ± 0.9 | 13.5 ± 6.7 3.5 ± 0.4 | 0.044 0.001 |
| eGFR, ml/min/1,73m2 | 103.7 ± 11.0 | 105.7 ± 11.7 | 0.563 |
| Uric acid, mg/dL | 5.4 ± 1.3 | 5.6 ± 1.0 | 0.776 |
| Total cholesterol, mg/dL | 180.7 ± 40.7 | 168.6 ± 34.6 | 0.438 |
| HDL cholesterol, mg/dL | 51.1 ± 15.4 | 52.3 ± 15.4 | 0.685 |
| LDL cholesterol, mg/dL | 115.3 ± 43.9 | 104.9 ± 27.8 | 0.546 |
| Triglycerides, mg/dL | 158.5 ± 62.3 | 113.0 ± 21.5 | 0.039 |
| hsCRP, mg/L | 5.6 ± 4.3 | 3.7 ± 1.2 | 0.044 |
| 25(OH)D, ng/mL | 17.5 ± 6.1 | 18.6 ± 6.8 | 0.645 |
| Hemodynamic | |||
| SBP, mmHg | 123.4 ± 16.9 | 119.8 ± 15.1 | 0.063 |
| DBP, mmHg | 73.5 ± 8.9 | 72.2 ± 7.6 | 0.067 |
| All (n = 56) r/P | VLCKD (n = 28) r/P | SHMD (n =24) r/P | |
|---|---|---|---|
| Anthropometric | |||
| Age, years | 0.250/0.334 | 0.305/0.221 | 0.088/0.187 |
| ΔBMI, Kg/m2 | 0.425/<0.0001 | 0.337/<0.0001 | 0.165/0.007 |
| ΔWC, cm | 0.338/<0.0001 | 0.219/<0.0001 | 0.178/0.005 |
| ΔFM, kg | 0.276/0.008 | 0.123/<0.0001 | 0.087/0.052 |
| ΔFFM, kg | 0.096/0.123 | 0.065/0.098 | 0.052/0.234 |
| ΔMM, kg | −0.111/0.164 | −0.094/0.078 | −0.045/0.198 |
| Biochemical | |||
| Total cholesterol, mg/dL | 0.065/0.043 | 0.127/0.020 | 0.099/0.326 |
| LDL-cholesterol, mg/dL | 0.104/0.047 | 0.153/0.139 | 0.039/0.346 |
| HDL-cholesterol, mg/dL | −0.175/0.002 | −0.101/0.237 | −0.107/0.139 |
| Triglycerides, mg/dL | 0.056/0.002 | 0.098/<0.0001 | 0.128/0.098 |
| HOMA | 0.312/<0.0001 | 0.249/<0.0001 | 0.369/0.004 |
| hsCRP, mg/L | 0.171/0.003 | 0.076/0.007 | 0.034/0.09 |
| Hemodynamic | |||
| SBP, mmHg | 0.331/0.067 | 0.220/0.043 | 0.245/0.544 |
| DBP, mmHg | 0.219/0.007 | 0.154/0.138 | 0.138/0.444 |
| r2 partial | r2 total | P | |
|---|---|---|---|
| All | |||
| ΔFM, kg | 15 | 15 | <0.0001 |
| ΔBMI, kg/m2 | 7.2 | 22.2 | <0.0001 |
| ΔWC, cm | 3.6 | 25.8 | 0.007 |
| HOMA | 1.1 | 26.9 | 0.002 |
| VLCKD | |||
| ΔFM, kg | 12.1 | 12.1 | <0.0001 |
| ΔBMI, kg/m2 | 6.9 | 19.0 | 0.009 |
| ΔWC, cm | 4.5 | 23.5 | 0.001 |
| SHMD | |||
| ΔBMI, kg/m2 | 9.6 | 9.6 | <0.0001 |
| ΔFM, kg | 2.8 | 12.4 | 0.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perticone, M.; Maio, R.; Sciacqua, A.; Suraci, E.; Pinto, A.; Pujia, R.; Zito, R.; Gigliotti, S.; Sesti, G.; Perticone, F. Ketogenic Diet-Induced Weight Loss is Associated with an Increase in Vitamin D Levels in Obese Adults. Molecules 2019, 24, 2499. https://doi.org/10.3390/molecules24132499
Perticone M, Maio R, Sciacqua A, Suraci E, Pinto A, Pujia R, Zito R, Gigliotti S, Sesti G, Perticone F. Ketogenic Diet-Induced Weight Loss is Associated with an Increase in Vitamin D Levels in Obese Adults. Molecules. 2019; 24(13):2499. https://doi.org/10.3390/molecules24132499
Chicago/Turabian StylePerticone, Maria, Raffaele Maio, Angela Sciacqua, Edoardo Suraci, Angelina Pinto, Roberta Pujia, Roberta Zito, Simona Gigliotti, Giorgio Sesti, and Francesco Perticone. 2019. "Ketogenic Diet-Induced Weight Loss is Associated with an Increase in Vitamin D Levels in Obese Adults" Molecules 24, no. 13: 2499. https://doi.org/10.3390/molecules24132499
APA StylePerticone, M., Maio, R., Sciacqua, A., Suraci, E., Pinto, A., Pujia, R., Zito, R., Gigliotti, S., Sesti, G., & Perticone, F. (2019). Ketogenic Diet-Induced Weight Loss is Associated with an Increase in Vitamin D Levels in Obese Adults. Molecules, 24(13), 2499. https://doi.org/10.3390/molecules24132499






