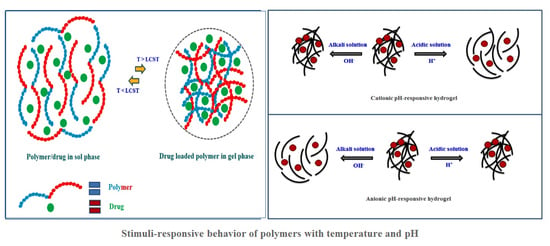
Review of Stimuli-Responsive Polymers in Drug Delivery and Textile Application
Abstract
:1. Introduction
2. Natural Stimuli-Responsive Polymers
2.1. Chitosan
2.2. Cellulose
2.3. Albumin
2.4. Gelatin
3. Synthetic Stimuli-Responsive Polymers
3.1. Pluronic F127
3.2. Poly(N-isopropylacrylamide)
3.3. Poly(ethylene glycol) (PEG)
3.4. Polyacrylic Acid
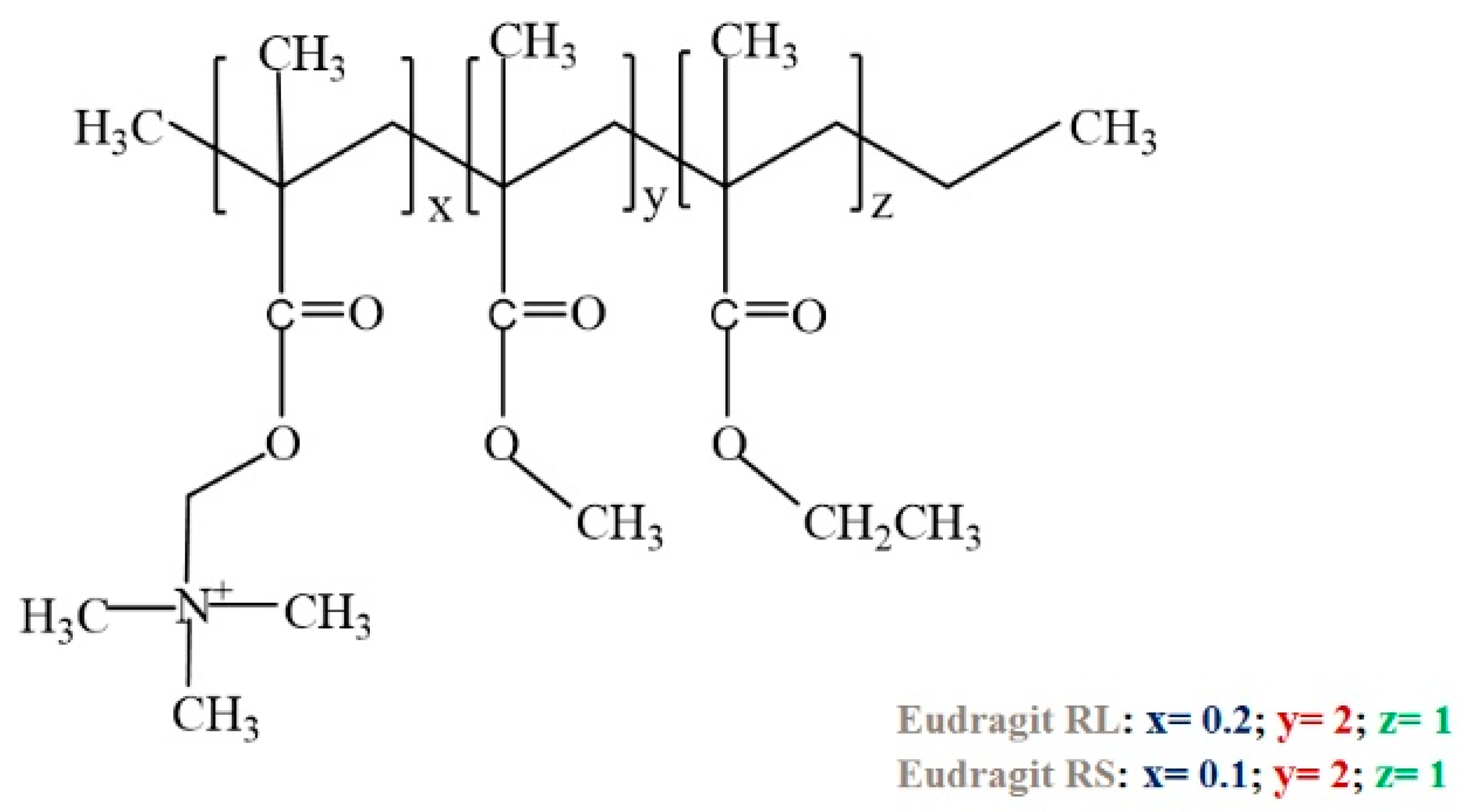
3.5. Poly(N,N-dialkylaminoethyl methacrylate) and Eudragit
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliver. Rev. 2002, 43, 3–12. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. Amini review on hydrogels classification and recent developments in miscellaneous applications. Mat. Sci. Eng. C-Mater. 2017, 79, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Wichterle, O.; Lím, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.N.; Ferreira, L.M.B.; Cardoso, V.M.O.; Boni, F.I.; Souza, A.L.R.; Gremião, M.P.D. Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef] [Green Version]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release. 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Peak, C.W.; Wilker, J.J.; Schmidt, G. A review on tough and sticky hydrogels. Colloid Polym. Sci. 2013, 291, 2031–2047. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Tsitsilianis, C. Responsive reversible hydrogels from associative “smart” macromolecules. Soft Matter 2010, 6, 2372–2388. [Google Scholar] [CrossRef]
- Peppas, N.A.; Wood, K.M.; Blanchette, J.O. Hydrogels for oral delivery of therapeuticproteins. Expert Opin. Biol. Ther. 2004, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, L.A.; Daily, A.M.; Horava, S.D.; Peppas, N.A. Therapeutic applications of hydrogels in oral drug delivery. Expert Opin. Drug Del. 2014, 11, 901–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Mastropietro, D.J.; Omidian, H.; Park, K. Drug delivery applications for superporous hydrogels. Expert Opin. Drug Del. 2012, 9, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.N.; Leporatti, S.; El-Kemary, M.A. Mucoadhesive hydrogel nanoparticles as smart biomedical drug delivery system. Appl. Sci. 2019, 9, 825. [Google Scholar] [CrossRef]
- Ahmed, T.A.; El-Say, K.M. Transdermal film-loaded finasteride microplates to enhance drug skin permeation: Two-step optimization study. Eur. J. Pharm. Sci. 2016, 88, 246–256. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. 2015, 93, 1–49. [Google Scholar] [CrossRef]
- Jeong, B.; Kibbey, M.R.; Birnbaum, J.C.; Won, Y.Y.; Gutowska, A. Thermogelling biodegradable polymers with hydrophilic backbones: PEG-g-PLGA. Macromolecules 2000, 33, 8317–8322. [Google Scholar] [CrossRef]
- Kuckling, D. Stimuli-responsive gels. Gels 2018, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H.M.N. Endogenous and exogenous stimuli-responsive drug delivery systems for programmed site-specific release. Molecules 2019, 24, 1117. [Google Scholar] [CrossRef] [PubMed]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, A.S. Stimuli-responsive polymers: Biomedical applications and challenges for clinical translation. Adv. Drug Deliver. Rev. 2013, 65, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Alsuraifi, A.; Curtis, A.; Lamprou, D.A.; Hoskins, C. Stimuli responsive polymeric systems for cancer therapy. Pharmaceutics 2018, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016, 23, 748–770. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Tong, L.; Feng, J.; Fu, J. Recent advances in stimuli-responsive release function drug delivery systems for tumor treatment. Molecules 2016, 21, 1715. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.; Banik, B.; Alexis, F. Stimulus responsive nanogels for drug delivery. Soft Matter 2011, 7, 5908–5916. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol-gel reversible hydrogels. Adv. Drug Deliver. Rev. 2002, 54, 37–51. [Google Scholar] [CrossRef]
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015, 10, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Gong, C.; Qi, T.; Wei, X.; Qu, Y.; Wu, Q.; Luo, F.; Qian, Z. Thermosensitive polymeric hydrogels as drug delivery systems. Curr. Med. Chem. 2013, 20, 79–94. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Leroux, J. In situ-forming hydrogels--review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef]
- Klouda, L.; Perkins, K.R.; Watson, B.M.; Hacker, M.C.; Bryant, S.J.; Raphael, R.M.; Kasper, F.K.; Mikos, A.G. Thermoresponsive, in situ crosslinkable hydrogels based on N-isopropylacrylamide: Fabrication, characterization and mesenchymal stem cell Encapsulation. Acta Biomater. 2011, 7, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Hui, P.C.-l.; Kan, C.-W. Thermoresponsive hydrogels and their biomedical applications: Special insight into their applications in textile based transdermal therapy. Polymers-Basel 2018, 10, 480. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Tang, S.; Floy, M.; Bhandari, R.; Sunkara, M.; Morris, A.J.; Dziubla, X.; Hilt, O.Z. Synthesis and characterization of thermoresponsive hydrogels Based on N-Isopropylacrylamide crosslinked with 4,4′-dihydroxybiphenyl diacrylate. ACS Omega 2017, 2, 8723–8729. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Kibbey, M.R.; Birnbaum, J.C.; Won, Y.Y.; Gutowska, A. pH-responsive hydrogels: Swelling model. Adv. Exp. Med. Biol. 2004, 553, 29–43. [Google Scholar]
- Xu, L.; Qiu, L.; Sheng, Y.; Sun, Y.; Deng, L.; Li, X.; Bradley, M.; Zhang, R. Biodegradable pH-responsive hydrogels for controlled dual-drug release. J. Mater. Chem. B. 2018, 6, 510–517. [Google Scholar] [CrossRef]
- Hibbins, A.R.; Kumar, P.; Choonara, Y.E.; Kondiah, P.P.D.; Marimuthu, T.; Toit, L.C.d.; Pillay, V. Design of a versatile pH-responsive hydrogel for potential oral delivery of gastric-sensitive bioactives. Polymers-Basel 2017, 9, 474. [Google Scholar] [CrossRef]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Yoshida, T.; Lai, T.C.; Kwon, G.S.; Sako, K. pH- and ion-sensitive polymers for drug delivery. Expert Opin. Drug Del. 2013, 10, 1497–1513. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wat, E.; Hui, P.C.L.; Chan, B.; Ng, F.S.F.; Kan, C.-W.; Wang, X.; Hu, H.; Wong, E.C.W.; Lau, C.B.S.; et al. Dual-functional transdermal drug delivery system with controllable drug loading based on thermosensitive poloxamer hydrogel for atopic dermatitis treatment. Sci. Rep. UK 2016, 6, 24112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Hui, P.C.L.; Wat, E.; Chan, B.; Ng, F.S.F.; Kan, C.-W.; Lau, C.B.S.; Leung, P.-C. Enhanced transdermal permeability via constructing the porous structure of poloxamer-based hydrogel. Polymers-Basel 2016, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hui, P.C.L.; Kan, C.W. Functionalized textile based therapy for the treatment of atopic dermatitis. Coatings 2017, 7, 82. [Google Scholar] [CrossRef]
- Wang, X.; Hu, H.; Yang, Z.; He, L.; Kong, Y.; Fei, B.; Xin, J.H. Smart hydrogel-functionalized textile system with moisture management property for skin application. Smart Mater. Struct. 2014, 23, 125027. [Google Scholar] [CrossRef]
- Hu, J.; Meng, H.; Li, G.; Ibekwe, S. A review of stimuli-responsive polymers for smart textile applications. Smart Mater. Struct. 2012, 21, 053001. [Google Scholar] [CrossRef]
- Liu, B.; Hu, J. The application of temperature-sensitive hydrogels to textiles: A review of Chinese and Japanese investigations. Fibres Text. East. Eur. 2005, 13, 45–49. [Google Scholar]
- Wang, J.; Zhong, Q.; Wu, J.; Chen, T. Thermo-responsive textiles. In Handbook of Smart Textiles; Tao, X., Ed.; Springer Science + Business Media: Singapore, 2015; ISBN 978-981-4451-46-8. [Google Scholar]
- Chatterjee, S.; Hui, P.C.-L. Stimuli-responsive hydrogels: An interdisciplinary review. In Hydrogels – Smart Materials for Biomedical Applications; Popa, L., Ghica, M.V., Dinu-Pîrvu, C.-E., Eds.; IntechOpen: London, UK, 2018; ISBN 978-1-78985-876-1. [Google Scholar]
- Chuang, C.-Y.; Don, T.-M.; Chiu, W.-Y. Synthesis of chitosan-based thermo- and pH-responsive porous nanoparticles by temperature-dependent self-assembly method and their application in drug release. J. Polym. Sci. A1. 2009, 47, 5126–5136. [Google Scholar] [CrossRef]
- Fathi, M.; Zangabad, P.S.; Majidi, S.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Stimuli-responsive chitosan-based nanocarriers for cancer therapy. Bioimpacts 2017, 7, 269–277. [Google Scholar] [CrossRef]
- Fang, J.Y.; Chen, J.P.; Leu, Y.L.; Hu, J.W. Temperature-sensitive hydrogels composed of chitosan and hyaluronic acid as injectable carriers for drug delivery. Eur. J. Pharm. Biopharm. 2008, 68, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.; Kim, J.; Vales, T.P.; Yang, S.K.; Kim, J.-K.; Sohn, H.; Kim, H.-J. Thermoresponsive drug controlled release from chitosan-based hydrogel embedded with poly(N-isopropylacrylamide) nanogels. J. Polym. Sci. A1. 2018, 56, 1907–1914. [Google Scholar] [CrossRef]
- Park, K.M.; Lee, S.Y.; Joung, Y.K.; Na, J.S.; Lee, M.C.; Park, K.D. Thermosensitive chitosan-pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater. 2009, 5, 1956–1965. [Google Scholar] [CrossRef]
- Bhattarai, N.; Matsen, F.A.; Zhang, M. PEG-grafted chitosan as an injectable thermoreversible hydrogel. Macromol. Biosci. 2005, 5, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Chen, X.G.; Kong, M.; Liu, C.S.; Cha, D.S.; Kennedy, J.F. Effect of molecular weight and degree of chitosan deacetylation on the preparation and characteristics of chitosan thermosensitive hydrogel as a delivery system. Carbohyd. Polym. 2008, 73, 265–273. [Google Scholar] [CrossRef]
- Wang, B.; Wu, X.; Li, J.; Hao, X.; Lin, J.; Cheng, D.; Lu, Y. Thermosensitive behavior and antibacterial activity of cotton fabric modified with a chitosan-poly(N-isopropylacrylamide) interpenetrating polymer network hydrogel. Polymers-Basel 2016, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Kondu, S.; Ji, H.F.; McShane, M.J. Study of the near-neutral pH-sensitivity of chitosan/gelatin hydrogels by turbidimetry and microcantilever deflection. Biotechnol. Bioeng. 2006, 95, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, Y.J.; Li, D.; Liu, Y.; Ma, Q.; Tan, Y.; Yue, Q.; Meng, F. Physically cross-linked pH-responsive chitosan-based hydrogels with enhanced mechanical performance for controlled drug delivery. RSC Adv. 2016, 6, 106035–106045. [Google Scholar] [CrossRef]
- Aycan, D.; Alemdar, N. Development of pH-responsive chitosan-based hydrogel modified with bone ash for controlled release of amoxicillin. Carbohyd. Polym. 2018, 184, 401–407. [Google Scholar] [CrossRef]
- Patel, V.R.; Amiji, M.M. Preparation and characterization of freeze-dried chitosan-poly(ethylene oxide) hydrogels for site-specific antibiotic delivery in the stomach. Pharm. Res. 1996, 13, 588–593. [Google Scholar] [CrossRef]
- Rasool, A.; Ata, S.; Islam, A. Stimuli responsive biopolymer (chitosan) based blend hydrogels for wound healing application. Carbohyd. Polym. 2019, 203, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X.A. review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Gopi, S.; Balakrishnan, P.; Geethamma, V.G.; Pius, A.; Thomas, S. Applications of cellulose nanofibrils in drug delivery. In Applications of Nanocomposite Materials in Drug Delivery; Woodhead Publishing Series in Biomaterials: Arlington, TX, USA, 2018; pp. 75–95. ISBN 9780128137581. [Google Scholar]
- Li, L.; Shan, H.; Yue, C.Y.; Lam, Y.C.; Tam, K.C.; Hu, X. Thermally induced association and dissociation of methylcellulose in aqueous solutions. Langmuir 2002, 18, 7291–7298. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, B.; Lu, W.W.; Li, X.; Zhu, D.; Yao, K.D.; Wang, Q.; Zhao, C.; Wang, C. A rapid temperature-responsive sol-gel reversible poly(N-isopropylacrylamide)-g-methylcellulose copolymer hydrogel. Biomaterials 2004, 25, 3005–3012. [Google Scholar] [CrossRef]
- Kim, J.K.; Won, Y.W.; Lim, K.S.; Kim, Y.H. Low-molecular-weight methylcellulose-based thermo-reversible gel/pluronic micelle combination system for local and sustained docetaxel delivery. Pharmaceut. Res. 2012, 2, 525–534. [Google Scholar] [CrossRef]
- Tomsic, M.; Prossnigg, F.; Glatter, O. A thermoreversible double gel: Characterization of a methylcellulose and kappa-carrageenan mixed system in water by SAXS, DSC and rheology. J. Colloid Interface Sci. 2008, 322, 41–50. [Google Scholar] [CrossRef]
- Oğuz, Ö.D.; Ege, D. Rheological and mechanical properties of thermoresponsive methylcellulose/calcium phosphate-based injectable bone substitutes. Materials 2018, 11, 604. [Google Scholar] [CrossRef]
- Tang, Y.F.; Wang, X.Y.; Li, Y.; Lei, M.; Du, Y.; Kennedy, J.F.; Knill, C.J. Production and characterisation of novel injectable chitosan/methylcellulose/salt blend hydrogels with potential application as tissue engineering scaffolds. Carbohyd. Polym. 2010, 82, 833–841. [Google Scholar] [CrossRef]
- Nayak, A.; Babla, H.; Han, T.; Das, D.B. Lidocaine carboxymethylcellulose with gelatine co-polymer hydrogel delivery by combined microneedle and ultrasound. Drug Deliv. 2016, 23, 668–679. [Google Scholar] [CrossRef]
- Liu, H.; Rong, L.; Wang, B.; Xie, R.; Sui, X.; Xu, H.; Zhang, L. Facile fabrication of redox/pH dual stimuli responsive cellulose hydrogel. Carbohyd. Polym. 2017, 176, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; He, M.; Zhou, J.; Zhang, L. Swelling behaviors of pH- and salt-responsive cellulose-based hydrogels. Macromolecules 2011, 44, 1642–1648. [Google Scholar] [CrossRef]
- Dutta, S.; Samanta, P.; Dhara, D. Temperature, pH and redox responsive cellulose based hydrogels for protein delivery. Int. J. Biol. Macromol. 2016, 87, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.S.; Kong, B.J.; Park, S.N. Physicochemical properties of pH-sensitive hydrogels based on hydroxyethyl cellulose–hyaluronic acid and for applications as transdermal delivery systems for skin lesions. Eur. J. Pharm. Biopharm. 2015, 92, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Buschle-Diller, G.; Wu, Y. Thermoresponsive hydrogels from BSA esterified with low molecular weight PEG. J. Appl. Polym. Sci. 2014, 131, 40946. [Google Scholar] [CrossRef]
- Iemma, F.; Spizzirri, U.G.; Puoci, F.; Cirillo, G.; Curcio, M.; Parisi, O.I.; Picci, N. Synthesis and release profile analysis of thermo-sensitive albumin hydrogels. Colloid. Polym. Sci. 2009, 287, 779–787. [Google Scholar] [CrossRef]
- Lou, J.; Hu, W.; Tian, R.; Zhang, H.; Jia, Y.; Zhang, J.; Zhang, L. Optimization and evaluation of a thermoresponsive ophthalmic in situ gel containing curcumin-loaded albumin nanoparticles. Int. J. Nanomed. 2014, 9, 2517–2525. [Google Scholar]
- Raja, S.T.K.; Thiruselvi, T.; Mandal, A.B.; Gnanamani, A. pH and redox sensitive albumin hydrogel: A self-derived biomaterial. Sci. Rep. UK. 2015, 5, 15977. [Google Scholar] [CrossRef]
- El-Sherif, H.; El-Masry, M.; Taleb, M.F.A. pH-sensitive hydrogels based on bovine serum albumin for anticancer drug delivery. J. Appl. Polym. Sci. 2010, 115, 2050–2059. [Google Scholar] [CrossRef]
- Iemma, F.; Spizzirri, U.G.; Puoci, F.; Muzzalupo, R.; Trombino, S.; Cassano, R.; Leta, S.; Picci, N. pH-sensitive hydrogels based on bovine serum albumin for oral drug delivery. Int. J. Pharmaceut. 2006, 312, 151–157. [Google Scholar] [CrossRef]
- Oliveira, K.A.L.d.; Sitta, D.L.A.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Design of pH-responsive albumin-alginate hydrogels for drug delivery. J. Control. Release 2017, 259, e5. [Google Scholar] [CrossRef]
- Pinho, E.; Soares, G. Functionalization of cotton cellulose for improved wound healing. J. Mater. Chem. B 2018, 6, 1887–1898. [Google Scholar] [CrossRef]
- Asghar, A.; Henrickson, R.L. Chemical, biochemical, functional, and nutritional characteristics of collagen in food systems. Adv. Food Res. 1982, 28, 231–372. [Google Scholar] [PubMed]
- Gandhi, S.S.; Yan, H.; Kim, C. Thermoresponsive gelatin nanogels. ACS Macro Lett. 2014, 3, 1210–1214. [Google Scholar] [CrossRef]
- Chang, Y.; Xiao, L.; Tang, Q. Preparation and characterization of a novel thermosensitive hydrogel based on chitosan and gelatin blends. J. Appl. Polym. Sci. 2009, 113, 400–407. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Yang, S.-H.; Su, W.-Y.; Chen, Y.C.; Yang, K.C.; Cheng, W.T.; Wu, S.C.; Lin, F.H. Thermosensitive chitosan-gelatin-glycerol phosphate hydrogels as a cell carrier for nucleus pulposus regeneration: An in vitro study. Tissue Eng. Pt. A 2010, 16, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Criado, M.; Rey, J.M.; Mijangos, C.M.; Hernández, R. Double-membrane thermoresponsive hydrogels from gelatin and chondroitin sulphate with enhanced mechanical properties. RSC Adv. 2016, 6, 105821–105826. [Google Scholar] [CrossRef]
- Curcio, M.; Altimari, I.; Spizzirri, U.G.; Cirillo, G.; Vittorio, O.; Puoci, F.; Picci, N.; Iemma, F. Biodegradable gelatin-based nanospheres as pH-responsive drug delivery systems. J. Nanopart. Res. 2013, 15, 1581. [Google Scholar] [CrossRef]
- Raafat, A.I. Gelatin based pH-sensitive hydrogels for colon-specific oral drug delivery: Synthesis, characterization, and in vitro release study. J. Appl. Polym. Sci. 2010, 118, 2642–2649. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Mohan, A.M. Novel pH sensitive dual drug loaded-gelatin methacrylate/methacrylic acid hydrogel for the controlled release of antibiotics. Int. J. Biol. Macromol. 2018, 110, 167–178. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Mohan, A.M. Novel pH switchable gelatin based hydrogel for the controlled delivery of the anticancer drug 5-fluorouracil. RSC Adv. 2014, 4, 12109–12118. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K. Recent progress in biomedical applications of pluronic (PF127): Pharmaceutical perspectives. J. Control. Release 2015, 209, 120–138. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.R.; Park, T.G. Temperature-responsive and degradable hyaluronic acid/pluronic composite hydrogels for controlled release of human growth hormone. J. Control. Release 2002, 80, 69–77. [Google Scholar] [CrossRef]
- Hsu, S.H.; Leu, Y.L.; Hu, J.W.; Fang, J.Y. Physicochemical characterization and drug release of thermosensitive hydrogels composed of a hyaluronic acid/pluronic f127 graft. Chem. Pharm. Bull. 2009, 57, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yao, P. Versatile injectable supramolecular hydrogels containing drug loaded micelles for delivery of various drugs. Polym. Chem. 2014, 5, 1072–1081. [Google Scholar] [CrossRef]
- Gong, C.Y.; Shi, S.; Dong, P.W.; Zheng, X.L.; Fu, S.Z.; Guo, G.; Yang, J.L.; Wei, Y.Q.; Qian, Z.Y. In vitro drug release behavior from a novel thermosensitive composite hydrogel based on Pluronic f127 and poly(ethylene glycol)-poly(ε-caprolactone)-poly(ethylene glycol) copolymer. BMC Biotechnol. 2009, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Rokhade, A.P.; Shelke, N.B.; Patil, S.A.; Aminabhavi, T.M. Novel hydrogel microspheres of chitosan and pluronic F-127 for controlled release of 5-fluorouracil. J. Microencapsul. 2007, 24, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Khateb, K.A.; Ozhmukhametova, E.K.; Mussin, M.N.; Seilkhanov, S.K.; Rakhypbekov, T.K.; Lau, W.M.; Khutoryanskiy, V.V. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int. J. Pharm. 2016, 502, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Fang, C.L.; Al-Suwayeh, S.A.; Leu, Y.L.; Fang, J.Y. Transdermal delivery of selegiline from alginate-pluronic composite thermogels. Int. J. Pharm. 2011, 415, 119–128. [Google Scholar] [CrossRef]
- Hacker, M.C.; Klouda, L.; Ma, B.B.; Kretlow, J.D.; Mikos, A.G. Synthesis and characterization of injectable, thermally and chemically gelable, amphiphilic poly(N-isopropylacrylamide)-based macromers. Biomacromolecules 2008, 9, 1558–1570. [Google Scholar] [CrossRef]
- Moghadam, S.; Larson, R.G. Assessing the efficacy of poly(N-isopropylacrylamide) for drug delivery applications using molecular dynamics simulations. Mol. Pharm. 2017, 14, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Gulyuz, U.; Okay, O. Self-healing poly(N-isopropylacrylamide) hydrogels. Eur. Polym. J. 2015, 72, 12–22. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Aloorkar, N.H. Smart polymers in drug delivery: An overview. J. Pharm. Res. 2010, 3, 100–108. [Google Scholar]
- Yin, X.; Hoffman, A.S.; Stayton, P.S. Poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers that respond sharply to temperature and pH. Biomacromolecules 2006, 7, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, Y.; Yoshida, R.; Sakai, K.; Okano, T.; Sakurai, Y. Swelling controlled zero-order and sigmoidal drug release from thermoresponsive poly(N-isopropylacrylamide-co-butyl methacrylate) hydrogel. J. Biomater. Sci. Polym. Ed. 1993, 4, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Song, X.; Wen, Y.; Zhu, J.L.; Li, J. Injectable thermoresponsive hydrogel formed by alginate-g-poly(N-isopropylacrylamide) that releases doxorubicin-encapsulated micelles as a smart drug delivery system. ACS Appl. Mater. Interfaces 2017, 9, 35673–35682. [Google Scholar] [CrossRef] [PubMed]
- Zubik, K.; Singhsa, P.; Wang, Y.; Manuspiya, H.; Narain, R. Thermo-responsive poly(N-Isopropylacrylamide)- cellulose nanocrystals hybrid hydrogels for wound dressing. Polymers-Basel 2017, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Derwent, J.J.K.; Mieler, W.F. Thermoresponsive hydrogels as a new ocular drug delivery platform to the posterior segment of the eye. Trans. Am. Ophthalmol. Soc. 2008, 106, 206–214. [Google Scholar]
- Schiphorst, J.T.; Broek, M.v.d.; Koning, T.d.; Murphy, J.N.; Schenning, A.P.H.J.; Esteves, A.C.C. Dual light and temperature responsive cotton fabric functionalized with a surface-grafted spiropyran-NIPAAm-hydrogel. J. Mater. Chem. A 2016, 4, 8676–8681. [Google Scholar] [CrossRef]
- Bashari, A.; Hemmatinejad, N.; Pourjavadi, A. Surface modification of cotton fabric with dual-responsive PNIPAAm/chitosan nano hydrogel. Polym. Adv. Technol. 2013, 24, 797–806. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Q.; Wang, T. Thermoresponsive PNIPAAm-modified cotton fabric surfaces that switch between superhydrophilicity and superhydrophobicity. Appl. Surf. Sci. 2012, 258, 4888–4892. [Google Scholar] [CrossRef]
- Huang, H.; Hou, L.; Zhu, F.; Li, J.; Xu, M. Controllable thermal and pH responsive behavior of PEG based hydrogels and applications for dye adsorption and release. RSC Adv. 2018, 8, 9334–9343. [Google Scholar] [CrossRef] [Green Version]
- Barkat, K.; Ahmad, M.; Minhas, M.U.; Khakid, I.; Nasir, B. Development and characterization of pH-responsive polyethylene glycol-co-poly(methacrylic acid)polymeric network system for colon target delivery of oxaliplatin: Its acute oral toxicity study. Adv. Polym. Technol. 2018, 37, 1806–1822. [Google Scholar] [CrossRef]
- Li, L.; Gu, J.; Zhang, J.; Xie, Z.; Lu, Y.; Shen, L.; Dong, Q.; Wang, Y. Injectable and biodegradable pH-responsive hydrogels for localized and sustained treatment of human fibrosarcoma. ACS Appl. Mater. Interfaces 2015, 7, 8033–8040. [Google Scholar] [CrossRef] [PubMed]
- Pourmoazzen, Z.; Bagheri, M.; Entezami, A.A.; Koshki, K.N. pH-responsive micelles composed of poly(ethylene glycol) and cholesterol-modified poly(monomethyl itaconate) as a nanocarrier for controlled and targeted release of piroxicam. J. Polym. Res. 2013, 20, 295. [Google Scholar] [CrossRef]
- Betancourt, T.; Pardo, J.; Soo, K.; Peppas, N.A. Characterization of pH-responsive hydrogels of poly(itaconic acid-g-ethylene glycol) prepared by UV-initiated free radical polymerization as biomaterials for oral delivery of bioactive agents. J. Biomed. Mater. Res. A. 2010, 93, 175–188. [Google Scholar] [CrossRef]
- Liu, R.; Li, D.; He, B.; Xu, X.; Sheng, M.; Lai, Y.; Wang, G.; Gu, Z. Anti-tumor drug delivery of pH-sensitive poly(ethylene glycol)-poly(L-histidine-)-poly(L-lactide) nanoparticles. J. Control. Release 2011, 152, 49–56. [Google Scholar] [CrossRef]
- Xiao, L.; Huang, L.; Moingeon, F.; Gauthier, M.; Yang, G. pH-Responsive poly(ethyleneglycol)-block-polylactide micelles for tumor-targeted drug delivery. Biomacromolecules 2017, 18, 2711–2722. [Google Scholar] [CrossRef]
- Gupta, B.; Arora, A.; Saxena, S.; Alam, M.S. Preparation of chitosan-polyethylene glycol coated cotton membranes for wound dressings: Preparation and characterization. Polym. Adv. Technol. 2009, 20, 58–65. [Google Scholar] [CrossRef]
- Swift, T.; Swanson, L.; Geoghegan, M.; Rimmer, S. The pH-responsive behaviour opoly(acrylic acid) in aqueous solution is dependent on molar mass. Soft Matter 2016, 12, 2542–2549. [Google Scholar] [CrossRef]
- Bromberg, L. Intelligent hydrogels for the oral delivery of chemotherapeutics. Expert. Opin. Drug Deliv. 2005, 2, 1003–1013. [Google Scholar] [CrossRef]
- Liu, L.; Yao, W.D.; Rao, Y.F.; Lu, X.Y.; Gao, J.Q. pH-Responsive carriers for oral drug delivery: Challenges and opportunities of current platforms. Drug Deliv. 2017, 24, 569–581. [Google Scholar] [CrossRef]
- Gao, X.; He, C.; Xiao, C.; Zhuang, X.; Chen, X. Synthesis and characterization of biodegradable pH-sensitive poly(acrylic acid) hydrogels crosslinked by 2-hydroxyethyl methacrylate modified poly(l-glutamic acid). Mater. Lett. 2012, 77, 74–77. [Google Scholar] [CrossRef]
- Li, G.; Song, S.; Guo, L.; Ma, S. Self-Assembly of thermo- and pH-responsive poly(acrylic acid)-b-poly(N-isopropylacrylamide) micelles for drug delivery. J. Polym. Sci. A1. 2008, 46, 5028–5035. [Google Scholar] [CrossRef]
- Tian, B.; Liu, S.; Wu, S.; Lu, W.; Wang, D.; Jin, L.; Hu, B.; Li, K.; Wang, Z.; Quan, Z. pH-responsive poly (acrylic acid)-gated mesoporous silica and its application in oral colon targeted drug delivery for doxorubicin. Colloid. Surface B 2017, 154, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; He, C.; Xiao, C.; Zhuang, X.; Chen, X. Biodegradable pH-responsive polyacrylic acid derivative hydrogels with tunable swelling behavior for oral delivery of insulin. Polymer 2013, 54, 1786–1793. [Google Scholar] [CrossRef]
- Pasche, S.; Angeloni, S.; Ischer, R.; Liley, M.; Luprano, J.; Voirin, G. Wearable biosensors for monitoring wound healing. Adv. Sci. Technol. 2008, 57, 80–87. [Google Scholar] [CrossRef]
- Deen, G.R.; Loh, X.J. Stimuli-responsive cationic hydrogels in drug delivery applications. Gels 2018, 4, 13. [Google Scholar] [CrossRef]
- Lim, Y.B.; Kim, S.M.; Lee, Y.; Lee, W.K.; Yang, T.G.; Lee, M.J.; Suh, H.; Park, J.S. Cationic hyperbranched poly(amino ester): A novel class of DNA condensing molecule with cationic surface, biodegradable three-dimensional structure, and tertiary amine group in the interior. J. Am. Chem. Soc. 2001, 123, 2460–2461. [Google Scholar] [CrossRef]
- Car, A.; Baumann, P.; Duskey, J.T.; Chami, M.; Bruns, N.; Meier, W. pH-responsive PDMS-b-PDMAEMA micelles for intracellular anticancer drug delivery. Biomacromolecules 2014, 15, 3235–3245. [Google Scholar] [CrossRef]
- Wu, W.; Liu, J.; Cao, S.; Tan, H.; Li, J.; Xu, F.; Zhang, X. Drug release behaviors of a pH sensitive semi-interpenetrating polymer network hydrogel composed of poly(vinylalcohol) and star poly 2-(dimethylamino)ethyl methacrylate. Int. J. Pharmaceut. 2011, 416, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Oishi, M.; Hayashi, H.; Iijima, M.; Nagasaki, Y. Endosomal release and intracellular delivery of anticancer drugs using pH-sensitive PEGylated nanogels. J. Mater. Chem. 2007, 17, 3720–3725. [Google Scholar] [CrossRef]
- Wang, D.; Tan, J.; Kang, H.; Ma, L.; Jin, X.; Liu, R.; Huang, Y. Synthesis, self-assembly and drug release behaviors of pH-responsive copolymers ethyl cellulose-graft-PDEAEMA through ATRP. Carbohyd. Polym. 2011, 84, 195–202. [Google Scholar] [CrossRef]
- Ou, K.; Wu, X.; Wang, B.; Meng, C.; Dong, X.; He, J. Controlled in situ graft polymerization of DMAEMA onto cotton surface via SI-ARGET ATRP for low-adherent wound dressings. Cellulose 2017, 24, 5211–5224. [Google Scholar] [CrossRef]
- Akhgari, A.; Tavakol, A. Prediction of optimum combination of Eudragit RS/Eudragit RL/ethyl cellulose polymeric free films based on experimental design for using as a coating system for sustained release theophylline pellets. Adv. Pharm. Bull. 2016, 6, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Cetin, M.; Atila, A.; Kadioglu, Y. Formulation and in vitro characterization of Eudragit® L100 andEudragit® L100-PLGA nanoparticles containing diclofenac sodium. AAPS Pharm. Sci. Tech. 2010, 11, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Mady, O. Ibuprofen encapsulation by Eudragit RS100 as microspheres: Preparation and drug release. MOJ Bioequiv. Availab. 2017, 4, 193–199. [Google Scholar] [CrossRef]
- Leo, V.D.; Milano, F.; Mancini, E.; Comparelli, R.; Giotta, L.; Nacci, A.; Longobardi, F.; Garbetta, A.; Agostiano, A.; Catucci, L. Encapsulation of curcumin-loaded liposomes for colonic drug delivery in a pH-responsive polymer cluster using a pH-driven and organic solvent-free process. Molecules 2018, 23, 739. [Google Scholar] [CrossRef]
- Tipre, D.; Vavia, P. Formulation optimization and stability study of transdermal therapeutic system of nicorandil. Pharm. Dev. Technol. 2002, 7, 325–332. [Google Scholar] [CrossRef]





| Polymer | Chemical Nature | Type of Stimuli-Responsiveness | Biomedical Applications |
|---|---|---|---|
| Chitosan | Natural (polysaccharide) | Thermo-responsive pH-responsive | Drug delivery, tissue engineering, textile application |
| Cellulose | Natural (polysaccharide) | Thermo-responsive pH-responsive | Drug delivery, tissue engineering, textile application |
| Albumin | Natural (polypeptide) | Thermo-responsive pH-responsive | Drug delivery, tissue engineering, textile application |
| Gelatin | Natural (polypeptide) | Thermo-responsive pH-responsive | Drug delivery, tissue engineering, textile application |
| PF127 | Synthetic | Thermo-responsive, in-situ gel formation | Drug delivery, textile based transdermal therapy |
| pNIPAAm | Synthetic | Thermo-responsive, in-situ gel formation | Drug delivery, textile application |
| PEO | Synthetic | pH-responsive (neutral) | Drug delivery, textile application |
| PAA | Synthetic | pH-responsive (anionic) | Drug delivery, textile application |
| PDMAEMA/PDEAEMA | Synthetic | pH-responsive (cationic) | Drug delivery, textile application |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
CHATTERJEE, S.; Chi-leung HUI, P. Review of Stimuli-Responsive Polymers in Drug Delivery and Textile Application. Molecules 2019, 24, 2547. https://doi.org/10.3390/molecules24142547
CHATTERJEE S, Chi-leung HUI P. Review of Stimuli-Responsive Polymers in Drug Delivery and Textile Application. Molecules. 2019; 24(14):2547. https://doi.org/10.3390/molecules24142547
Chicago/Turabian StyleCHATTERJEE, Sudipta, and Patrick Chi-leung HUI. 2019. "Review of Stimuli-Responsive Polymers in Drug Delivery and Textile Application" Molecules 24, no. 14: 2547. https://doi.org/10.3390/molecules24142547
APA StyleCHATTERJEE, S., & Chi-leung HUI, P. (2019). Review of Stimuli-Responsive Polymers in Drug Delivery and Textile Application. Molecules, 24(14), 2547. https://doi.org/10.3390/molecules24142547