Discovery of New Selective Butyrylcholinesterase (BChE) Inhibitors with Anti-Aβ Aggregation Activity: Structure-Based Virtual Screening, Hit Optimization and Biological Evaluation
Abstract
1. Introduction
2. Results and Discussion
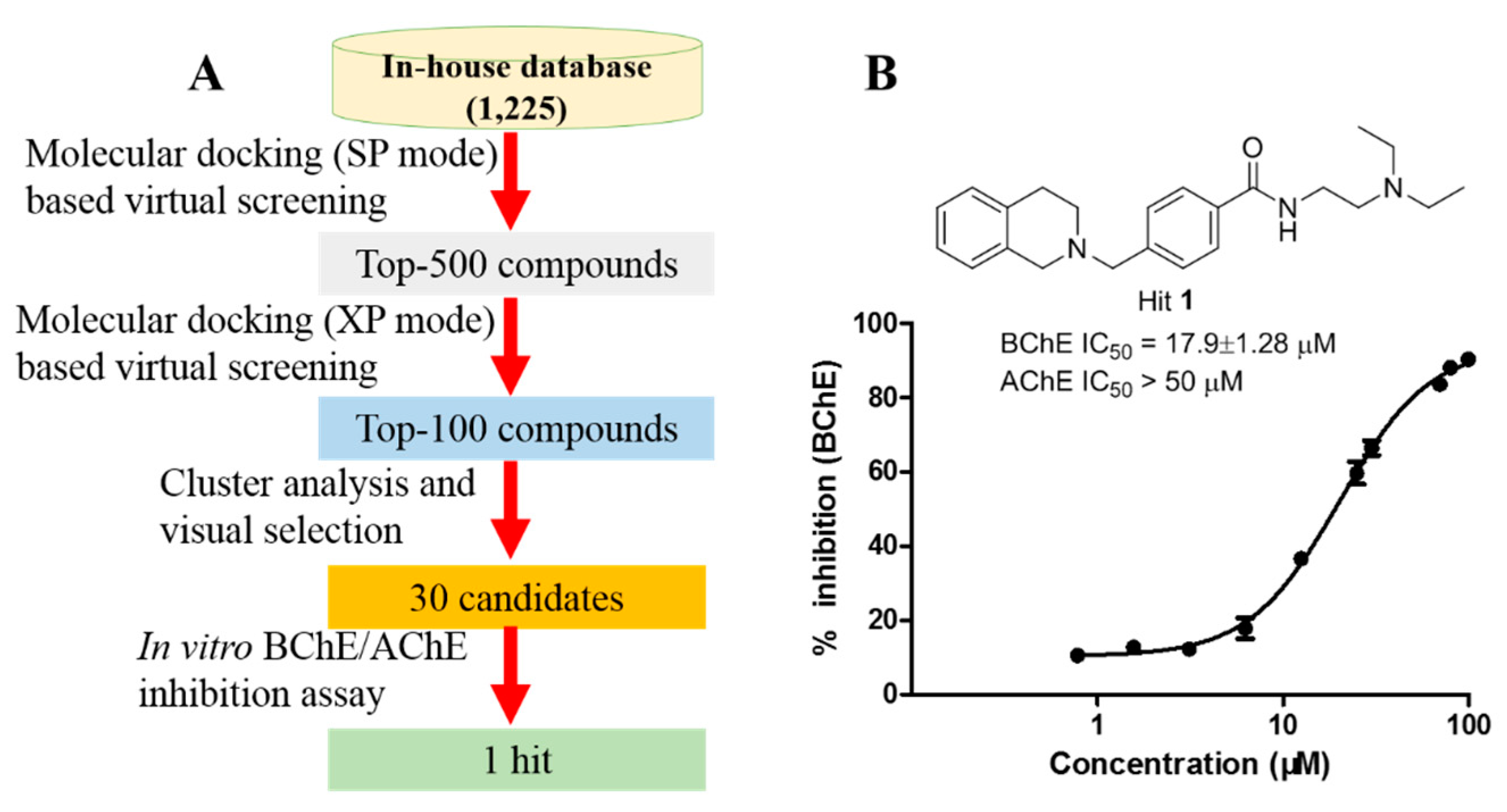
2.1. Molecular Docking-Based Virtual Screening to Identify Hit 1 as Promising Selective BChE Inhibitor
2.2. Binding mode of 1 Provides Insights for Hit Optimization
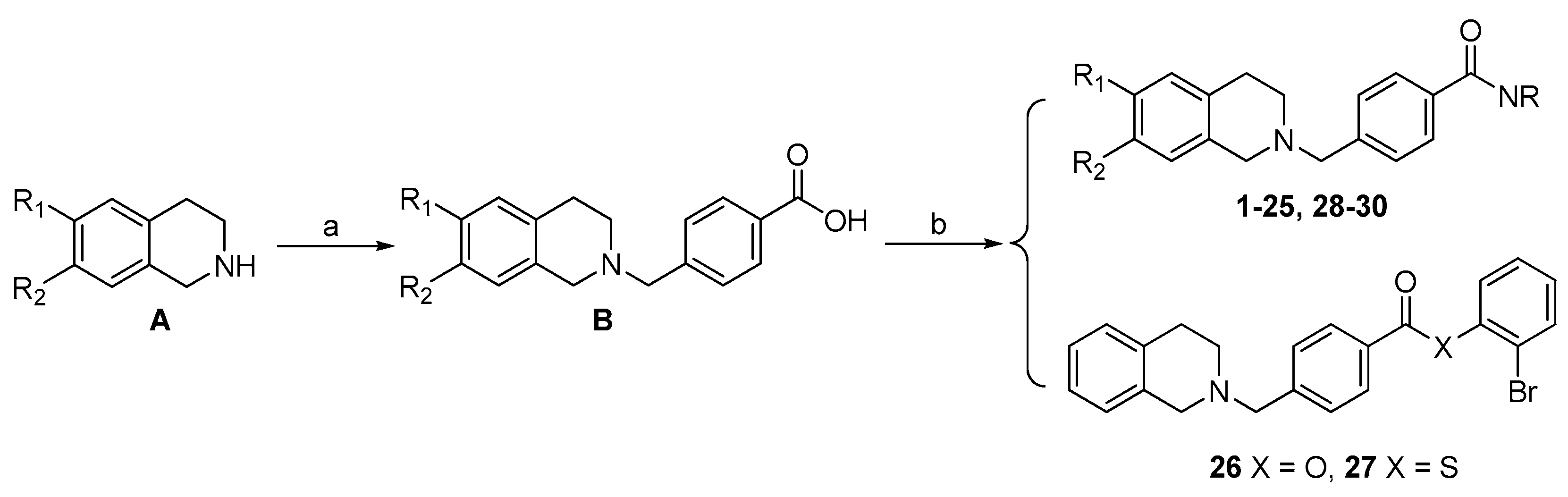
2.3. Chemcial Synthesis
2.4. Biological Evaluation and SAR Study
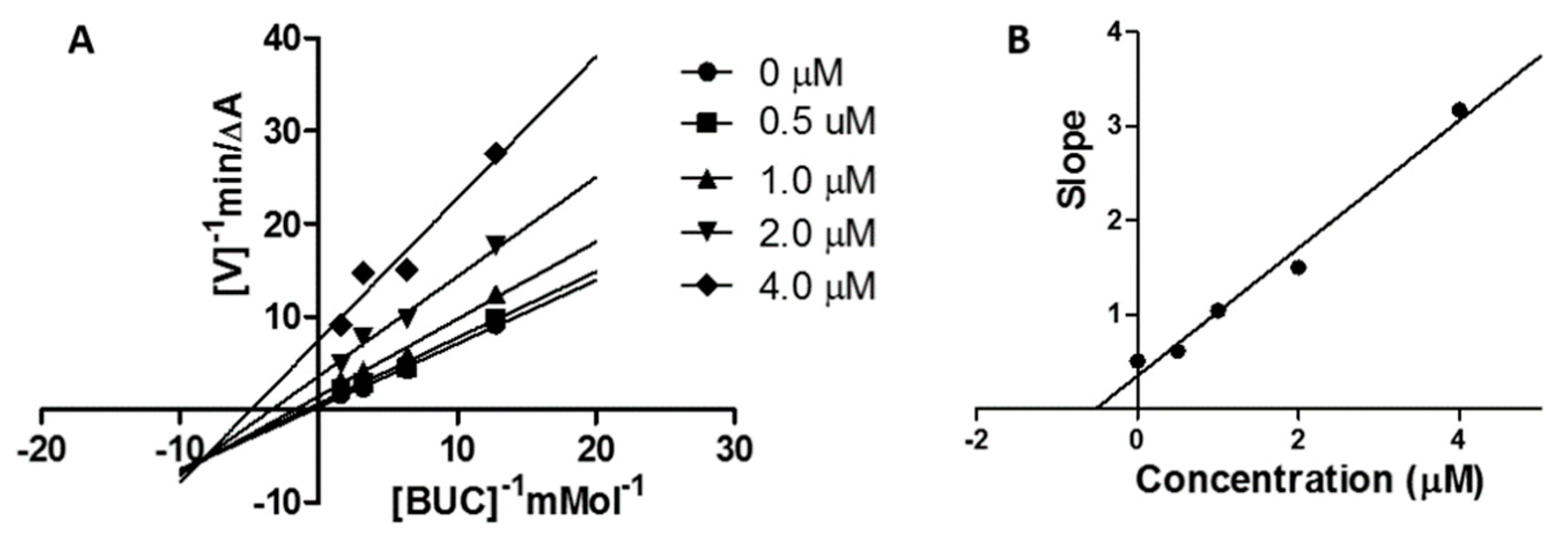
2.5. Kinetic Study on BChE Inhibition
2.6. Binding Mode Analysis
2.7. Molecular Dynamics
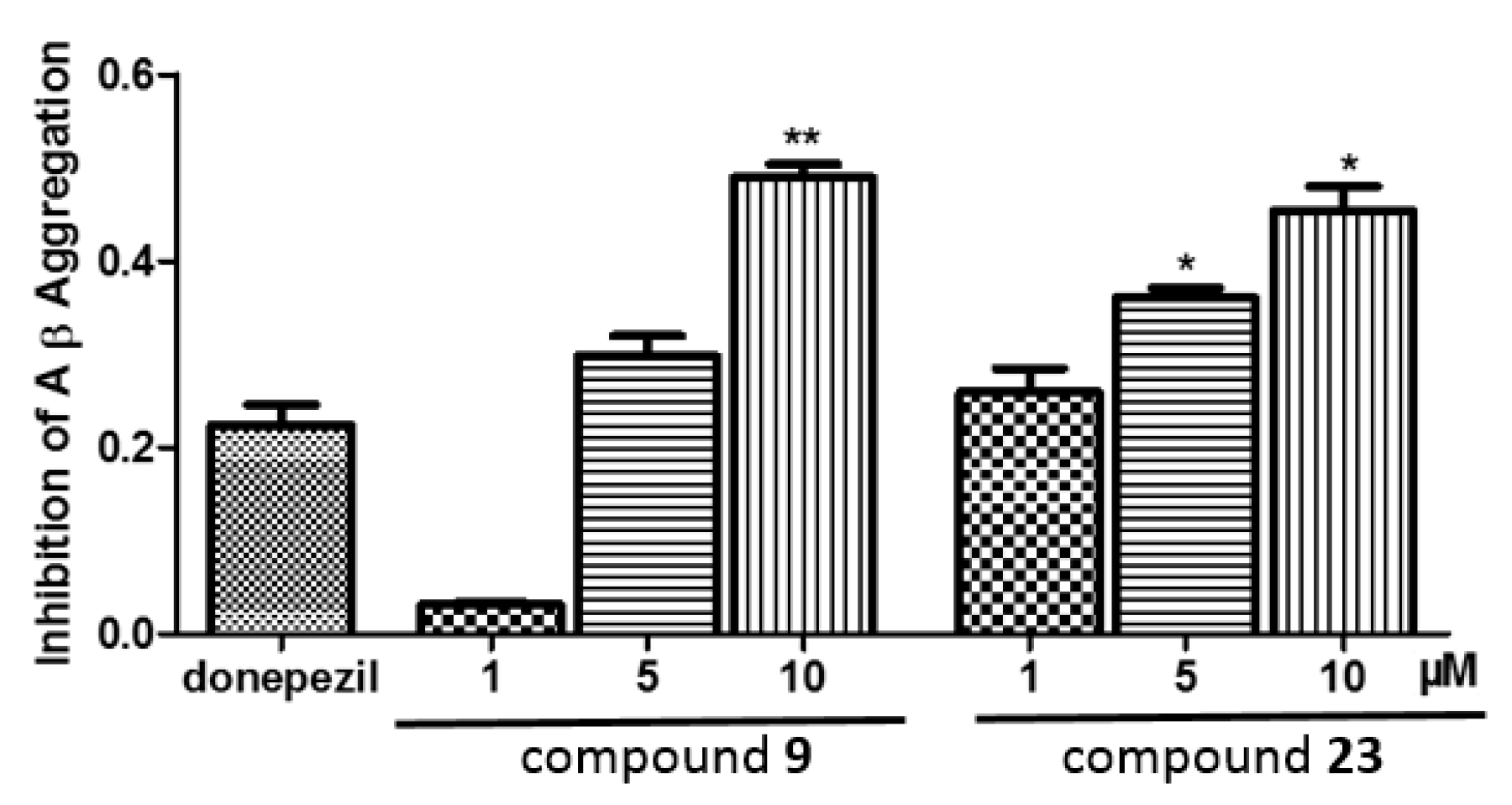
2.8. Inhibition on Aβ Aggregation
2.9. Cell Viability Assay
2.10. Protective Activity of 9 and 23 Against Aβ1-42-Induced Toxicity in SH-SY5Y Cells
3. Materials and Methods
3.1. Virtural Screening
3.2. Chemistry
3.2.1. General Methods
3.2.2. General Procedure for the Synthesis of B
3.2.3. General Procedure for the Synthesis of 1–30
3.3. BChE and AChE Bioassay
3.4. Kinetic Assay
3.5. Molecular Docking
3.6. Molecular Dynamics Simulation
3.7. Binding Free Energy Calculation with MM-PBSA Method
3.8. Determination of the Inhibitory Potency on Aβ1–42 Aggregation
3.9. Cell Viability Assay and Neuroprotective Activity Evaluation
3.10. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Alzheimer’s disease | (AD) |
| acetylcholine | (ACh) |
| beta-amyloid | (Aβ) |
| cholinesterase | (ChE) |
| butyrylcholinesterase | (BChE) |
| acetylcholinesterase | (AChE) |
| catalytic active site | (CAS) |
| peripheral anionic site | (PAS) |
| structure-activity relationship | (SAR) |
| molecular dynamics | (MD) |
| Molecular Mechanics Poisson-Boltzmann Surface Area | (MM-PBSA) |
| root-mean-square deviations | (RMSDs) |
| thioflavin T | (ThT) |
| epigallocatechin gallate | (EGCG) |
| butyrylthiocholine | (BUC) |
| acetylthiocholine | (ATC) |
References
- Wiemann, J.; Loesche, A.; Csuk, R. Novel dehydroabietylamine derivatives as potent inhibitors of acetylcholinesterase. Bioorg. Chem. 2017, 74, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Mattila, J.; Soininen, H.; Koikkalainen, J.; Rueckert, D.; Wolz, R.; Waldemar, G.; Lötjönen, J. Optimizing the diagnosis of early Alzheimer's disease in mild cognitive impairment subjects. J. Alzheimer’s Dis. 2012, 32, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.alz.co.uk/research/world-report-2018 (accessed on 20 May 2019).
- Yu, Y.F.; Huang, Y.D.; Zhang, C.; Wu, X.N.; Zhou, Q.; Wu, D.; Wu, Y.; Luo, H.B. Discovery of Novel Pyrazolopyrimidinone Derivatives as Phosphodiesterase 9A Inhibitors Capable of Inhibiting Butyrylcholinesterase for Treatment of Alzheimer's Disease. ACS Chem. Neurosci. 2017, 8, 2522–2534. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yang, H.; Li, Q.; Chen, Y.; Li, Q.; Zhou, Y.; Feng, F.; Liu, W.; Guo, Q.; Sun, H. Expansion of the scaffold diversity for the development of highly selective butyrylcholinesterase (BChE) inhibitors: Discovery of new hits through the pharmacophore model generation, virtual screening and molecular dynamics simulation. Bioorg. Chem. 2019, 85, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Scarpini, E.; Scheltens, P.; Feldman, H. Treatment of Alzheimer’s disease: Current status and new perspectives. Lancet Neurol. 2003, 2, 539–547. [Google Scholar] [CrossRef]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef]
- Kodamullil, A.T.; Zekri, F.; Sood, M.; Hengerer, B.; Canard, L.; McHale, D.; Hofmann-Apitius, M. Trial watch: Tracing investment in drug development for Alzheimer disease. Nat. Rev. Drug Discov. 2017, 16, 819. [Google Scholar] [CrossRef]
- Bartus, R.T.; Dean, R.L.; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef]
- Anand, P.; Singh, B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch Pharm. Res. 2013, 36, 375–399. [Google Scholar] [CrossRef]
- Feng, B.; Li, X.; Xia, J.; Wu, S. Discovery of novel isoflavone derivatives as AChE/BuChE dual-targeted inhibitors: Synthesis, biological evaluation and molecular modelling. J. Enzyme Inhib. Med. Chem. 2017, 32, 968–977. [Google Scholar] [CrossRef]
- De Vita, D.; Pandolfi, F.; Ornano, L.; Feroci, M.; Chiarotto, I.; Sileno, I.; Pepi, F.; Costi, R.; Di Santo, R.; Scipione, L. New N,N-dimethylcarbamate inhibitors of acetylcholinesterase: Design synthesis and biological evaluation. J. Enzyme Inhib. Med. Chem. 2016, 31, 106–113. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.K.; Tomlinson, B.E.; Blessed, G.; Bergmann, K.; Gibson, P.H.; Perry, R.H. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br. Med. J. 1978, 2, 1457–1459. [Google Scholar] [CrossRef] [PubMed]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, H.; Chen, Y.; Sun, H. Recent progress in the identification of selective butyrylcholinesterase inhibitors for Alzheimer's disease. Eur. J. Med. Chem. 2017, 32, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Thomas, R.G.; Grundman, M.; Bennett, D.; Doody, R.; Ferris, S.; Galasko, D.; Jin, S.; Kaye, J.; Levey, A.; et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N. Engl. J. Med. 2005, 352, 2379–2388. [Google Scholar] [CrossRef]
- Dileep, K.V.; Remya, C.; Tintu, I.; Sadasivan, C. Inhibition, ADME and structure based modification of IAA and IBA against acetylcholinesterase: An attempt towards new drug development for Alzheimer’s disease. Front. Life Sci. 2013, 7, 164–173. [Google Scholar] [CrossRef][Green Version]
- Li, B.; Duysen, E.G.; Carlson, M.; Lockridge, O. The butyrylcholinesterase knockout mouse as a model for human butyrylcholinesterase deficiency. J. Pharmacol. Exp. Ther. 2008, 324, 1146–1154. [Google Scholar] [CrossRef]
- Hartmann, J.; Kiewert, C.; Duysen, E.G.; Lockridge, O.; Greig, N.H.; Klein, J. Excessive hippocampal acetylcholine levels in acetylcholinesterase-deficient mice are moderated by butyrylcholinesterase activity. J. Neurochem. 2007, 100, 1421–1429. [Google Scholar] [CrossRef]
- Fang, L.; Pan, Y.; Muzyka, J.L.; Zhan, C.G. Active site gating and substrate specificity of butyrylcholinesterase and acetylcholinesterase: Insights from molecular dynamics simulations. J. Phys. Chem. B 2011, 115, 8797–8805. [Google Scholar] [CrossRef]
- Sawatzky, E.; Wehle, S.; Kling, B.; Wendrich, J.; Bringmann, G.; Sotriffer, C.A.; Heilmann, J.; Decker, M. Discovery of Highly Selective and Nanomolar Carbamate-Based Butyrylcholinesterase Inhibitors by Rational Investigation into Their Inhibition Mode. J. Med. Chem. 2016, 59, 2067–2082. [Google Scholar] [CrossRef] [PubMed]
- Heller, L.; Kahnt, M.; Loesche, A.; Grabandt, P.; Schwarz, S.; Brandt, W.; Csuk, R. Amino derivatives of platanic acid act as selective and potent inhibitors of butyrylcholinesterase. Eur. J. Med. Chem. 2017, 126, 652–668. [Google Scholar] [CrossRef] [PubMed]
- Košak, U.; Brus, B.; Knez, D.; Žakelj, S.; Trontelj, J.; Pišlar, A.; Šink, R.; Jukič, M.; Živin, M.; Podkowa, A.; et al. The Magic of Crystal Structure-Based Inhibitor Optimization: Development of a Butyrylcholinesterase Inhibitor with Picomolar Affinity and in Vivo Activity. J. Med. Chem. 2018, 61, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Holloway, H.W.; Utsuki, T.; Brossi, A.; Greig, N.H. Synthesis of Novel Phenserine-Based-Selective Inhibitors of Butyrylcholinesterase for Alzheimer’s Disease. J. Med. Chem. 1999, 42, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Carolan, C.G.; Dillon, G.P.; Khan, D.; Ryder, S.A.; Gaynor, J.M.; Reidy, S.; Marquez, J.F.; Jones, M.; Holland, V.; Gilmer, J.F. Isosorbide-2-benzyl carbamate-5-salicylate, a peripheral anionic site binding subnanomolar selective butyrylcholinesterase inhibitor. J. Med. Chem. 2010, 53, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Wu, G.; Kang, D.; Zhou, Z.; Song, Y.; Liu, X.; Zhan, P. Contemporary medicinal-chemistry strategies for the discovery of selective butyrylcholinesterase inhibitors. Drug Discov. Today 2019, 24, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Neves, B.J.; Braga, R.C.; Melo-Filho, C.C.; Moreira-Filho, J.T.; Muratov, E.N.; Andrade, C.H. QSAR-Based Virtual Screening: Advances and Applications in Drug Discovery. Front. Pharmacol. 2018, 9, 1275. [Google Scholar] [CrossRef]
- Beesu, M.; Caruso, G.; Salyer, A.C.; Khetani, K.K.; Sil, D.; Weerasinghe, M.; Tanji, H.; Ohto, U.; Shimizu, T.; David, S.A. Structure-Based Design of Human TLR8-Specific Agonists with Augmented Potency and Adjuvanticity. J. Med. Chem. 2015, 58, 7833–7849. [Google Scholar] [CrossRef] [PubMed]
- Beesu, M.; Caruso, G.; Salyer, A.C.; Shukla, N.M.; Khetani, K.K.; Smith, L.J.; Fox, L.M.; Tanji, H.; Ohto, U.; Shimizu, T.; et al. Identification of a Human Toll-like Receptor (TLR) 8-Specific Agonist and a Functional Pan-TLR inhibitor in 2-Aminoimidazoles. J. Med. Chem. 2016, 59, 3311–3330. [Google Scholar] [CrossRef]
- Cheng, Z.Q.; Zhu, K.K.; Zhang, J.; Song, J.L.; Muehlmann, L.A.; Jiang, C.S.; Liu, C.L.; Zhang, H. Molecular-docking-guided design and synthesis of new IAA-tacrine hybrids as multifunctional AChE/BChE inhibitors. Bioorg. Chem. 2019, 83, 277–288. [Google Scholar] [CrossRef]
- Cheng, Z.Q.; Song, J.L.; Zhu, K.; Zhang, J.; Jiang, C.S.; Zhang, H. Total Synthesis of Pulmonarin B and Design of Brominated Phenylacetic Acid/Tacrine Hybrids: Marine Pharmacophore Inspired Discovery of New ChE and Aβ Aggregation Inhibitors. Mar. Drugs 2018, 16, 293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.C.; Song, J.L.; Cheng, Z.Q.; Sun, J.Z.; Jiang, C.S. Synthesis and evaluation of coumarin/1,2,4-oxadiazole hybrids as selective BChE inhibitors with neuroprotective activity. J. Asian Nat. Prod. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, C.S. Synthesis and evaluation of coumarin/piperazine hybrids as acetylcholinesterase inhibitors. Med. Chem. Res. 2018, 27, 1717–1727. [Google Scholar] [CrossRef]
- Li, J.C.; Zhang, J.; Rodrigues, M.C.; Ding, D.J.; Longo, J.P.; Azevedo, R.B.; Muehlmann, L.A.; Jiang, C.S. Synthesis and evaluation of novel 1,2,3-triazole-based acetylcholinesterase inhibitors with neuroprotective activity. Bioorg. Med. Chem. Lett. 2016, 26, 3881–3885. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.S.; Ge, Y.X.; Cheng, Z.Q.; Song, J.L.; Wang, Y.Y.; Zhu, K.; Zhang, H. Discovery of new multifunctional selective acetylcholinesterase inhibitors: Structure-based virtual screening and biological evaluation. J. Comput. Aided Mol. Des. 2019, 33, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Nachon, F.; Carletti, E.; Ronco, C.; Trovaslet, M.; Nicolet, Y.; Jean, L.; Renard, P.Y. Crystal structures of human cholinesterases in complex with huprine W and tacrine: Elements of specificity for anti-Alzheimer's drugs targeting acetyl- and butyryl-cholinesterase. Biochem. J. 2013, 453, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Ghafary, S.; Najafi, Z.; Mohammadi-Khanaposhtani, M.; Nadri, H.; Edraki, N.; Ayashi, N.; Larijani, B.; Amini, M.; Mahdavi, M. Novel cinnamic acid-tryptamine hybrids as potent butyrylcholinesterase inhibitors: Synthesis, biological evaluation, and docking study. Arch. Pharm. 2018, 351, e1800115. [Google Scholar] [CrossRef]
- Razzokov, J.; Yusupov, M.; Bogaerts, A. Oxidation destabilizes toxic amyloid beta peptide aggregation. Sci. Rep. 2019, 9, 5476. [Google Scholar] [CrossRef]
- Yuan, C.; Gao, Z. Aβ interacts with both the iron center and the porphyrin ring of heme: Mechanism of heme’s action on Aβ aggregation and disaggregation. Chem. Res. Toxicol. 2013, 26, 262–269. [Google Scholar] [CrossRef]
- Caruso, G.; Distefano, D.A.; Parlascino, P.; Fresta, C.G.; Lazzarino, G.; Lunte, S.M.; Nicoletti, V.G. Receptor-mediated toxicity of human amylin fragment aggregated by short- and long-term incubations with copper ions. Mol. Cell. Biochem. 2017, 425, 85–93. [Google Scholar] [CrossRef]
- Selkoe, D.J. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav. Brain Res. 2008, 192, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, V.K.; Risacher, S.L.; Nho, K.; Kim, S.; Swaminathan, S.; Shen, L.; Foroud, T.M.; Hakonarson, H.; Huentelman, M.J.; Aisen, P.S.; et al. APOE and BCHE as modulators of cerebral amyloid deposition: A florbetapir PET genome-wide association study. Mol. Psychiatry 2014, 19, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.A.; Darvesh, S. Butyrylcholinesterase-knockout reduces brain deposition of fibrillar β-amyloid in an Alzheimer mouse model. Neuroscience 2015, 298, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.C.; Myers, J.B.; Folta, T.; Shoja, V.; Heath, L.S.; Onufriev, A. H++: A server for estimating pKas and adding missing hydrogens to macromolecules. Nucleic Acids Res. 2005, 33, W368–W371. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Bayly, C.I.; Cieplak, P.; Cornell, W.D.; Kollman, P.A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Case, D.A.; Cheatham, T.E.; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef]
- Ryckaert, J.P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Numerical-integration of Cartesian equations of motion of a system with constraints: Molecular-dynamics of nalkanes. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Fang, L.; Fang, X.; Gou, S.; Lupp, A.; Lenhardt, I.; Sun, Y.; Huang, Z.; Chen, Y.; Zhang, Y.; Fleck, C. Design, synthesis and biological evaluation of D-ring opened galantamine analogs as multifunctional anti-Alzheimer agents. Eur. J. Med. Chem. 2014, 76, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.W.; Ji, S.S.; Xiao, W.; Wang, X.D.; Jiang, C.S.; Ma, W.Q.; Zhang, H.Y.; Gong, J.X.; Guo, Y.W. Synthesis and biological evaluation of benzothiazol-based 1,3,4-oxadiazole derivatives as amyloid β-targeted compounds against Alzheimer’s disease. Monatsh. Chem. 2017, 148, 1807–1815. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–30 are available from the authors. |




| No. | R | BChE | AChE | ||
|---|---|---|---|---|---|
| IR a(%) | IC50 (μM) | IR(%) | IC50 (μM) | ||
| 1 | 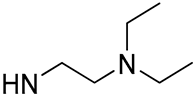 | 68.4 | 17.9 ± 1.86 | 26.6 | -b |
| 2 | 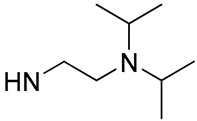 | 76.4 | 9.98 ± 0.89 | 56.1 | 21.46 ± 0.98 |
| 3 | 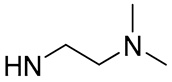 | 36.9 | - | 11.4 | - |
| 4 |  | 27.2 | - | 8.8 | - |
| 5 | 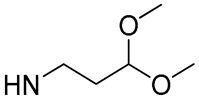 | 56.6 | 38.10 ± 1.68 | 2.7 | - |
| 6 |  | 19.1 | - | 13.4 | - |
| 7 | 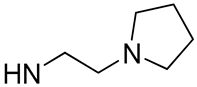 | 45.4 | 15.2 ± 2.33 | 4.4 | - |
| 8 | 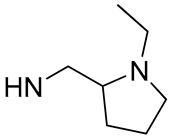 | 63.0 | 24.98 ± 1.27 | 13.3 | - |
| 9 | 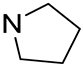 | 95.1 | 2.68 ± 0.28 | 29.1 | - |
| 10 | 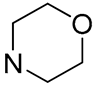 | 11.2 | - | 3.4 | - |
| Tacrine | 98.4 | 0.14 ± 0.01 | 96.2 | 0.11 ± 0.03 | |
| No. | 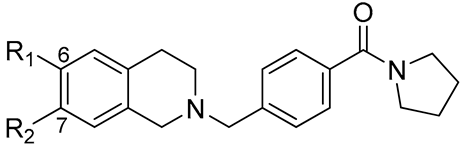 | BChE IC50 (μM) | AChE IC50 (μM) |
|---|---|---|---|
| 11 | R1 = Br, R2 = H | 8.38 ± 0.65 | 30.61 ± 1.82 |
| 12 | R1 = H, R2 = Br | 39.44 ± 2.25 | >50 |
| 13 | R1 = OMe, R2 = H | 12.62 ± 0.88 | 12.13 ± 0.90 |
| 14 | R1 = OMe, R2 = OMe | - | 18.24 ± 0.86 |
| No. | R | BChE | AChE | ||
|---|---|---|---|---|---|
| IR a(%) | IC50 (μM) | IR(%) | IC50 (μM) | ||
| 15 | 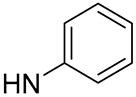 | 23.7 | -b | 8 | - |
| 16 | 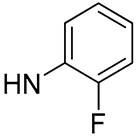 | 74.7 | 6.93 ± 0.48 | 2.1 | - |
| 17 | 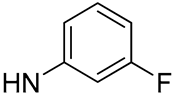 | 10.0 | - | 7.7 | - |
| 18 | 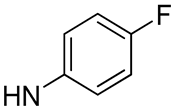 | 14.1 | - | 7.0 | - |
| 19 | 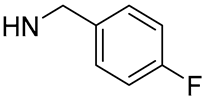 | 83.9 | 14.78 ± 0.58 | 2.3 | - |
| 20 | 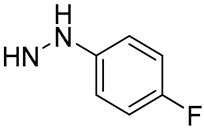 | 13.2 | - | 21.8 | - |
| 21 | 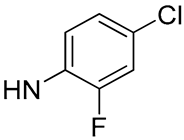 | 38.6 | - | 5 | - |
| 22 | 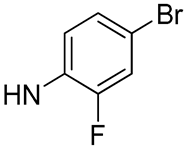 | 15.4 | - | 28.3 | - |
| 23 | 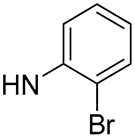 | 97.9 | 0.83 ± 0.08 | 22.7 | - |
| 24 |  | 35.1 | - | 21.6 | - |
| 25 | 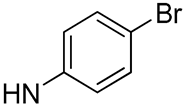 | 8.1 | - | 40.6 | - |
| 26 |  | 11 | - | 26.2 | - |
| 27 | 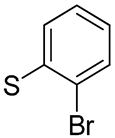 | 1.5 | - | 22.8 | - |
| 28 | 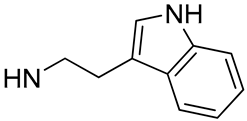 | 1.3 | - | 8.4 | - |
| 29 |  | 6.5 | - | 4.6 | - |
| 30 | 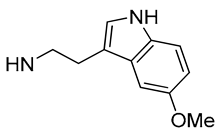 | 1.1 | - | 8.3 | - |
| Inhibitor | ΔGgas | ΔGsolv | ΔG |
|---|---|---|---|
| 23 | −59.52 ± 10.41 | 33.59 ± 9.06 | −25.93 ± 5.07 |
| Residue | Occupancy Rate (%) | Residue | Occupancy Rate (%) |
|---|---|---|---|
| D70 | 79 | P285 | 61 |
| W82 | 100 | A328 | 57 |
| G115 | 58 | F329 | 80 |
| G116 | 56 | Y332 | 78 |
| Y128 | 68 | H438 | 59 |
| E197 | 55 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.-S.; Ge, Y.-X.; Cheng, Z.-Q.; Wang, Y.-Y.; Tao, H.-R.; Zhu, K.; Zhang, H. Discovery of New Selective Butyrylcholinesterase (BChE) Inhibitors with Anti-Aβ Aggregation Activity: Structure-Based Virtual Screening, Hit Optimization and Biological Evaluation. Molecules 2019, 24, 2568. https://doi.org/10.3390/molecules24142568
Jiang C-S, Ge Y-X, Cheng Z-Q, Wang Y-Y, Tao H-R, Zhu K, Zhang H. Discovery of New Selective Butyrylcholinesterase (BChE) Inhibitors with Anti-Aβ Aggregation Activity: Structure-Based Virtual Screening, Hit Optimization and Biological Evaluation. Molecules. 2019; 24(14):2568. https://doi.org/10.3390/molecules24142568
Chicago/Turabian StyleJiang, Cheng-Shi, Yong-Xi Ge, Zhi-Qiang Cheng, Yin-Yin Wang, Hong-Rui Tao, Kongkai Zhu, and Hua Zhang. 2019. "Discovery of New Selective Butyrylcholinesterase (BChE) Inhibitors with Anti-Aβ Aggregation Activity: Structure-Based Virtual Screening, Hit Optimization and Biological Evaluation" Molecules 24, no. 14: 2568. https://doi.org/10.3390/molecules24142568
APA StyleJiang, C.-S., Ge, Y.-X., Cheng, Z.-Q., Wang, Y.-Y., Tao, H.-R., Zhu, K., & Zhang, H. (2019). Discovery of New Selective Butyrylcholinesterase (BChE) Inhibitors with Anti-Aβ Aggregation Activity: Structure-Based Virtual Screening, Hit Optimization and Biological Evaluation. Molecules, 24(14), 2568. https://doi.org/10.3390/molecules24142568






