Comprehensive Chemical Profiling and Multidirectional Biological Investigation of Two Wild Anthemis Species (Anthemis tinctoria var. Pallida and A. cretica subsp. tenuiloba): Focus on Neuroprotective Effects
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenolic and Flavonoid Contents
2.2. LC-MS Results
2.2.1. Acylquinic Acids
2.2.2. Flavonoids
2.2.3. Sesquiterpenes
2.3. Antioxidant Activity
2.4. Enzyme Inhibitory Activity
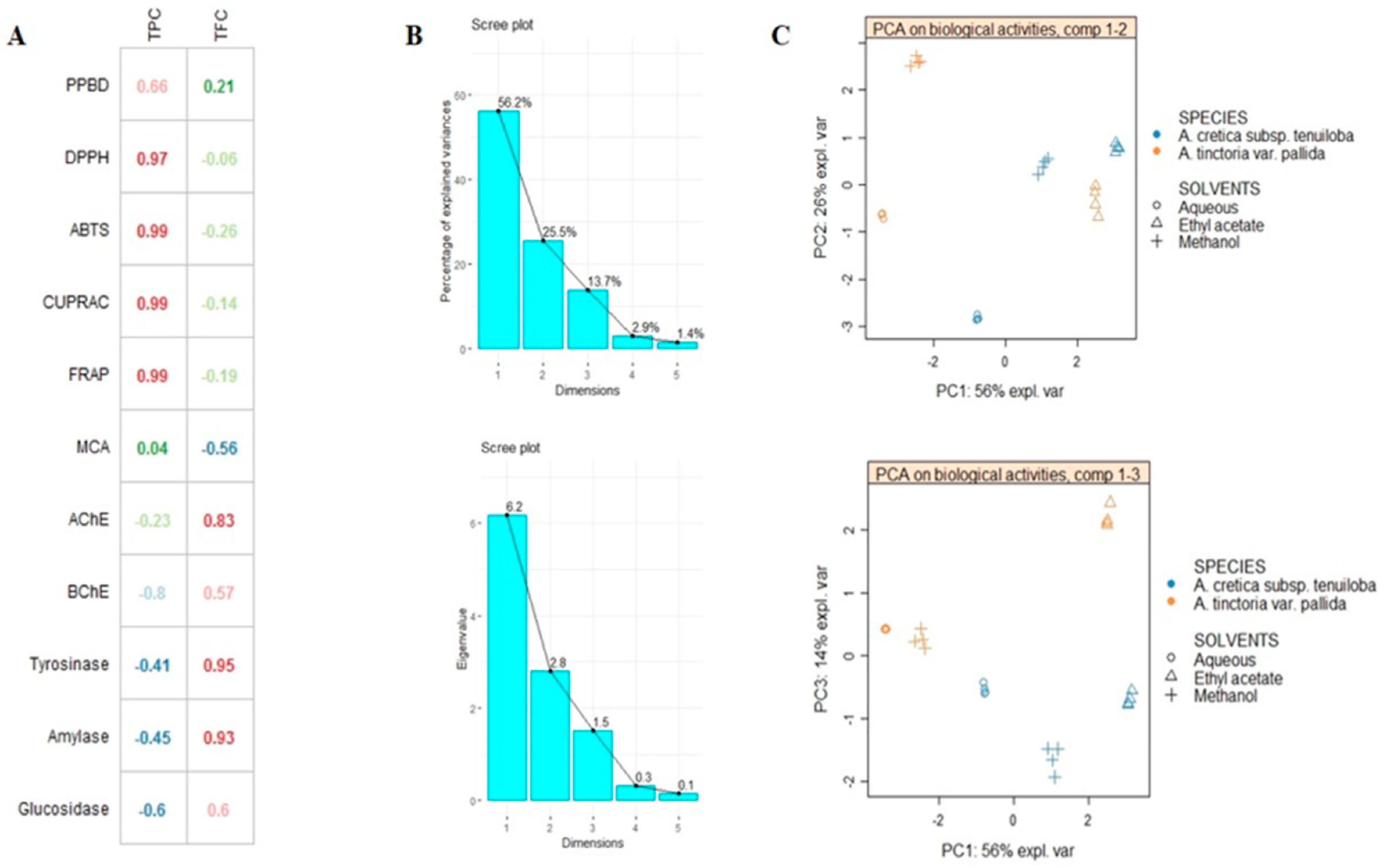
2.5. Multivariate Analysis
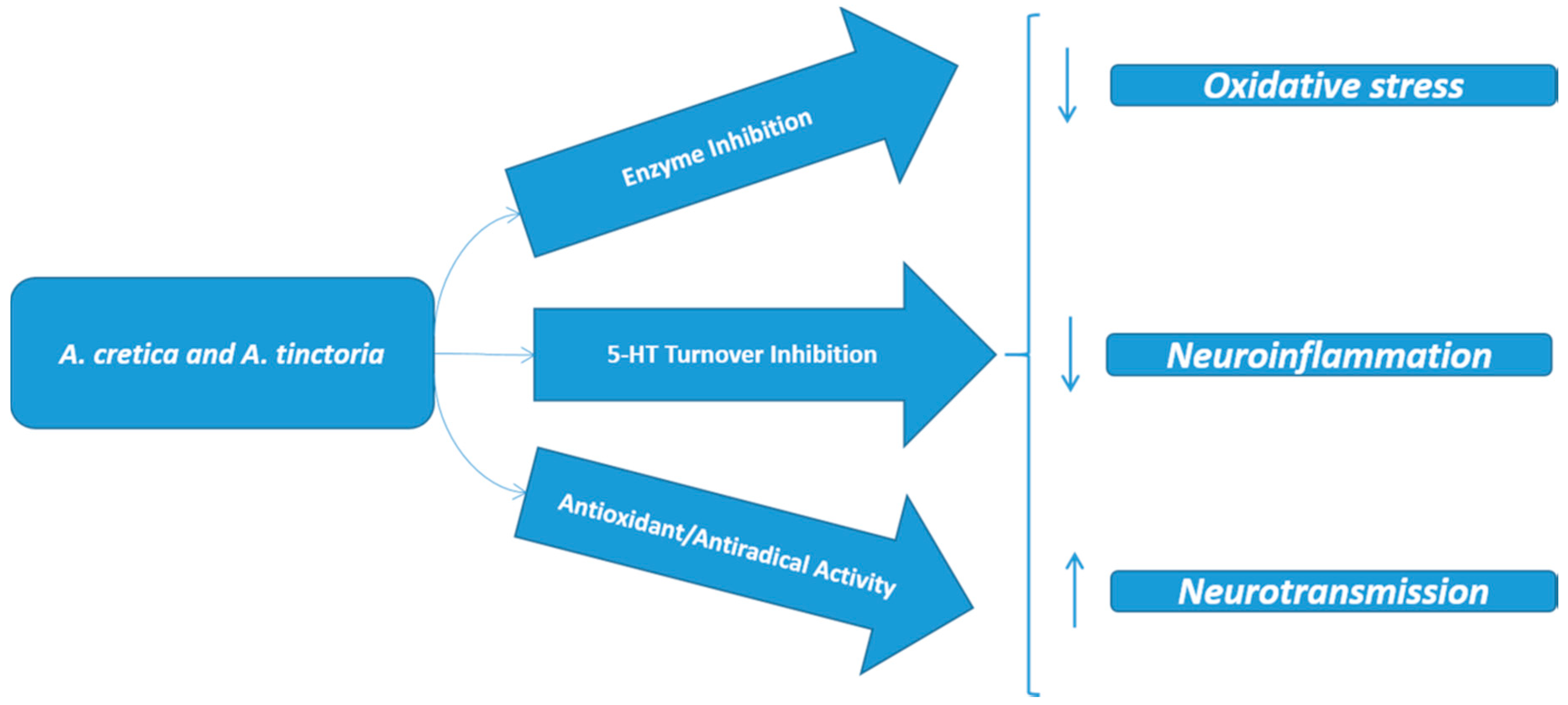
2.6. Multidirectional Biological Evaluation
3. Materials and Methods
3.1. Plant Material and Preparation of Extracts
3.2. Assays for Total Phenolic and Flavonoids
3.3. Antioxidant and Enzyme Inhibition Assays
3.4. UHPLC-ESI/HRMS Analysis
3.5. Statistical Analysis for Antioxidant and Enzyme Inhibitory Assays
3.6. Pharmacological Assays
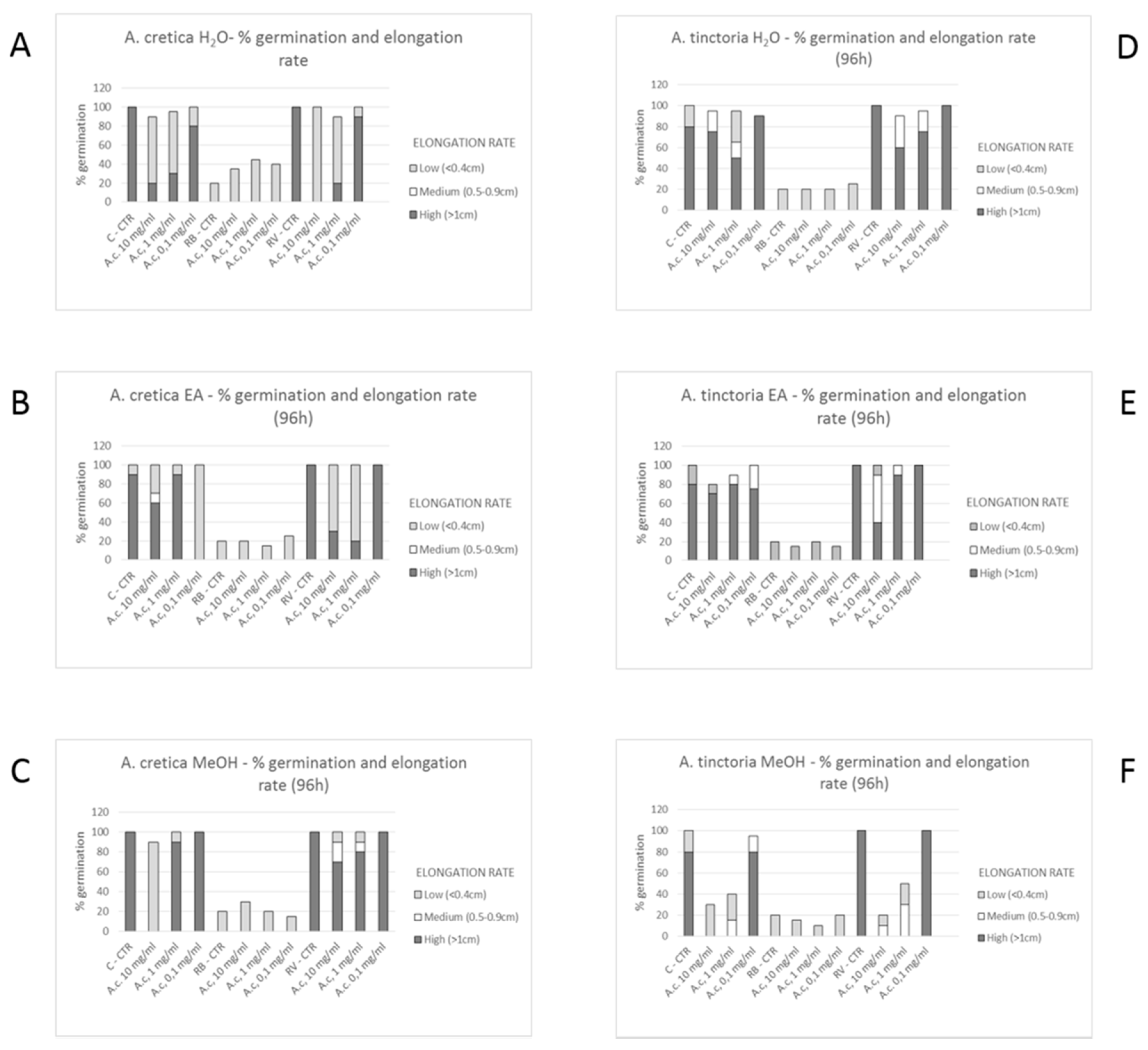
3.6.1. Allelopathy Bioassay
3.6.2. Artemia salina Lethality Bioassay
3.6.3. In Vitro Studies
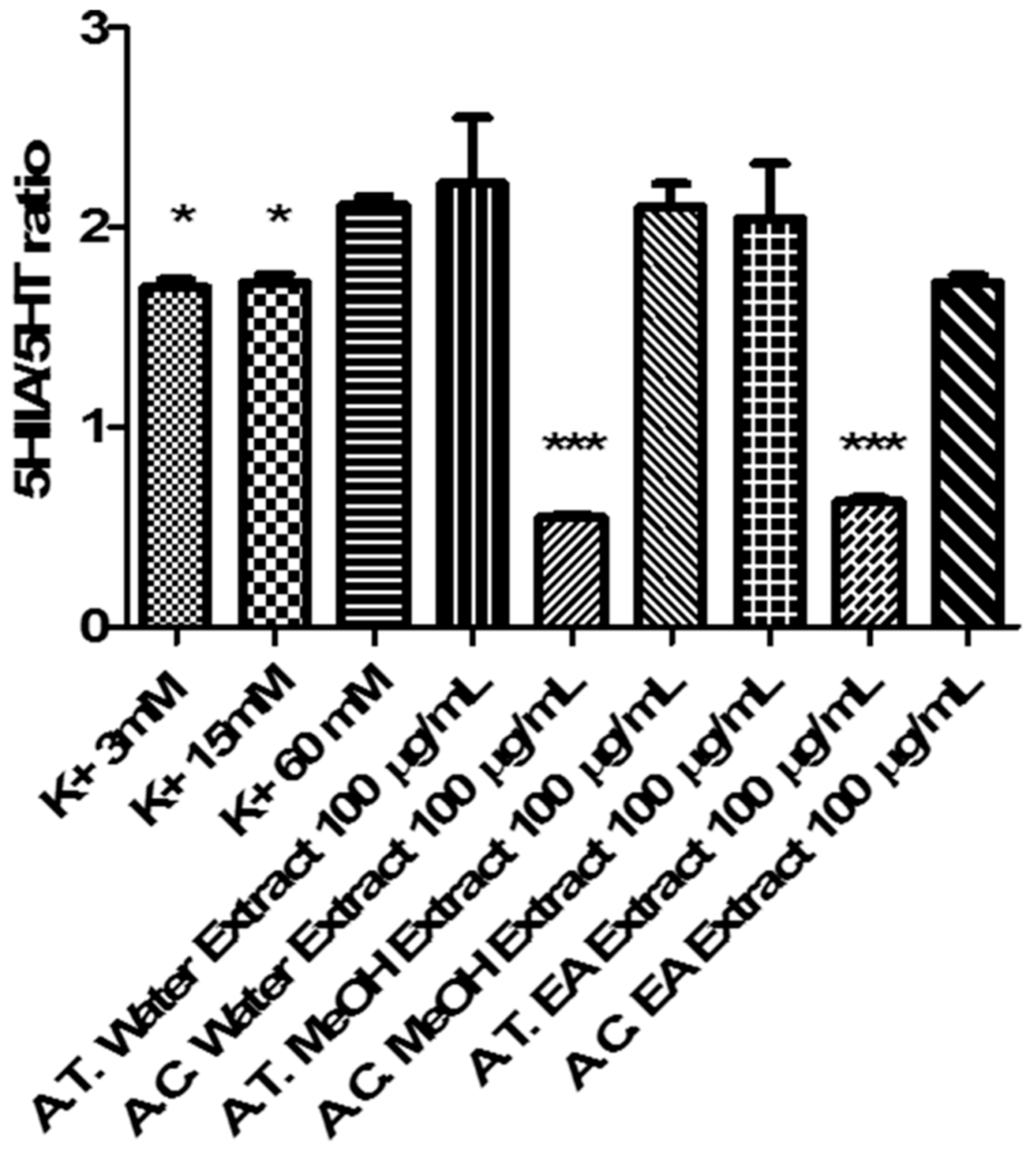
3.6.4. Ex Vivo Cortical Spreading Depression Paradigm
- K+ 3 mM: corresponding to basal condition;
- K+ 15 mM: corresponding to physiologic depolarizing-stimulus;
- K+ 60 mM: corresponding to excitotoxicity depolarizing-stimulus.
3.7. Protein Extraction and Filter-aided Sample Preparation
3.8. Statistical Analysis for Pharmacological Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Davis, P. Flora of Turkey and the Aegean Islands; Edinburgh University Press: Edinburgh, UK, 1975; Volume 5. [Google Scholar]
- Doğan, G.; Demirpolat, A.; Bağcı, E. Composition of the Volatile Oils of Anthemis coelopoda var. coelopoda from Turkey. Hacettepe J. Biol. Chem. 2015, 4, 259–265. [Google Scholar] [CrossRef]
- Staneva, J.D.; Todorova, M.N.; Evstatieva, L.N. Sesquiterpene lactones as chemotaxonomic markers in genus Anthemis. Phytochemistry 2008, 69, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Gonenc, T.; Argyropoulou, C.; Erdogan, T.; Gousiadou, C.; Juergenliemk, G.; Kıvçak, B.; Skaltsa, H. Chemical constituents from Anthemis wiedemanniana Fisch. & Mey. Biochem. Syst. Ecol. 2011, 39, 51–55. [Google Scholar]
- Kilic, O.; Kocak, A.; Bagci, E. Composition of the volatile oils of two Anthemis L. taxa from Turkey. Z. Für Nat. C 2011, 66, 535–540. [Google Scholar] [CrossRef]
- Vaverkova, S.; Habán, M.; Eerna, K. Qualitative Properties of Anthemis Tinctoria and Anthemis nobilis, (Chamaemelum nobile) under Different Environmental Conditions. Ecophysiology of Plant Production Processes in Stress Conditions. In Proceedings of the Fourth International Conference, Rackova Dolina, Slovakia, 12–14 September 2001; pp. 1–2. [Google Scholar]
- Aboee-Mehrizi, F.; Rustaiyan, A.; Zandi, H.; Ashkezari, M.D.; Zare, M. Chemical Composition and Antimicrobial Activity of the Essential Oil of Anthemis gayana Growing in Iran. J. Essent. Oil Bear. Plants 2016, 19, 1557–1560. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Tawaha, K.A.; Hudaib, M.M. Antioxidant, antimicrobial and antiproliferative activities of Anthemis palestina essential oil. Bmc Complementary Altern. Med. 2014, 14, 297. [Google Scholar] [CrossRef] [PubMed]
- Kurtulmus, A.; Fafal, T.; Mert, T.; Saglam, H.; Kivcak, B.; Ozturk, T.; Demirci, B.; Baser, K. Chemical composition and antimicrobial activity of the essential oils of three Anthemis species from Turkey. Chem. Nat. Compd. 2009, 45, 900–904. [Google Scholar] [CrossRef]
- Samadi, N.; Manayi, A.; Vazirian, M.; Samadi, M.; Zeinalzadeh, Z.; Saghari, Z.; Abadian, N.; Mozaffarian, V.-O.-A.; Khanavi, M. Chemical composition and antimicrobial activity of the essential oil of Anthemis altissima L. var. altissima. Nat. Prod. Res. 2012, 26, 1931–1934. [Google Scholar] [CrossRef] [PubMed]
- Eser, F.; Sahin Yaglioglu, A.; Dolarslan, M.; Aktas, E.; Onal, A. Dyeing, fastness, and cytotoxic properties, and phenolic constituents of Anthemis tinctoria var. tinctoria (Asteraceae). J. Text. Inst. 2017, 108, 1489–1495. [Google Scholar] [CrossRef]
- Kizil, S.; Kayabaşi, N.; Arslan, N. Determination of some agronomical and dyeing properties of dyer’s chamomile (Anthemis Tinctoria L.). J. Cent. Eur. Agric. 2006, 6, 403–408. [Google Scholar]
- Kültür, Ş. Medicinal plants used in Kırklareli province (Turkey). J. Ethnopharmacol. 2007, 111, 341–364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Won, Y.-K.; Ong, C.-N.; Shen, H.-M. Anti-cancer potential of sesquiterpene lactones: bioactivity and molecular mechanisms. Curr. Med. Chem. -Anti-Cancer Agents 2005, 5, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Gonenc, T.M.; Erdogan, T.F.; Demirci, B.; Baser, K.; Kivcak, B. Chemical composition of the essential oils of Anthemis coelopoda var. bourgaei and A. aciphylla var. aciphylla. Chem. Nat. Compd. 2012, 48, 332–334. [Google Scholar] [CrossRef]
- Pavlović, M.; Kovačević, N.; Tzakou, O.; Couladis, M. Essential oil composition of Anthemis triumfetti (L.) DC. Flavour Fragr. J. 2006, 21, 297–299. [Google Scholar]
- Uysal, I.; Celik, S.; Oldacay, M. Antimicrobial activity of Anthemis coelopoda Var. bourgaei Boiss. and Anthemis tinctoria Var. pallida DC. species having ethnobotanical features. J. Appl. Sci. 2005, 5, 639–642. [Google Scholar]
- Özüdoğru, B.; Akaydın, G.; Erik, S.; Yesilada, E. Inferences from an ethnobotanical field expedition in the selected locations of Sivas and Yozgat provinces (Turkey). J. Ethnopharmacol. 2011, 137, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Banagozar Mohammadi, A.; Torbati, M.; Farajdokht, F.; Sadigh-Eteghad, S.; Fazljou, S.M.B.; Vatandoust, S.M.; Golzari, S.E.J.; Mahmoudi, J. Sericin alleviates restraint stress induced depressive- and anxiety-like behaviors via modulation of oxidative stress, neuroinflammation and apoptosis in the prefrontal cortex and hippocampus. Brain Res. 2019, 1715, 47–56. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MS n identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MS n. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Gevrenova, R.; Zaharieva, M.M.; Najdenski, H.; Ruseva, S.; Lozanov, V.; Balabanova, V.; Yagi, S.; Momekov, G.; Mitev, V. HPLC-UV and LC–MS analyses of acylquinic acids in Geigeria alata (DC) Oliv. & Hiern. and their contribution to antioxidant and antimicrobial capacity. Phytochem. Anal. 2017, 28, 176–184. [Google Scholar] [PubMed]
- Justesen, U. Collision-induced fragmentation of deprotonated methoxylated flavonoids, obtained by electrospray ionization mass spectrometry. J. Mass Spectrom. 2001, 36, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Smelcerovic, A.; Lamshoeft, M.; Radulovic, N.; Ilic, D.; Palic, R. LC–MS Analysis of the Essential Oils of Achillea millefolium and Achillea crithmifolia. Chromatographia 2010, 71, 113–116. [Google Scholar] [CrossRef]
- Priestap, H.A.; Abboud, K.A.; Velandia, A.E.; Lopez, L.A.; Barbieri, M.A. Dehydroleucodin: a guaiane-type sesquiterpene lactone. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, o3470. [Google Scholar] [CrossRef] [PubMed]
- Michalska, K.; Żylewski, M.; Kisiel, W. Structure elucidation and complete NMR spectral assignments of two new sesquiterpene lactone xylosides from Lactuca triangulata. Magn. Reson. Chem. 2008, 46, 1185–1187. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Wu, W.; Kirkpatrick, J.; Kuhnert, N. Profiling the chlorogenic acids and other caffeic acid derivatives of herbal Chrysanthemum by LC− MS n. J. Agric. Food Chem. 2007, 55, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Zengin, G.; Lobine, D.; Mollica, A.; Locatelli, M.; Carradori, S.; Mahomoodally, M.F. Multiple pharmacological approaches on Fibigia eriocarpa extracts by in vitro and computational assays. Fundam. Clin. Pharmacol. 2018. [Google Scholar] [CrossRef]
- Ma, Y.-L.; Zhu, D.-Y.; Thakur, K.; Wang, C.-H.; Wang, H.; Ren, Y.-F.; Zhang, J.-G.; Wei, Z.-J. Antioxidant and antibacterial evaluation of polysaccharides sequentially extracted from onion (Allium cepa L.). Int. J. Biol. Macromol. 2018, 111, 92–101. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Calisi, A.; Giordano, M.E.; Schettino, T. Acetylcholinesterase as a biomarker in environmental and occupational medicine: new insights and future perspectives. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Greig, N.H.; Lahiri, D.K.; Sambamurti, K. Butyrylcholinesterase: an important new target in Alzheimer’s disease therapy. Int. Psychogeriatr. 2002, 14, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Pintus, F.; Sabatucci, A.; Maccarrone, M.; Dainese, E.; Medda, R. Amine oxidase from Euphorbia characias: Kinetic and structural characterization. Biotechnol. Appl. Biochem. 2018, 65, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Houghton, P.; Soumyanath, A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J. Ethnopharmacol. 2006, 107, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Ouassou, H.; Zahidi, T.; Bouknana, S.; Bouhrim, M.; Mekhfi, H.; Ziyyat, A.; Aziz, M.; Bnouham, M. Inhibition of α-Glucosidase, Intestinal Glucose Absorption, and Antidiabetic Properties by Caralluma europaea. Evid. -Based Complementary Altern. Med. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Ohikhena, F.U.; Wintola, O.A.; Afolayan, A.J. Toxicity Assessment of Different Solvent Extracts of the Medicinal Plant, Phragmanthera capitata (Sprengel) Balle on Brine Shrimp (Artemia salina). Int. J. Pharmacol. 2016, 12, 701–710. [Google Scholar]
- Richter, F.; Eitner, A.; Leuchtweis, J.; Bauer, R.; Ebersberger, A.; Lehmenkühler, A.; Schaible, H.-G. The potential of substance P to initiate and perpetuate cortical spreading depression (CSD) in rat in vivo. Sci. Rep. 2018, 8, 17656. [Google Scholar] [CrossRef] [PubMed]
- Raiteri, L.; Stigliani, S.; Zedda, L.; Raiteri, M.; Bonanno, G. Multiple mechanisms of transmitter release evoked by ‘pathologically’elevated extracellular [K+]: involvement of transporter reversal and mitochondrial calcium. J. Neurochem. 2002, 80, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Sbrenna, S.; Marti, M.; Morari, M.; Calo, G.; Guerrini, R.; Beani, L.; Bianchi, C. Modulation of 5-hydroxytryptamine efflux from rat cortical synaptosomes by opioids and nociceptin. Br. J. Pharmacol. 2000, 130, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Supornsilpchai, W.; Sanguanrangsirikul, S.; Maneesri, S.; Srikiatkhachorn, A. Serotonin depletion, cortical spreading depression, and trigeminal nociception. Headache J. Head Face Pain 2006, 46, 34–39. [Google Scholar] [CrossRef]
- Close, L.N.; Eftekhari, S.; Wang, M.; Charles, A.C.; Russo, A.F. Cortical spreading depression as a site of origin for migraine: Role of CGRP. Cephalalgia 2018, 0333102418774299. [Google Scholar] [CrossRef]
- Zarcone, D.; Corbetta, S. Shared mechanisms of epilepsy, migraine and affective disorders. Neurol. Sci. 2018, 38, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chang, C.; Liu, I.; Chi, T.; Yu, H.; Cheng, J. Changes in endogenous monoamines in aged rats. Clin. Exp. Pharmacol. Physiol. 2001, 28, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Orlando, G.; Ferrante, C.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A. Peripheral chemerin administration modulates hypothalamic control of feeding. Peptides 2014, 51, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Francisco, E.D.S.; Guedes, R.C. Sub-convulsing dose administration of pilocarpine reduces glycemia, increases anxiety-like behavior and decelerates cortical spreading depression in rats suckled on various litter sizes. Front. Neurosci. 2018, 12, 897. [Google Scholar] [CrossRef] [PubMed]
- Mollica, A.; Stefanucci, A.; Zengin, G.; Locatelli, M.; Macedonio, G.; Orlando, G.; Ferrante, C.; Menghini, L.; Recinella, L.; Leone, S. Polyphenolic composition, enzyme inhibitory effects ex-vivo and in-vivo studies on two Brassicaceae of north-central Italy. Biomed. Pharmacother. 2018, 107, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.; Recinella, L.; Locatelli, M.; Guglielmi, P.; Secci, D.; Leporini, L.; Chiavaroli, A.; Leone, S.; Martinotti, S.; Brunetti, L. Protective effects induced by microwave-assisted aqueous Harpagophytum extract on rat cortex synaptosomes challenged with amyloid β-peptide. Phytother. Res. 2017, 31, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Pace, L.; Tempesta, B.; Lavecchia, A.M.; Macheda, T.; Bedse, G.; Petrella, A.; Cifani, C.; Serviddio, G.; Vendemiale, G. Depressive-like behavior is paired to monoaminergic alteration in a murine model of Alzheimer’s disease. Int. J. Neuropsychopharmacol. 2015, 18. [Google Scholar] [CrossRef]
- Ramis, M.R.; Sarubbo, F.; Terrasa, J.L.; Moranta, D.; Aparicio, S.; Miralles, A.; Esteban, S. Chronic α-Tocopherol Increases Central Monoamines Synthesis and Improves Cognitive and Motor Abilities in Old Rats. Rejuvenation Res. 2016, 19, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, T.; Yue, F.; Li, X.; Wang, P.; Li, Y.; Chan, P.; Yu, S. Tea polyphenols alleviate motor impairments, dopaminergic neuronal injury, and cerebral α-synuclein aggregation in MPTP-intoxicated parkinsonian monkeys. Neuroscience 2015, 286, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Leporini, L.; Vecchiotti, G.; Locatelli, M.; Carradori, S.; Ferrante, C.; Zengin, G.; Recinella, L.; Chiavaroli, A.; Leone, S. Crocus sativus L. stigmas and byproducts: qualitative fingerprint, antioxidant potentials and enzyme inhibitory activities. Food Res. Int. 2018, 109, 91–98. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Fernández-Tajes, J.; Pásaro, E.; Méndez, J.; Laffon, B. Identification of differentially expressed genes in SHSY5Y cells exposed to okadaic acid by suppression subtractive hybridization. Bmc Genom. 2012, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Jahn, R.; Südhof, T.C. Membrane fusion and exocytosis. Annu. Rev. Biochem. 1999, 68, 863–911. [Google Scholar] [CrossRef] [PubMed]
- Manzur, A.; Sosa, M.; Seltzer, A.M. Transient increase in rab 3A and synaptobrevin immunoreactivity after mild hypoxia in neonatal rats. Cell. Mol. Neurobiol. 2001, 21, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Valdez, S.R.; Patterson, S.I.; Ezquer, M.E.; Torrecilla, M.; Lama, M.C.; Seltzer, A.M. Acute sublethal global hypoxia induces transient increase of GAP-43 immunoreactivity in the striatum of neonatal rats. Synapse 2007, 61, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wang, S.; Qin, G.; Xie, J.; Tan, G.; Zhou, J.; Chen, L. Protein kinase C γ contributes to central sensitization in a rat model of chronic migraine. J. Mol. Neurosci. 2017, 63, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Varadarajulu, J.; Lebar, M.; Krishnamoorthy, G.; Habelt, S.; Lu, J.; Bernard Weinstein, I.; Li, H.; Holsboer, F.; Turck, C.W.; Touma, C. Increased anxiety-related behaviour in Hint1 knockout mice. Behav Brain Res. 2011, 220, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef]
- Zengin, G.; Aktumsek, A.; Ceylan, R.; Uysal, S.; Mocan, A.; Guler, G.O.; Mahomoodally, M.F.; Glamoclija, J.; Ciric, A.; Sokovic, M. Shedding light on the biological and chemical fingerprints of three Achillea species (A. biebersteinii, A. millefolium and A. teretifolia). Food Funct. 2017, 8, 1152–1165. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef]
- Ferrante, C.; Orlando, G.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; Vacca, M. Central inhibitory effects on feeding induced by the adipo-myokine irisin. Eur. J. Pharmacol. 2016, 791, 389–394. [Google Scholar] [CrossRef]
Sample Availability: Samples of the extracts are available from the authors. |




| Plant Names | Solvents | Total Phenol Content (mg GAE/g) | Total Flavonoid Content (mg RE/g) |
|---|---|---|---|
| A. tinctoria var. pallida | EA | 26.46 ± 1.11 d | 45.82 ± 0.40 b |
| MeOH | 100.09 ± 2.83 a | 48.54 ± 0.57 a | |
| Aqueous | 86.74 ± 1.80 b | 23.10 ± 0.13 d | |
| A. cretica subsp. tenuiloba | EA | 21.31 ± 1.58 e | 46.26 ± 0.25 b |
| MeOH | 46.73 ± 0.80 c | 45.08 ± 0.26 c | |
| Aqueous | 47.61 ± 1.89 c | 21.17 ± 0.24 e |
| Peak № | Accurate Mass [M − H]− m/z | Molecular Formula | MS/MS Data m/z | tR min | Exact Mass [M − H]− m/z | Delta ppm | Tentative Structure | Ref. |
|---|---|---|---|---|---|---|---|---|
| Acylquinnic acids | ||||||||
| Monoacylquinic acids | ||||||||
| 1 | 337.0946 | C16H17O8 | 337.0946 (3.1), 191.0557 (7.7), 173.0453 (2.5), 163.0390 (100), 119.0488 (22.8) | 3.05 | 337.0929 | 5.071 | 3-p-coumaroyl-quinic acid 1,2,6 | [19] |
| 2 | 337.0932 | C16H17O8 | 337.0932 (7.2), 191.0554 (100), 173.0445 (6.7), 163.0390 (5.8), 119.0489 (5.5), 93.0329 (17.6) | 4.07 | 337.0929 | 0.829 | 5-p-coumaroyl-quinic acid 1,2,3,5,6 | [19] |
| 3 | 337.0930 | C16H17O8 | 337.0930 (5.9), 191.0554 (100), 173.0446 (1.9), 163.0391 (2.4), 127.0391 (1.5), 119.0487 (1.4), 111.0439 (1.3), 93.0331 (5.3), 85.0280 (7.1) | 4.74 | 337.0929 | 0.473 | 1-p-coumaroyl-quinic acid 1,2,5,6 | [19] |
| 4 | 353.0879 | C16H17O9 | 353.0879 (32.4), 191.0553 (100), 179.0341 (60.7), 173.0443 (4.1),161.0233 (3.9), 135.0439 (50.7), 111.0438 (0.9), 93.0333 (4.5), 85.0279 (8.7) | 2.47 | 353.0867 | 0.325 | neochlorogenic (3-caffeoylquinic) acid 1,2,3,4,6 | * |
| 5 | 353.0879 | C16H17O9 | 353.0879 (29.9), 191.0554 (47.7), 179.0341 (70.1), 173.0447 (100), 135.0439 (57.7), 111.0436 (3.3), 93.331 (22.6), 85.0280 (8.6) | 2.48 | 353.0867 | 0.240 | 1-caffeoylquinic acid 1,2,3,4,6 | [19] |
| 6 | 353.0880 | C16H17O9 | 353.0880 (3.8), 191.0554 (100), 179.0338 (1.4), 173.0446 (0.9), 161.0234 (2.1), 135.0441 (1.1), 111.0435 (1.2), 93.0331 (2.7), 85.0279 (0.4) | 3.30 | 353.0867 | 0.495 | chlorogenic (5-caffeoylquinic) acid 1,2,3,4,5,6 | * |
| 7 | 353.0880 | C16H17O9 | 353.0880 (28.1), 203.6057 (0.1), 191.0554 (100), 179.0341 (58.3), 173.0446 (4.6), 161.0233 (3.9), 135.0438 (51.8), 111.0437 (2.2), 93.0330 (4.82), 85.0279 (10.2) | 5.92 | 353.0867 | 0.665 | 4-caffeoylquinic acid 1,2,3,4,6 | [19] |
| 8 | 367.1043 | C17H19O9 | 367.1043 (12.5), 193.0500 (100), 173.0453 (3.9), 149.0598 (2.9), 134.0361 (65.7), 127.0395 (1.0), 111.0439 (1.4), 93.0331 (2.9) | 3.54 | 367.1034 | 2.410 | 3-feruloylquinic acid 1,2,5,6 | [19] |
| 9 | 367.1039 | C17H19O9 | 367.1039 (49.3), 161.0234 (100), 127.0390 (1.7), 85.0281 (13.4) | 3.97 | 367.1034 | 1.238 | 1-feruloylquinic acid 1,2,5,6 | [19] |
| 10 | 367.1035 | C17H19O9 | 367.1035 (14.1), 193.0499 (6.7), 191.0555 (100), 173.0447 (18.2), 134.0447 (10.9), 111.0437 (3.9), 93.0331 (26.2), 85.0280 (5.3) | 4.52 | 367.1034 | −0.015 | 5-feruloylquinic acid 1,2,3,4,5,6 | |
| 11 | 367.1030 | C17H19O9 | 367.1030 (11.8), 193.0499 (17.1), 173.0446 (100), 111.0435 (3.1), 93.0331 (24.0) | 4.78 | 367.1034 | -1.322 | 4-feruloylquinic acid 1,2,5,6 | [19] |
| Diacylquinic acids | ||||||||
| 12 | 515.1196 | C25H23O12 | 515.1196 (100), 353.0880 (18.5), 335.0788 (6.4), 203.0331 (1.3), 191.0554 (29.5), 179.0341 (58.1), 173.0446 (65.7), 161.0235 (19.8), 135.0439 (57.5), 127.0389 (3.6), 111.0436 (4.7), 93.0330 (18.7), 85.0277 (4.3) | 5.79 | 515.1184 | 0.137 | 3,4-dicaffeoylquinic acid 1,2,3,4,5,6 | * |
| 13 | 515.1199 | C25H23O12 | 515.1199 (21.8), 353.0881 (83.1), 335.0782 (2.6), 191.0554 (100), 179.0341 (48.6), 173.0445 (10.6), 161.0233 (12.4), 135.0439 (57.5), 127.0387 (2.8), 93.0332 (6.4), 85.0280 (9.0) | 5.96 | 515.1184 | 1,5-dicaffeoylquinic acid 1,2,3,4,5,6 | * | |
| 14 | 515.1204 | C25H23O12 | 515.1204 (22.6), 353.0880 (87.4), 191.0555 (100), 179.0342 (52.0), 173.0455 (9.8), 161.0235 (8.9), 135.0438 (51.7), 93.0333 (2.8), 85.0281 (8.6), | 6.14 | 515.1184 | 1.787 | 3,5-dicaffeoylquinic acid 3,4 | [28] |
| 15 | 515.1199 | C25H23O12 | 515.1199 (75.6), 353.0880 (60.6), 203.0343 (3.1), 191.0555 (34.7), 179.0342 (72.3), 173.0447 (100), 135.0440 (48.1), 127.0384 (1.2), 111.0439 (3.7), 93.0330 (17.2), 85.0280 (4.2) | 6.34 | 515.1184 | 0.720 | 4,5-dicaffeoylquinic acid 1,2,3,4,5,6 | [28] |
| 16 | 529.1356 | C26H25O12 | 529.1356 (100), 367.1037 (8.3), 353.0889 (7.4), 349.0935 (5.2), 335.0774 (11.8), 193.0499 (60.5), 191.0555 (9.2), 179.0342 (42.1), 173.0446 (48.1), 161.0235 (24.6), 149.0596 (1.1), 134.0361 (56.0), 111.0437 (8.9), 93.0331 (14.7), 85.0276 (2.7) | 6.00 | 529.1351 | 0.889 | 3-feruloyl-5-caffeoylquinic acid 1,2,4,5,6 | [28] |
| 17 | 529.1357 | C26H25O12 | 529.1357 (55.4), 367.1038 (25.6), 193.0499 (2.6), 179.0342 (3.0), 173.0449 (1.9), 161.0234 (100), 135.0441 (12.7), 134.0367 (4.3), 127.0380 (0.5), 93.0331 (1.1), 85.0279 (3.2) | 6.92 | 529.1351 | 1.003 | 1-feruloyl-5-caffeoylquinic acid 1,2,3,5 | [28] |
| 18 | 529.1354 | C26H25O12 | 529.1354 (38.3), 367.1040 (39.6), 353.0883 (44.99), 193.0506 (15.6), 191.0555 (100), 179.0343 (41.61), 173.0454 (14.8), 161.0239 (13.0), 135.0440 (52.3), 134.0363 (21.5), 93.0332 (14.2), 85.0281 (7.4) | 7.00 | 529.1351 | 0.436 | 3-caffeoyl-5-feruloylquinic acid 1,2,3,4 | [28] |
| 19 | 529.1352 | C26H25O12 | 529.1352 (66.9), 367.1044 (100), 193.0504 (12.1), 179.0333 (57.2), 173.0447 (76.9), 161.0236 (15.6), 135.0439 (73.7), 134.0365 (49.6), 93.0331 (76.7) | 7.12 | 529.1351 | 0.077 | 4-feruloyl-5-caffeoylquinic acid 4,5,6 | [28] |
| 20 | 529.1361 | C26H25O12 | 529.1361 (14.2), 367.1036 (60.3), 193.0499 (14.5), 173.0447 (100), 161.0239 (1.1), 134.0362 (17.5), 127.0392 (1.0), 111.0436 (3.4), 93.0330 (24.9), | 7.23 | 529.1351 | 1.816 | 3-caffeoyl-4-feruloylquinic acid 1,2,3,6 | [28] |
| 21 | 529.1388 | C26H25O12 | 529.1388 (83.7), 353.0898 (56.5), 191.0550 (75.9), 179.0346 (79.9), 173.0448 (100), 161.0237 (11.9), 135.0439 (69.8), 93.0330 (38.2) | 7.28 | 529.1351 | 6.880 | 4-caffeoyl-5-feruloylquinic acid 4,5,6 | [28] |
| 22 | 529.1356 | C26H25O12 | 529.1356 (74.2), 367.1031 (21.5), 349.0925 (2.1), 191.0554 (1.2), 179.0341 (30.5), 173.0446 (4.94), 161.0234 (100), 135.0439 (49.6), 93.0331 (1.9) | 7.34 | 529.1351 | 0.776 | 1-feruloyl-3-caffeoylquinic acid 1,2,3,5 | [28] |
| 23 | 529.1348 | C26H25O12 | 529.1348 (100), 367.1052 (45.3), 179.0348 (10.3), 173.0449 (60.2), 161.0237 (24.0), 135.0441 (16.4), 111.0439 (13.0), 93.0331 (77.1) | 7.44 | 529.1351 | −0.717 | 4-feruloyl-?-caffeoylquinic acid 4,6 | ** |
| 24 | 529.1353 | C26H25O12 | 529.1353 (74.9), 367.1037(19.5), 349.0932 (4.4), 179.0341 (73.22), 161.0234 (62.3), 135.0439 (100), 134.0364 (12.4), 93.0331 (0.6), 85.0276 (0.5) | 7.71 | 529.1351 | 0.304 | 1-feruloyl-3-caffeoylquinic acid-isomer 1,2,5,6 | ** |
| Triacylquinic acids | ||||||||
| 25 | 677.1523 | C34H29O15 | 677.1523 (2.9), 515.1105 (44.5), 353.0903 (3.7), 341.0888 (4.4), 191.0552 (9.6), 179.0341 (100), 173.0446 (6.0), 161.0230 (4.6), 135.0439 (79.8), 111.0443 (1.0), 93.0331 (1.6) | 4.85 | 677.1512 | 1.634 | 1,3,5-tricaffeoylquinic acid 1,2,4,5,6 | [28] |
| 26 | 677.1524 | C34H29O15 | 677.1524 (89.2), 515.1190 (17.3), 353.0879 (25.83), 341.0876 (8.0), 335.0772 (23.0), 323.0791 (5.1), 203.1523 (0.9), 191.0552 (30.6), 179.0341 (71.0), 173.0446 (54.7), 161.0233 (46.0), 135.0439 (100), 127.0388 (4.3), 111.0437 (7.8), 93.0330 (19.3), 85.0280 (1.9) | 5.26 | 677.1512 | 1.812 | 1,3,4-tricaffeoylquinic acid 1,2,3,5,6 | [28] |
| 27 | 677.1529 | C34H29O15 | 677.1529 (52.3), 515.1200 (55.7), 353.0878 (26.0), 341.0900 (15.6), 191.0553 (57.3), 179.0338 (75.3), 173.0449 (31.1), 161.0236 (34.66), 135.0439(100), 93.0328 (7.5) | 5.37 | 677.1512 | 2.447 | 1,3,5-tricaffeoylquinic acid 2,5,6 isomer | ** |
| 28 | 677.1524 | C34H29O15 | 677.1524 (87.1), 515.1281 (24.3), 353.0878 (26.2), 341.0880 (31.5), 335.0787 (2.3), 323.0779 (10.6), 191.0555 (34.4), 179.0342 (94.0), 173.0447 (78.7), 161.0235 (21.9), 135.0439 (100), 111.0439 (2.2), 93.0331 (24.8), 85.0279 (4.8) | 5.66 | 677.1512 | 1.812 | 1,4,5-tricaffeoylquinic acid 1,2,5,6 | [28] |
| 29 | 677.1519 | C34H29O15 | 677.1519 (88.4), 515.1203 (30.3), 353.0881 (55.6), 335.0779 (19.1), 203.0344 (1.3), 191.0554 (57.4), 179.0341 (79.9), 173.0447 (100), 161.0234 (33.1), 135.0439 (95.5), 111.0437 (1.1), 93.0331 (28.3), 85.0278 (6.3) | 7.85 | 677.1512 | 0.985 | 3,4,5-tricaffeoylquinic acid 1,2,3,4,5 | |
| Flavonoids | ||||||||
| 30 | 269.0457 | C15H9O5 | 269.0457 (100), 151.0027 (6.39), 149.0233 (5.74), 117.0332 (22.24), 107.0124 (5.35) | 8.73 | 269.0444 | 0.644 | apigenin 1,2,3,4,5,6 | * |
| 31 | 285.0406 | C15H9O6 | 285.0406 (100), 151.0028 (5.92), 133.0282 (25.08), 107.0126 (3.32) | 7.82 | 285.0393 | 0.452 | luteolin 1,2,3,4,5,6 | * |
| 32 | 287.0566 | C15H11O6 | 287.0566 (14.91), 151.0025 (100), 135.0435 (89.99), 125.0231 (5.03), 107.0124 (13.58) | 7.53 | 287.0550 | 1.842 | eriodictyol 1,2,3,4,5,6 | [24] |
| 33 | 299.0561 | C16H11O6 | 299.0561 (58.00), 284.0328 (100), 256.0382 (0.86), 227.0350 (3.45), 211.0393 (2.22) | 8.91 | 299.0550 | 0.029 | diosmetin 1,2,3,4,5,6 | * |
| 34 | 299.0562 | C16H11O6 | 299.0562 (95.99), 284.9335 (100), 227.047 (2.78), 151.0033 (3.67), 107.0123 (2.84) | 9.09 | 299.0550 | 0.430 | 3,4′,7-trihydroxy-3′-methoxyflavone 1,2,3,4,5,6 | [23] |
| 35 | 301.0352 | C15H9O7 | 301.0352 (100), 300.0273 (24.24), 178.9976 (21.40), 151.0025 (49.72), 121,0282 (16.11), 107.0124 (14.15) | 7.83 | 301.0342 | −0.717 | quercetin 1,2,3,4,5,6 | * |
| 36 | 315.0512 | C16H11O7 | 315.0512 (61.55), 300.0279 (100), 271.0252 (28.24), 255.030 (11.23), 227.0349 (2.28), 136.9872 (2.51), | 8.34 | 315.0499 | 0.584 | nepetin 4,5,6 | Mass bank |
| 37 | 315.0513 | C16H11O7 | 315.0513 (86.15), 301.0315 (11.76), 300.0276 (100), 243. 0303 (0.70), 165.9890 (1.63), 136.9868 (9.78) | 7.90 | 315.0499 | 0.965 | rhamnetin 1,2,3,4,5,6 | * |
| 38 | 315.0514 | C16H11O7 | 315.0514 (100), 301.0316 (3.73), 300.0273 (41.59), 243. 0298 (1.08), 151.0025 (7.85), 107.0126 (6.32) | 9.26 | 315.0499 | 1.156 | isorhamnetin 1,2,3,4,5,6 | * |
| 39 | 329.0670 | C17H13O7 | 329.0607 (14.45), 314.0436 (100), 299.0198 (25.08), 271.0250 (47.23),133.0282 (5.34), 107.2971 (0.52) | 9.25 | 329.0655 | 0.954 | jaceosidin 1,3,4,6 | Mass bank |
| 40 | 331.0463 | C16H11O8 | 331.0463 (100), 316.0226 (56.40), 287.0199 (15.97), 271.0246 (5.47), 270.0176 (4.09), 165.9897 (19.03) | 7.80 | 331.0448 | 1.086 | patuletin 1,3,4,6 | [23] |
| 41 | 345.0618 | C17H13O8 | 345.0618 (91.18), 330.0384 (100), 315.0150 (50.33), 287.0201 (15.30), 121.0280 (1.86) | 8.36 | 345.0604 | 0.694 | eupatuletin 4,5,6 | ** |
| 42 | 345.0618 | C17H13O8 | 345.0618 (100), 330.0385 (95.66), 315.0150 (46.41), 287.0198 (14.78), 121.0284 (7.72) | 8.40 | 345.0604 | 0.694 | spinatoside 1,2,3 | [23] |
| 43 | 345.0619 | C17H13O8 | 345.0619 (100), 330.0385 (42.35), 315.0145 (4.01), 301.0388 (6.46), 287.0199 (40.72) | 9.38 | 345.0604 | 0.694 | spinacetin 1,2,3 | [23] |
| 44 | 359.0775 | C18H15O8 | 359.0775 (100), 344.0539 (49.89), 329.0304 (52.64), 301.0359 (6.67), 287.0139 (4.46) | 9.95 | 359.0761 | 0.750 | jaceidin 1,2,3,4,5,6 | [23] |
| 45 | 431.0981 | C21H19O10 | 431.0981 (100), 269.0440 (27.72), 268.0378 (57.01) | 6.17 | 431.0972 | −0.673 | apigenin-7-O-glucoside 1,2,3,4,5,6 | * |
| 46 | 447.0934 | C21H19O11 | 447.0934 (100), 327.0507 (0.95), 285.0405 (99.96), 151.0030 (7.20), 133.0280 (5.74), 107.0123 (4.57) | 5.45 | 447.0921 | 0.348 | luteolin-7-O-glucoside 1,2,3,4,5,6 | * |
| 47 | 461.0725 | C21H17O12 | 461.0725 (42.84), 285.0406 (100), 151.0025 (3.72), 133.0280 (10.72), 107.0121 (1.50) | 5.46 | 461.0714 | −0.150 | luteolin-7-O-glucuronide 1,2,3 | * |
| 48 | 461.1094 | C22H21O11 | 461.1094 (100), 446.0858 (28.76), 299.0554 (12.48), 284.0313 (9.73), 283.0250 (21.41), 269.0467 (2.08), 255.0300 (73.76), 227.0345 (0.67), 151,0028 (0.86) | 6.37 | 461.1078 | 2.220 | diosmetin-O-glucoside 1,2,3 | ** |
| 49 | 463.0887 | C21H19O12 | 463.0887 (100), 372.1873 (4.84), 301.0360 (93.71), 300.0282 (27.20) | 4.77 | 463.0871 | 1.038 | isoquercitrin 1,2,3,4,5,6 | * |
| 50 | 463.0903 | C21H19O12 | 463.0903 (100), 301.0349 (47.50), 300.0276 (66.34) | 5.29 | 463.0871 | 4.601 | hyperoside 1,2,3,4,5,6 | * |
| 51 | 477.1041 | C22H21O12 | 477.1041 (100), 315.0496 (14.04), 314.0434 (53.04), 300.0274 (5.68), 285.0411 (6.84), 243.0297 (22.73), 271.0249 (27.98), 151.0032 (3.66) | 6.10 | 477.1027 | 0.442 | isorhamnetin-7-O-glucoside 1,2,3,4,5,6 | * |
| 52 | 493.0777 | C25H17O11 | 493.0777 (11.58), 315.0512 (99.41), 314.0435 (100), 300.0277 (14.30), 285.0411 (13.30), 243.0293 (34.06), 227.0341 (4.04), 177.0182 (16.10), 151.0030 (4.86), 133.0283 (12.50) | 9.12 | 493.0765 | 0.194 | caffeoyl-O-isorhamnetin 1,3 | ** |
| 53 | 493.0974 | C22H21O13 | 493.0974 (100), 331.0463 (96.71), 316.0224 (22.61), 287.0196 (26.93), 271.0253 (9.58), 165.9891 (8.24) | 5.63 | 493.0976 | −0.257 | patuletin-O-hexoside 4,5,6 | ** |
| 54 | 593.1504 | C27H29O15 | 593.1504 (97.39), 318.6214 (6.24), 285.0405 (100), 284.0327 (60.74), 227.0352 (29.58) | 5.71 | 593.1500 | −1.354 | luteolin-7-O-rutinoside 1,2,3,4,5,6 | * |
| 55 | 609.1467 | C27H29O16 | 609.1467 (72.52), 343.0477 (1.65), 301.0354 (100), 300.0278 (38.67), 178.9970 (1.59), 151.0027 (13.46),121.0279 (1.84), 107.0123 (4.76) | 5.17 | 609.1450 | 0.923 | rutin 1,2,3,4,5,6 | * |
| 56 | 609.1472 | C27H29O16 | 609.1472 (96.84), 411.8945 (5.23), 315.0517 (100), 300.0275 (48.87), 133.0284 (7.86) | 5.43 | 609.1450 | 2.149 | isorhamnetin-O-pentosyl-hexoside 4,5,6 | ** |
| 57 | 623.1613 | C28H31O16 | 623.1613 (5.57), 315.0514 (100), 301.0306 (1.22), 300.0279 (16.19), 151.0025 (7.85), 107.0125 (3.48) | 6.08 | 623.1606 | −0.703 | isorhamnetin-3-O-rutinoside 1,2,3,4,5,6 | * |
| Sesquiterpenes | ||||||||
| 58 | 229.1220 | C15H17O2 | 229.1220 (100), 211.1116 (12.69), 201.1272 (19.57), 183.1167 (40.99), 91.0548 (6.40), | 6.10 | 229.1223 | −3.643 | chamazulene carboxylic acid 1,2,3 | [25] |
| 59 | 245.1170 | C15H17O3 | 245.1170 (100), 227.1067 (44.35), 217.1222 (20.20), 201.0910 (20.68), 199.1116 (75.08), 185.0959 (28.15), 95.0497 (66.93) | 10.48 | 245.1172 | −0.942 | dehydroleucodin/isodehydroleucodin 1,2,3 | [26] |
| 60 | 245.1171 | C15H17O3 | 245.1171 (84.77), 227.1065 (44.23), 217.1225 (17.55), 201.0910 (17.75), 199.1116 (63.86), 185.0960 (18.51), 95.0497 (100) | 10.48 | 245.1172 | −0.942 | dehydroleucodin/isodehydroleucodin 1,2,3 | [26] |
| 61 | 247.1325 | C15H19O3 | 247.1325 (100), 229.1220 (90.72), 211.1113 (8.83), 201.1271 (37.99), 187.0752 (37.99), 183.1167 (22.85), 91.0548 (9.16) | 9.79 | 247.1328 | −1.663 | desacetoxymatricarin 1,2,3,4,5,6 | ** |
| 62 | 249.1481 | C15H21O3 | 249.1481 (100), 231.1378 (30.41), 213.1277 (9.04), 203.1431 (10.25), 193.0856 (5.87), 189.1272 (11.79), 159.1167 (22.04), 119.0857 (16.94), 105.0702 (18.62), 95.0860 (10.61) | 10.42 | 249.1485 | −0.451 | parthenolide 1,2,3,4,5,6 | ** |
| 63 | 263.1275 | C15H19O4 | 263.1275 (100), 245.1170 (55.39), 227.1061 (9.88), 219.1021 (15.59), 217.1223 (47.56), 203.1065 (10.39), 199.1115 (21.45), 191.0704 (28.72), 95.0497 (96.99) | 10.52 | 263.1277 | −0.316 | hydroxyleucodin 1,2,3 | ** |
| 64 | 263.1274 | C15H19O4 | 263.1274 (100), 245.1171 (33.08), 227.1063 (4.95), 219.1012 (14.30), 217.1224 (16.65), 203.1065 (10.12), 199.1117 (15.19), 191.0702 (79.26), 95.0497 (20.99) | 11.22 | 263.1277 | −0.406 | hydroxyleucodin isomer 1,2,3 | ** |
| 65 | 263.1275 | C15H19O4 | 263.1275(100), 245.1170 (51.98), 227.1063 (11.55), 219.1014 (34.60), 217.1223 (47.24), 203.1067 (12.57), 199.1117 (22.59), 191.0702 (32.46), 95.0497 (81.14) | 11.22 | 263.1277 | −0.406 | hydroxyleucodin isomer 1,2,3 | ** |
| 66 | 265.1432 | C15H21O4 | 265.1432 (8.16), 247.1326 (34.45), 229.1221 (100), 211.1115 (3.94), 201.1273 (20.45), 187.0752 (14.30), 183.1168 (7.67), 91.0548 (5.66) | 6.04 | 265.1434 | −0.813 | stizolin 1,3 | ** |
| 67 | 265.1429 | C15H21O4 | 265.1429 (24.21), 247.1325 (100), 229.1222 (71.63), 219.1372 (36.25), 201.1272 (55.14), 187.1111 (17.50), 183.1174 (17.90), 91.0548 (13.40) | 9.78 | 265.1434 | −1.982 | stizolin isomer 1,3 | ** |
| 68 | 305.1360 | C17H21O5 | 305.1360 (44.97), 287.1268 (3.27), 269.1206 (1.63), 263.1274 (42.58), 245.1170 (100), 227.1065 (63.42), 217.1220 (60.59), 109.1116 (23.36), 201.0903 (12.29), 185.0958 (19.24), 181.1010 (44.17), 171.1166 (56.51), 105.0703 (50.85), 95.0496 (25.16) | 7.05 | 305.1383 | −7.538 | matricarin 3 | ** |
| 69 | 305.1363 | C17H21O5 | 305.1363 (23.50), 263.1279 (20.85), 245.1170 (100), 227.1063 (29.92), 217.1216 (19.51), 201.0911 (2.62), 185.0961 (16.47), 181.1009 (28.17), 171.1167 (31.70), 131.0857 (46.50), 95.0496 (3.40) | 5.42 | 305.1383 | −6.654 | matricarin isomer 3 | ** |
| 70 | 307.1544 | C17H21O5 | 307.1544 (2.15), 289.1433 (0.75), 267.2721 (0.18), 247.1325 (2.63), 229.1219 (100), 211.1115 (0.95), 183.1168 (2.88) | 9.67 | 307.1540 | 1.366 | ludalbin 1,2,3 | ** |
| Plant Names | Solvents | Phosphomolybdenum (mmol TE/g) | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mgTE/g) | FRAP (mgTE/g) | Metal Chelating Abilitiy (mg EDTAE/g) |
|---|---|---|---|---|---|---|---|
| A. tinctoria var. pallida | Ethyl acetate | 2.59 ± 0.19 b | 40.30 ± 0.78 e | 45.52 ± 5.53 f | 113.31 ± 2.26 d | 47.63 ± 3.77 f | 39.01 ± 4.42 a |
| Methanol | 2.99 ± 0.14 a | 407.07 ± 8.88 a | 320.11 ± 5.67 a | 691.17 ± 12.07 a | 362.12 ± 2.63 a | 28.28 ± 1.81 c | |
| Aqueous | 2.65 ± 0.02 b | 298.40 ± 6.74 b | 303.16 ± 8.57 b | 584.01 ± 8.71 b | 316.34 ± 4.15 b | 33.59 ± .16 b | |
| A. cretica subsp. tenuiloba | Ethyl acetate | 1.69 ± 0.08 cd | 45.47 ± 2.16 e | 57.13 ± 3.89 e | 112.87 ± 4.41 d | 55.74 ± 2.27 e | 21.90 ± 0.81 d |
| Methanol | 1.77 ± 0.08 c | 97.22 ± 0.22 c | 112.41 ± 2.35 d | 223.09 ± 6.17 c | 143.21 ± 1.77 d | 20.93 ± 1.70 d | |
| Aqueous | 1.56 ± 0.05 d | 86.74 ± 2.46 d | 127.68 ± 0.45 c | 214.45 ± 1.39 c | 130.86 ± 1.81 c | 39.64 ± 1.34 a |
| Plant Names | Solvents | AChE Inhibition (mg GALAE/g) | BChE Inhibition (mg GALAE/g) | Tyrosinase Inhibition (mg KAE/g) | Amylase Inhibition (mmol ACAE/g) | Glucosidase Inhibition (mmol ACAE/g) |
|---|---|---|---|---|---|---|
| A. tinctoria var. pallida | Ethyl acetate | 1.33 ± 0.03 c | 3.48 ± 0.21 a | 124.60 ± 0.15 b | 0.78 ± 0.05 a | 21.94 ± 1.91 b |
| Methanol | 3.28 ± 0.43 b | na | 124.48 ± 1.23 b | 0.54 ± 0.02 c | 9.15 ± 2.26 c | |
| Aqueous | na | na | 72.10 ± 1.64 d | 0.11 ± 0.01 d | 4.95 ± 0.25 d | |
| A. cretica subsp. tenuiloba | Ethyl acetate | 4.68 ± 0.21 a | 2.51 ± 0.34 b | 128.73 ± 0.71 a | 0.65 ± 0.01 b | 24.16 ± 0.12 a |
| Methanol | 3.45 ± 0.26 b | 1.15 ± 0.05 c | 128.85 ± 1.41 a | 0.52 ± 0.06 c | 4.49 ± 0.93 d | |
| Aqueous | na | na | 88.95 ± 0.49 c | 0.09 ± 0.01 d | 2.49 ± 0.17 e |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlando, G.; Zengin, G.; Ferrante, C.; Ronci, M.; Recinella, L.; Senkardes, I.; Gevrenova, R.; Zheleva-Dimitrova, D.; Chiavaroli, A.; Leone, S.; et al. Comprehensive Chemical Profiling and Multidirectional Biological Investigation of Two Wild Anthemis Species (Anthemis tinctoria var. Pallida and A. cretica subsp. tenuiloba): Focus on Neuroprotective Effects. Molecules 2019, 24, 2582. https://doi.org/10.3390/molecules24142582
Orlando G, Zengin G, Ferrante C, Ronci M, Recinella L, Senkardes I, Gevrenova R, Zheleva-Dimitrova D, Chiavaroli A, Leone S, et al. Comprehensive Chemical Profiling and Multidirectional Biological Investigation of Two Wild Anthemis Species (Anthemis tinctoria var. Pallida and A. cretica subsp. tenuiloba): Focus on Neuroprotective Effects. Molecules. 2019; 24(14):2582. https://doi.org/10.3390/molecules24142582
Chicago/Turabian StyleOrlando, Giustino, Gokhan Zengin, Claudio Ferrante, Maurizio Ronci, Lucia Recinella, Ismail Senkardes, Reneta Gevrenova, Dimitrina Zheleva-Dimitrova, Annalisa Chiavaroli, Sheila Leone, and et al. 2019. "Comprehensive Chemical Profiling and Multidirectional Biological Investigation of Two Wild Anthemis Species (Anthemis tinctoria var. Pallida and A. cretica subsp. tenuiloba): Focus on Neuroprotective Effects" Molecules 24, no. 14: 2582. https://doi.org/10.3390/molecules24142582
APA StyleOrlando, G., Zengin, G., Ferrante, C., Ronci, M., Recinella, L., Senkardes, I., Gevrenova, R., Zheleva-Dimitrova, D., Chiavaroli, A., Leone, S., Di Simone, S., Brunetti, L., Picot-Allain, C. M. N., Mahomoodally, M. F., Sinan, K. I., & Menghini, L. (2019). Comprehensive Chemical Profiling and Multidirectional Biological Investigation of Two Wild Anthemis Species (Anthemis tinctoria var. Pallida and A. cretica subsp. tenuiloba): Focus on Neuroprotective Effects. Molecules, 24(14), 2582. https://doi.org/10.3390/molecules24142582















