Lipidomic Profiling of the Olive (Olea europaea L.) Fruit towards Its Valorisation as a Functional Food: In-Depth Identification of Triacylglycerols and Polar Lipids in Portuguese Olives
Abstract
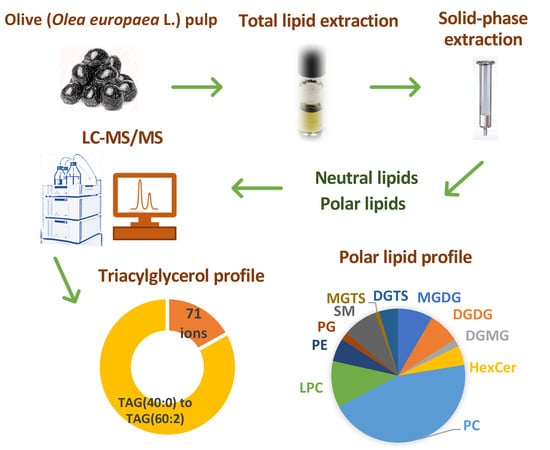
:1. Introduction
2. Results and Discussion
2.1. Triacylglycerol Profile
2.2. Polar Lipid Profile
2.2.1. Phospholipids
2.2.2. Glycosphingolipids
2.2.3. Glycolipids
2.2.4. Betaine Lipids
3. Materials and Methods
3.1. Reagents
3.2. Sampling and Sample Preparation
3.3. Total Lipid Extraction
3.4. Total Lipid Fractionation
3.5. HPLC-MS and HPLC-MS/MS Analysis of the Neutral Lipid Fraction
3.6. HPLC-MS and HPLC-MS/MS Analysis of the Polar Lipid Fraction
3.7. Data Analysis
4. Conclusions and Future Perspective
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Olive Council. World table olive figures—consumption. Available online: http://www.internationaloliveoil.org/estaticos/view/132-world-table-olive-figures (accessed on 20 January 2019).
- Grounta, A.; Tassou, C.C.; Panagou, E.Z. Greek-style table olives and their functional value. In Olives and Olive Oil as Functional Foods: Bioactivity, Chemistry and Processing, 1st ed.; Shahidi, F., Kiritsakis, A., Eds.; Wiley: Oxford, UK, 2017; pp. 325–342. [Google Scholar]
- Boskou, D.; Camposeo, S.; Clodoveo, M.L. Table olives as sources of bioactive compunds. In Olive and Olive Oil Bioactive Constituents; Boskou, D., Ed.; AOCS Press: Urbana, IL, USA, 2015; pp. 215–259. [Google Scholar]
- Bianchi, G. Lipids and phenols in table olives. Eur. J. Lipid Sci. Technol. 2003, 105, 229–242. [Google Scholar] [CrossRef]
- Bianchi, G.; Vlahov, G. Composition of the lipid classes in the morphologically different parts of the olive fruit, cv. Coratina (Olea europaea Linn.) Fett/Lipid 1994, 96, 72–77. [Google Scholar] [CrossRef]
- Sakouhi, F.; Absalon, C.; Kallel, H.; Boukhchina, S. Comparative analysis of triacylglycerols from Olea europaea L. fruits using HPLC and MALDI-TOFMS. Eur. J. Lipid Sci. Technol. 2010, 112, 574–579. [Google Scholar] [CrossRef]
- Vlahov, G.; Schiavone, C.; Simone, N. Triacylglycerols of the olive fruit (Olea europaea L.): Characterization of mesocarp and seed triacylglycerols in different cultivars by liquid chromatography and 13C NMR spectroscopy. Fett/Lipid 1999, 101, 146–150. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids and risk of cardiovascular disease: Synopsis of the evidence available from systematic reviews and meta-analyses. Nutrients 2012, 4, 1989–2007. [Google Scholar] [CrossRef] [PubMed]
- Takenaga, F.; Matsuyama, K.; Abe, S.; Torii, Y.; Itoh, S. Lipid and fatty acid composition of mesocarp and seed of avocado fruits harvested at northern range in Japan. J. Oleo Sci. 2008, 57, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Pacetti, D.; Boselli, E.; Lucci, P.; Frega, N. Simultaneous analysis of glycolipids and phospholids molecular species in avocado (Persea americana Mill) fruit. J. Chromatogr. A. 2007, 1150, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Domingues, M.R.M.; Domingues, P. Polar lipids from olives and olive oil: A review on their identification, significance and potential biotechnological applications. Foods 2018, 7, 109. [Google Scholar] [CrossRef]
- Moussaoui, R.; Labbaci, W.; Hemar, N.; Youyou, A.; Amir, Y. Physico-chemical characteristics of oils extracted from three compartments of the olive fruit (pulp, endocarp and seed) of variety Chemlal cultivated in Kabylia (Algeria). J. Food Agric. Environ. 2008, 6, 52–55. [Google Scholar]
- Bianco, A.; Mazzei, R.; Melchioni, C.; Scarpati, M.; Romeo, G.; Uccella, N. Microcomponents of olive oil. Part II: Digalactosyldiacylglycerols from Olea europaea. Food Chem. 1998, 62, 343–346. [Google Scholar] [CrossRef]
- Sun, N.; Chen, J.; Wang, D.; Lin, S. Advance in food-derived phospholipids: Sources, molecular species and structure as well as their biological activities. Trends Food Sci. Technol. 2018, 80, 199–211. [Google Scholar] [CrossRef]
- Larsen, E.; Christensen, L. Common vegetables and fruits as a source of 1,2-di-O-α-linolenoyl-3-O-beta-d-galactopyranosyl-sn-glycerol, a potential anti-inflammatory and antitumor agent. J. Food Lipids 2007, 14, 272–279. [Google Scholar] [CrossRef]
- Hou, C.; Chen, Y.; Wu, J.; Huang, C.; Wang, S.; Yang, N.; Shyur, L. A galactolipid possesses novel cancer chemopreventive effects by suppressing inflammatory mediators and mouse b16 melanoma. Cancer Res. 2007, 67, 6907–6915. [Google Scholar] [CrossRef] [PubMed]
- Tsantila, N.; Karantonis, H.; Perrea, D.; Theocharis, S.; Iliopoulos, D.; Antonopoulou, S.; Demopoulos, C. Antithrombotic and antiatherosclerotic properties of olive oil and olive pomace polar extracts in rabbits. Med. Inflamm. 2007, 2007, 11. [Google Scholar] [CrossRef] [PubMed]
- Karantonis, H.; Antonopoulou, S.; Demopoulos, C. Antithrombotic lipid minor constituents from vegetable oils. Comparison between olive oils and others. J. Agric. Food Chem. 2002, 50, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.C. Tandem Mass Spectrometry of Lipids: Molecular Analysis of Complex Lipids; Royal Society of Chemistry: Cambridge, UK, 2014. [Google Scholar]
- Mu, H.; Porsgaard, T. The metabolism of structured triacylglycerols. Prog. Lipid Res. 2005, 44, 430–448. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.E. Triacylglycerol structure and interesterification of palmitic and stearic acid-rich fats: An overview and implications for cardiovascular disease. Nutr. Res. Rev. 2009, 22, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Zaeem, M.; Fillier, T.A.; Nadeem, M.; Vidal, N.P.; Manful, C.; Cheema, S.; Cheema, M.; Thomas, R.H. Targeting modified lipids during routine lipidomics analysis using HILIC and C30 reverse phase liquid chromatography coupled to mass spectrometry. Sci. Rep. 2019, 9, 5048. [Google Scholar] [CrossRef]
- Lee, J.; Nagai, T.; Gotoh, N.; Fukusaki, E.; Bamba, T. Profiling of regioisomeric triacylglycerols in edible oils by supercritical fluid chromatography/tandem mass spectrometry. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2014, 966, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Narvaez-Rivas, M.; Zhang, Q. Comprehensive untargeted lipidomic analysis using core-shell C30 particle column and high field orbitrap mass spectrometer. J. Chromatogr. A. 2016, 1440, 123–134. [Google Scholar] [CrossRef]
- Ranalli, A.; Pollastri, L.; Contento, S.; Di Loreto, G.; Iannucci, E.; Lucera, L.; Russi, F. Acylglycerol and fatty acid components of pulp, seed, and whole olive fruit oils. Their use to characterize fruit variety by chemometrics. J. Agric. Food Chem. 2002, 50, 3775–3779. [Google Scholar] [CrossRef] [PubMed]
- Lisa, M.; Holcapek, M. Triacylglycerols profiling in plant oils important in food industry, dietetics and cosmetics using high-performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A. 2008, 1198, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.; Moreira, A.S.P.; Rey, F.; da Costa, E.; Melo, T.; Maciel, E.; Rego, A.; Abreu, M.H.; Domingues, P.; Calado, R.; et al. Lipidomic signature of the green macroalgae Ulva rigida farmed in a sustainable integrated multi-trophic aquaculture. J. Appl. Phycol. 2019, 31, 1369–1381. [Google Scholar] [CrossRef]
- Lopes, C.; Barbosa, J.; Maciel, E.; da Costa, E.; Alves, E.; Domingues, P.; Mendo, S.; Domingues, M.R.M. Lipidomic signature of Bacillus licheniformis I89 during the different growth phases unravelled by high-resolution liquid chromatography-mass spectrometry. Arch. Biochem. Biophys. 2019, 664, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Montealegre, C.; Sanchez-Hernandez, L.; Crego, A.; Marina, M. Determination and characterization of glycerophospholipids in olive fruit and oil by nonaqueous capillary electrophoresis with electrospray-mass spectrometric detection. J. Agric. Food Chem. 2013, 61, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Mancha, M. Fosfolípidos y glicolípidos de la pulpa de aceitunas maduras. Grasas Aceites (Sevilla, Spain) 1974, 159–164. [Google Scholar]
- Vioque, E.; Maza, M. Glicerolípidos de la aceituna. Grasas Aceites (Sevilla, Spain) 1973, 24, 226–235. [Google Scholar]
- Fedeli, E. Lipids of olives. Prog. Chem. Fats Other Lipids 1977, 15, 57–74. [Google Scholar] [CrossRef]
- Castro-Gomez, P.; Garcia-Serrano, A.; Visioli, F.; Fontecha, J. Relevance of dietary glycerophospholipids and sphingolipids to human health. Prostaglandins Leukot. Essent. Fatty Acids 2015, 101, 41–51. [Google Scholar] [CrossRef]
- Küllenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11, 3. [Google Scholar] [CrossRef]
- Schmelz, E.; Dillehay, D.; Webb, S.; Reiter, A.; Adams, J.; Merrill, A. Sphingomyelin consumption suppresses aberrant colonic crypt foci and increases the proportion of adenomas versus adenocarcinomas in CF1 mice treated with 1,2-dimethylhydrazine: Implications for dietary sphingolipids and colon carcinogenesis. Cancer Res. 1996, 56, 4936–4941. [Google Scholar] [PubMed]
- Vesper, H.; Schmelz, E.; Nikolova-Karakashian, M.; Dillehay, D.; Lynch, D.; Merrill, A. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J. Nutr. 1999, 129, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Ho, C.; Sang, S.; Rosen, R. Determination of sphingolipids in nuts and seeds by a single quadrupole liquid chromatography-mass spectrometry method. J. Food Lipids 2005, 12, 327–343. [Google Scholar] [CrossRef]
- Barreto-Bergter, E.; Sassaki, G.L.; de Souza, L.M. Structural analysis of fungal cerebrosides. Front. Microbiol. 2011, 2, 239. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, B. Cerebrosides in mature-green and red-ripe bell pepper and tomato fruits. Phytochemistry 1996, 42, 627–632. [Google Scholar] [CrossRef]
- Garcia-Barros, M.; Coant, N.; Truman, J.; Snider, A.; Hannun, Y. Sphingolipids in colon cancer. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 2014, 1841, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Symolon, H.; Schmelz, E.; Dillehay, D.; Merrill, A. Dietary soy sphingolipids suppress tumorigenesis and gene expression in 1,2-dimethylhydrazine-treated CF1 mice and Apc(Min/+) mice. J. Nutr. 2004, 134, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Aida, K.; Takakuwa, N.; Kinoshita, M.; Sugawara, T.; Imai, H.; Ono, J.; Ohnishi, M.; Murata, N.; Yamada, M.; Nishida, I.; et al. Properties and physiological effects of plant cerebroside species as functional lipids. Adv. Res. Plant Lipids 2003, 233–236. [Google Scholar]
- Duivenvoorden, I.; Voshol, P.J.; Rensen, P.C.; van Duyvenvoorde, W.; Romijn, J.A.; Emeis, J.J.; Havekes, L.M.; Nieuwenhuizen, W.F. Dietary sphingolipids lower plasma cholesterol and triacylglycerol and prevent liver steatosis in APOE*3Leiden mice. Am. J. Clin. Nutr. 2006, 84, 312–321. [Google Scholar] [CrossRef]
- Tsuji, K.; Mitsutake, S.; Ishikawa, J.; Takagi, Y.; Akiyama, M.; Shimizu, H.; Tomiyama, T.; Igarashi, Y. Dietary glucosylceramide improves skin barrier function in hairless mice. J. Dermatol. Sci. 2006, 44, 101–107. [Google Scholar] [CrossRef]
- Ma, Y.; Kind, T.; Yang, D.; Leon, C.; Fiehn, O. MS2Analyzer: A software for small molecule substructure annotations from accurate tandem mass spectra. Anal. Chem. 2014, 86, 10724–10731. [Google Scholar] [CrossRef] [PubMed]
- Murakami, C.; Kumagai, T.; Hada, T.; Kanekazu, U.; Nakazawa, S.; Kamisuki, S.; Maeda, N.; Xu, X.; Yoshida, H.; Sugawara, F.; et al. Effects of glycolipids from spinach on mammalian DNA polymerases. Biochem. Pharmacol. 2003, 65, 259–267. [Google Scholar] [CrossRef]
- Akasaka, H.; Mizushina, Y.; Yoshida, K.; Ejima, Y.; Mukumoto, N.; Wang, T.; Inubushi, S.; Nakayama, M.; Wakahara, Y.; Sasaki, R. MGDG extracted from spinach enhances the cytotoxicity of radiation in pancreatic cancer cells. Radiat. Oncol. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Larsen, E.; Kharazmi, A.; Christensen, L.P.; Christensen, S.B. An antiinflammatory galactolipid from rose hip (Rosa canina) that inhibits chemotaxis of human peripheral blood neutrophils in vitro. J. Nat. Prod. 2003, 66, 994–995. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P. Galactolipids as potential health promoting compounds in vegetable foods. Recent Pat. Food Nutr. Agric. 2009, 1, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Benning, C.; Huang, Z.H.; Gage, D.A. Accumulation of a novel glycolipid and a betaine lipid in cells of Rhodobacter sphaeroides grown under phosphate limitation. Arch. Biochem. Biophys. 1995, 317, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V. Betaine ether-linked glycerolipids: Chemistry and biology. Prog. Lipid Res. 1996, 35, 1–51. [Google Scholar] [CrossRef]
- Alves, E.; Melo, T.; Rey, F.; Moreira, A.S.F.; Domingues, P.; Domingues, M.R. Polar lipid profiling of olive oils as a useful tool in helping to decipher their unique fingerprint. LWT Food Sci. Technol. 2016, 74, 371–377. [Google Scholar] [CrossRef]
- Du, J.; Shen, L.; Tan, Z.; Zhang, P.; Zhao, X.; Xu, Y.; Gan, M.; Yang, Q.; Ma, J.; Jiang, A.; et al. Betaine supplementation enhances lipid metabolism and improves insulin resistance in mice fed a high-fat diet. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Airaksinen, K.; Jokkala, J.; Ahonen, I.; Auriola, S.; Kolehmainen, M.; Hanhineva, K.; Tiihonen, K. High-fat diet, betaine, and polydextrose induce changes in adipose tissue inflammation and metabolism in C57BL/6J Mice. Mol. Nutr. Food Res. 2018, 62, e1800455. [Google Scholar] [CrossRef]
- Tiihonen, K.; Saarinen, M.; Alhoniemi, E.; Mitsuya, N.; Yamaki, G. Effect of dietary betaine on metabolic syndrome risk factors in Asian males with mild fatty liver. J. Diabetes Metab. 2016, 7. [Google Scholar] [CrossRef]
- Badami, R.; Patil, K. Structure and occurrence of unusual fatty acids in minor seed oils. Prog. Lipid Res. 1980, 19, 119–153. [Google Scholar] [CrossRef]
- Koiwai, A.; Matsuzaki, T. Hydroxy and normal fatty-acid distribution in stigmas of Nicotiana and other plants. Phytochemistry 1988, 27, 2827–2830. [Google Scholar] [CrossRef]
- Christie, W. The LipidWeb. Fatty acids: Hydroxy and other oxygenated. Available online: http://www.lipidhome.co.uk/lipids/fa-eic/fa-oxy/index.htm (accessed on 28 June 2019).
- Cyberlipid. Hydroxy fatty acids. Available online: http://cyberlipid.gerli.com/lipids/hydroxy-fatty-acids/ (accessed on 28 June 2019).
- Jalal, M.; Read, D. The organic acid composition of Calluna heathland soil with special reference to phyto- and fungitoxicity. 1. Isolation and identification of organic acids. Plant Soil 1983, 70, 257–272. [Google Scholar] [CrossRef]
- Mittendorf, V.; Robertson, E.; Leech, R.; Kruger, N.; Steinbuchel, A.; Poirier, Y. Synthesis of medium-chain-length polyhydroxyalkanoates in Arabidopsis thaliana using intermediates of peroxisomal fatty acid beta-oxidation. Proc. Natl. Acad. Sci. USA 1998, 95, 13397–13402. [Google Scholar] [CrossRef] [PubMed]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef] [PubMed]
- Shearer, G.; Walker, R. An overview of the biologic effects of omega-6 oxylipins in humans. Prostaglandins Leukot. Essent. Fatty Acids 2018, 137, 26–38. [Google Scholar] [CrossRef]
- Diedrich, M.; Henschel, K. The natural occurrence of unusual fatty acids. Part 1. Odd numbered fatty acids. Nahrung-Food 1990, 34, 935–943. [Google Scholar] [CrossRef]
- Tsydendambaev, V.; Christie, W.; Brechany, E.; Vereshchagin, A. Identification of unusual fatty acids of four alpine plant species from the Pamirs. Phytochemistry 2004, 65, 2695–2703. [Google Scholar] [CrossRef]
- Kendel, M.; Barnathan, G.; Fleurence, J.; Rabesaotra, V.; Wielgosz-Collin, G. Non-methylene interrupted and hydroxy fatty acids in polar lipids of the alga Grateloupia turuturu over the four seasons. Lipids 2013, 48. [Google Scholar] [CrossRef]
- Hildebrand, D. AOCS Lipid Library. Production of unusual fatty acids in plants. Available online: http://lipidlibrary.aocs.org/chemistry/physics/plant-lipid/production-of-unusual-fatty-acids-in-plants) (accessed on 28 June 2019).
- Millar, A.; Smith, M.; Kunst, L. All fatty acids are not equal: Discrimination in plant membrane lipids. Trends Plant Sci. 2000, 5, 95–101. [Google Scholar] [CrossRef]
- Bligh, E.; Dyer, W. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Ruiz, J.; Antequera, T.; Andres, A.; Petron, M.; Muriel, E. Improvement of a solid phase extraction method for analysis of lipid fractions in muscle foods. Anal. Chim. Acta 2004, 520, 201–205. [Google Scholar] [CrossRef]
- Rouser, G.; Fleischer, S.; Yamamoto, A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 1970, 5, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Oresic, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Lipid extracts are available from the authors. |




| Lipid name | m/z | tR (min) | Acyl Chain Composition |
|---|---|---|---|
| TAG(C:N) | [M + NH4]+ | ||
| TAG(40:0) | 712.6446 | 23.02 | 12:0-12:0-16:0 and 12:0-14:0-14:0 |
| TAG(41:0) | 726.6595 | 23.29 | 13:0-14:0-14:0 |
| TAG(42:1) | 738.6597 | 23.02 | 12:0-12:0-18:1 and 12:0-14:0-16:1 |
| TAG(42:0) | 740.6758 | 23.78 | 12:0-14:0-16:0 and 13:0-14:0-15:0 |
| TAG(43:1) | 752.6757 | 23.39 | 13:0-14:0-16:1 |
| TAG(43:0) | 754.6914 | 23.95 | 14:0-14:0-15:0 |
| TAG(44:2) | 764.6763 | 23.18 | 14:0-14:1-16:1 |
| TAG(44:1) | 766.6916 | 23.88 | 14:0-14:0-16:1 |
| TAG(44:0) | 768.7071 | 24.67 | 14:0-15:0-15:0 |
| TAG(45:2) | 778.6913 | 23.56 | 14:1-15:0-16:1 |
| TAG(45:1) | 780.7065 | 24.33 | 14:0-15:0-16:1 |
| TAG(45:0) | 782.7230 | 25.20 | 14:0-15:0-16:0 |
| TAG(46:3) | 790.6922 | 23.32 | 14:1-16:1-16:1 |
| TAG(46:2) | 792.7069 | 23.98 | 15:0-15:1-16:1 |
| TAG(46:1) | 794.7226 | 24.84 | 14:0-16:0-16:1 and 14:1-16:0-16:0 |
| TAG(46:0) | 796.7386 | 25.77 | 14:0-16:0-16:0 and 15:0-15:0-16:0 |
| TAG(47:3)* | 804.7074 | 23.71 | 47:3 |
| TAG(47:2) | 806.7227 | 24.46 | 15:0-16:1-16:1 |
| TAG(47:1) | 808.7380 | 25.31 | 15:0-16:0-16:1 |
| TAG(47:0) | 810.7541 | 26.38 | 15:0-16:0-16:0 |
| TAG(48:3) | 818.7231 | 24.09 | 14:1-16:1-18:1 |
| TAG(48:2) | 820.7386 | 24.98 | 16:0-16:1-16:1 |
| TAG(48:1) | 822.7544 | 25.77 | 16:0-16:0-16:1 |
| TAG(48:0) | 824.7700 | 27.07 | 16:0-16:0-16:0 |
| TAG(49:3) | 832.7384 | 24.52 | 16:1-16:1-17:1 |
| TAG(49:2) | 834.7539 | 25.39 | 15:1-16:0-18:1 |
| TAG(49:1) | 836.7698 | 26.38 | 15:0-16:0-18:1 |
| TAG(49:0) | 838.7851 | 27.85 | 16:0-16:0-17:0 |
| TAG(50:4) | 844.7388 | 24.33 | 16:1-16:1-18:2 |
| TAG(50:3) | 846.7541 | 24.98 | 16:0-16:1-18:2 |
| TAG(50:2) | 848.7708 | 25.77 | 16:0-16:1-18:1 |
| TAG(50:1) | 850.7866 | 27.04 | 16:0-16:0-18:1 |
| TAG(50:0) | 852.8004 | 28.70 | 16:0-16:0-18:0 |
| TAG(51:3) | 860.7699 | 25.48 | 16:0-17:1-18:2 and 16:1-17:0-18:2 and 16:1-17:1-18:1 |
| TAG(51:2) | 862.7856 | 26.34 | 16:0-17:0-18:2 and 16:0-17:1-18:1 |
| TAG(51:1) | 864.8012 | 27.76 | 16:0-17:0-18:1 |
| TAG(51:0) | 866.8158 | 29.72 | 16:0-17:0-18:0 |
| TAG(52:4) | 872.7700 | 25.27 | 16:0-18:2-18:2 |
| TAG(52:3) | 874.7866 | 25.77 | 16:1-18:1-18:1 |
| TAG(52:2) | 876.8027 | 27.00 | 16:0-18:1-18:1 |
| TAG(52:1) | 878.8169 | 28.63 | 16:0-18:0-18:1 |
| TAG(52:0) | 880.8319 | 30.75 | 16:0-18:0-18:0 |
| TAG(53:6)* | 882.7509 | 25.77 | 53:6 |
| TAG(53:4) | 886.7861 | 25.42 | 17:1-18:1-18:2 |
| TAG(53:3) | 888.8013 | 26.34 | 17:1-18:1-18:1 |
| TAG(53:2) | 890.8167 | 27.76 | 17:0-18:1-18:1 |
| TAG(53:1) | 892.8316 | 27.11 | 17:0-18:0-18:1 |
| TAG(54:6) | 896.7697 | 24.49 | 18:2-18:2-18:2 |
| TAG(54:5) | 898.7859 | 25.24 | 18:1-18:1-18:3 |
| TAG(54:4) | 900.8021 | 26.00 | 18:1-18:1-18:2 |
| TAG(54:3) | 902.8182 | 26.94 | 18:1-18:1-18:1 |
| TAG(54:2) | 904.8333 | 28.57 | 18:0-18:1-18:1 |
| TAG(54:1) | 906.8480 | 30.63 | 18:0-18:0-18:1 |
| TAG(54:0) | 908.8640 | 33.39 | 14:0-16:0-24:0 |
| TAG(55:4) | 914.8184 | 25.27 | 18:1-18:2-19:1 |
| TAG(55:2) | 918.8482 | 27.04 | 18:1-18:1-19:0 |
| TAG(55:1) | 920.8642 | 28.63 | 16:0-18:1-21:0 |
| TAG(56:5) | 926.8155 | 26.00 | 18:1-18:3-20:1 |
| TAG(56:4) | 928.8332 | 27.00 | 18:1-18:1-20:2 |
| TAG(56:3) | 930.8482 | 28.41 | 18:1-18:1-20:1 |
| TAG(56:2) | 932.8640 | 30.59 | 18:1-18:1-20:0 |
| TAG(56:1) | 934.8802 | 22.45 | 16:0-18:1-22:0 and 18:0-18:1-20:0 |
| TAG(57:5)* | 940.8334 | 25.24 | 57:5 |
| TAG(57:4)* | 942.8490 | 26.00 | 57:4 |
| TAG(57:3)* | 944.8640 | 26.91 | 57:3 |
| TAG(57:2) | 946.8792 | 28.57 | 18:1-18:1-21:0 |
| TAG(58:3) | 958.8793 | 31.25 | 18:1-18:2-22:0 |
| TAG(58:2) | 960.8956 | 33.19 | 18:1-18:1-22:0 |
| TAG(58:1) | 962.9106 | 23.08 | 16:0-18:1-24:0 |
| TAG(60:3) | 986.9110 | 33.98 | 18:1-18:2-24:0 |
| TAG(60:2) | 988.9265 | 36.51 | 18:1-18:1-24:0 |
| Lipid Name | m/z | tR (min) | Acyl Chain Composition |
|---|---|---|---|
| Class(C:N) | [M + H]+ | ||
| PC(24:0(OH)) | 638.4399 | 11.93 | 16:0-8:0(OH) |
| PC(26:2) | 646.4436 | 10.13 | 8:1-18:1 |
| PC(16:0/C8CHO)* | 650.4400 | 10.93 | 16:0/C8CHO |
| PC(26:2(OH)) | 662.4403 | 10.83 | 18:1-8:1(OH) |
| PC(26:1(OH)) | 664.4585 | 11.63 | 18:1-8:0(OH) |
| PC(16:0/C8COOH)* | 666.4348 | 10.83 | 16:0/C8COOH |
| PC(27:2(OH)) | 676.4560 | 10.63 | 18:1-9:1(OH) |
| PC(28:3(OH)) | 688.4554 | 10.53 | 18:1-10:2(OH) |
| PC(30:3)a) | 700.4895 | 10.03 | 30:3 |
| PC(29:2(OH)) | 704.4867 | 10.43 | 18:1-11:1(OH) |
| PC(32:2) | 730.5379 | 9.63 | 16:1-16:1 |
| PC(32:1) | 732.5539 | 9.63 | 16:0-16:1 and 14:0-18:1 |
| PC(32:2(OH)) | 746.5316 | 9.83 | 16:0-16:2(OH) and 16:1-16:1(OH) and 18:1-14:1(OH) |
| PC(33:1) | 746.5671 | 9.53 | 16:0-17:1 |
| PC(32:1(OH)) | 748.5476 | 9.83 | 18:1-14:0(OH) and 16:0-16:1(OH) |
| PC(34:4) | 754.5372 | 9.53 | 16:1-18:3 |
| PC(34:3) | 756.5545 | 9.43 | 16:0-18:3 and 16:1-18:2 |
| PC(34:2) | 758.5699 | 9.43 | 16:0-18:2 and 16:1-18:1 |
| PC(34:1) | 760.5854 | 9.43 | 16:0-18:1 |
| PC(34:4(OH)) | 770.5338 | 9.73 | 17:1-17:3(OH) and 18:1-16:3(OH) and 18:2-16:2(OH) |
| PC(35:3) | 770.5697 | 9.43 | 18:2-17:1 |
| PC(35:2) | 772.5848 | 9.23 | 18:1-17:1 |
| PC(34:3(OH)) | 772.5487 | 9.63 | 16:0-18:3(OH) and 16:1-18:2(OH) and 18:1-16:2(OH) |
| PC(34:2(OH)) | 774.5646 | 9.63 | 16:1-18:1(OH) and 16:0-18:2(OH) and 18:1-16:1(OH) |
| PC(35:1) | 774.5998 | 9.23 | 18:1-17:0 |
| PC(34:1(OH)) | 776.5799 | 9.63 | 16:0-18:1(OH) |
| PC(36:6) | 778.5380 | 9.43 | 18:3-18:3 |
| PC(36:5) | 780.5512 | 9.43 | 18:3-18:2 |
| PC(36:4) | 782.5689 | 9.33 | 18:2-18:2 and 18:3-18:1 |
| PC(36:3) | 784.5853 | 9.23 | 18:1-18:2 |
| PC(36:2) | 786.6009 | 9.03 | 18:1-18:1 |
| PC(34:1(2OH)) | 792.5756 | 8.83 | 16:0-18:1(2OH) |
| PC(36:5(OH)) | 796.5476 | 9.63 | 18:2-18:3(OH) |
| PC(37:4) | 796.5829 | 9.13 | 18:1-19:3 and 18:2-19:2 |
| PC(36:4(OH)) | 798.5647 | 9.43 | 18:1-18:3(OH) and 18:2-18:2(OH) |
| PC(36:3(OH)) | 800.5798 | 9.33 | 18:1-18:2(OH) and 18:2-18:1(OH) |
| PC(37:2) | 800.6156 | 9.03 | 19:0-18:2 and 18:1-19:1 |
| PC(36:2(OH)) | 802.5949 | 9.43 | 18:1-18:1(OH) |
| PC(38:3) | 812.6140 | 8.93 | 18:1-20:2 and 18:2-20:1 |
| PC(38:2) | 814.6318 | 8.93 | 18:1-20:1 |
| PC(38:1) | 816.6452 | 8.83 | 18:1-20:0 |
| PC(36:3(2OH)) | 816.5755 | 8.73 | 18:1-18:2(2OH) |
| PC(36:2(2OH)) | 818.5908 | 8.73 | 18:1-18:1(2OH) |
| PC(40:2) | 842.6611 | 8.83 | 18:1-22:1 and 18:2-22:0 |
| PC(40:1) | 844.6812 | 8.73 | 18:1-22:0 |
| PC(41:1) | 858.6943 | 8.66 | 18:1-23:0 |
| PC(42:1) | 872.7111 | 8.63 | 18:1-24:0 |
| PC(43:1) | 886.7261 | 8.53 | 18:1-25:0 |
| [M + H]+ | |||
| LPC(16:1) | 494.3245 | 14.03 | 16:1 |
| LPC(16:0) | 496.3402 | 13.73 | 16:0 |
| LPC(18:3) | 518.3224 | 13.73 | 18:3 |
| LPC(18:2) | 520.3404 | 13.63 | 18:2 |
| LPC(18:1) | 522.3559 | 13.43 | 18:1 |
| LPC(18:2(OH)) | 536.3355 | 14.53 | 18:2(OH) |
| LPC(18:1(OH)) | 538.3511 | 14.63 | 18:1(OH) |
| LPC(20:1) | 550.3876 | 12.93 | 20:1 |
| LPC(20:0) | 552.4025 | 12.73 | 20:0 |
| LPC(18:1(2OH)) | 554.3457 | 13.13 | 18:1(2OH) |
| LPC(22:0) | 580.4359 | 12.42 | 22:0 |
| LPC(24:0) | 608.4664 | 11.93 | 24:0 |
| [M + H]+ | |||
| PE(30:3)a) | 658.4426 | 4.54 | |
| PE(34:2) | 716.5216 | 4.34 | 16:1-18:1 and 16:0-18:2 |
| PE(34:1) | 718.5380 | 4.34 | 16:0-18:1 |
| PE(36:4)* | 740.5216 | 4.34 | |
| PE(36:3) | 742.5388 | 4.34 | 18:1-18:2 |
| PE(36:2) | 744.5538 | 4.24 | 18:1-18:1 and 18:0-18:2 and 17:1-19:1 |
| [M − H]− | |||
| PG(34:1) | 747.5175 | 2.49 | 16:0-18:1 |
| PG(36:2)* | 773.5332 | 2.49 | |
| [M + H]+ | |||
| SM(d34:1) | 703.5755 | 11.33 | |
| SM(d36:1) | 731.6054 | 11.13 | |
| SM(t38:1)* | 775.6330 | 10.63 | |
| SM(d41:1) | 801.6875 | 10.53 | |
| SM(t40:0) | 805.6792 | 10.83 | |
| SM(d42:2) | 813.6870 | 10.53 | |
| SM(d42:1) | 815.6969 | 10.53 | |
| SM(t41:1)* | 817.6834 | 10.33 | |
| SM(t41:0) | 819.6953 | 10.73 | |
| [M + NH4]+ | |||
| MGDG(34:4) | 768.5644 | 3.34 | 16:1-18:3 |
| MGDG(34:2)* | 772.5932 | 3.34 | |
| MGDG(34:1)* | 774.6076 | 3.34 | |
| MGDG(36:6) | 792.5622 | 3.34 | 18:3-18:3 and 18:4-18:2 |
| MGDG(36:4) | 796.5934 | 3.34 | 18:2-18:2 and 18:3-18:1 |
| MGDG(36:3) | 798.6091 | 3.24 | 18:1-18:2 and 18:3-18:0 |
| MGDG(36:2) | 800.6250 | 3.34 | 18:1-18:1 |
| MGDG(38:1)* | 830.6714 | 3.24 | |
| MGDG(40:4)* | 852.6562 | 3.24 | |
| [M + NH4]+ | |||
| DGMG(18:3)* | 694.4022 | 3.64 | 18:3 |
| DGMG(18:1)* | 698.4317 | 3.64 | 18:1 |
| [M + NH4]+ | |||
| DGDG(34:4)* | 930.6154 | 3.54 | |
| DGDG(34:3) | 932.6315 | 3.54 | 16:0-18:3 |
| DGDG(34:2) | 934.6437 | 3.54 | 16:1-18:1 and 16:0-18:2 |
| DGDG(34:1) | 936.6621 | 3.54 | 16:0-18:1 |
| DGDG(36:6) | 954.6150 | 3.54 | 18:3-18:3 and 18:4-18:2 |
| DGDG(36:4) | 958.6467 | 3.54 | 18:1-18:3 and 18:2-18:2 |
| DGDG(36:3) | 960.6582 | 3.54 | 18:2-18:1 and 18:0-18:3 |
| DGDG(36:2) | 962.6775 | 3.54 | 18:1-18:1 and 18:2-18:0 |
| [M + H]+ | |||
| HexCer(d34:2(OH)) | 714.5518 | 3.34 | d18:2-16:0-OH |
| HexCer(d34:1(OH)) | 716.5669 | 3.34 | d18:1-16:0-OH |
| HexCer(t40:1(OH)) | 816.6555 | 3.24 | t18:1-22:0-OH |
| HexCer(t42:1(OH)) | 844.6874 | 3.24 | t18:1-24:0-OH |
| HexCer(t44:1((OH)2)) | 872.7195 | 3.24 | t18:1-26:0-((OH)2) |
| [M + H]+ | |||
| MGTS(16:0) | 474.3793 | 6.44 | 16:0 |
| [M + H]+ | |||
| DGTS(34:2) | 736.6082 | 5.04 | 16:0-18:2 and 16:1-18:1 |
| DGTS(34:1) | 738.6213 | 5.04 | 16:0-18:1 |
| DGTS(36:4) | 760.6095 | 4.94 | 18:2-18:2 |
| DGTS(36:3) | 762.6238 | 4.94 | 18:2-18:1 |
| DGTS(36:2) | 764.6404 | 4.94 | 18:1-18:1 |
| Relative Abundance (%) | ||||
|---|---|---|---|---|
| Mean | S.D. | |||
| Phospholipids | 71.69 | 2.20 | ||
| PC | 96.66 | 1.63 | ||
| Non-Hydroxylated | 85.42 | 6.32 | ||
| Hydroxylated | 14.58 | 6.32 | ||
| LPC | 2.61 | 1.55 | ||
| Non-Hydroxylated | 97.11 | 1.39 | ||
| Hydroxylated | 2.89 | 1.39 | ||
| PE | 0.65 | 0.10 | ||
| SM | 0.06 | 0.03 | ||
| PG | 0.01 | 0.00 | ||
| Glycolipids | 25.44 | 1.94 | ||
| MGDG | 52.29 | 3.14 | ||
| DGDG | 46.97 | 2.87 | ||
| DGMG | 0.74 | 0.29 | ||
| Glycosphingolipids | 2.33 | 0.81 | ||
| HexCer | 2.33 | 0.81 | ||
| Betaines | 0.54 | 0.26 | ||
| DGTS | 91.56 | 2.63 | ||
| MGTS | 8.44 | 2.63 | ||
| Sum | 100.00 | 3.32 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, E.; Melo, T.; Barros, M.P.; Domingues, M.R.M.; Domingues, P. Lipidomic Profiling of the Olive (Olea europaea L.) Fruit towards Its Valorisation as a Functional Food: In-Depth Identification of Triacylglycerols and Polar Lipids in Portuguese Olives. Molecules 2019, 24, 2555. https://doi.org/10.3390/molecules24142555
Alves E, Melo T, Barros MP, Domingues MRM, Domingues P. Lipidomic Profiling of the Olive (Olea europaea L.) Fruit towards Its Valorisation as a Functional Food: In-Depth Identification of Triacylglycerols and Polar Lipids in Portuguese Olives. Molecules. 2019; 24(14):2555. https://doi.org/10.3390/molecules24142555
Chicago/Turabian StyleAlves, Eliana, Tânia Melo, Madalena P. Barros, M. Rosário M. Domingues, and Pedro Domingues. 2019. "Lipidomic Profiling of the Olive (Olea europaea L.) Fruit towards Its Valorisation as a Functional Food: In-Depth Identification of Triacylglycerols and Polar Lipids in Portuguese Olives" Molecules 24, no. 14: 2555. https://doi.org/10.3390/molecules24142555
APA StyleAlves, E., Melo, T., Barros, M. P., Domingues, M. R. M., & Domingues, P. (2019). Lipidomic Profiling of the Olive (Olea europaea L.) Fruit towards Its Valorisation as a Functional Food: In-Depth Identification of Triacylglycerols and Polar Lipids in Portuguese Olives. Molecules, 24(14), 2555. https://doi.org/10.3390/molecules24142555