Epoxy-Containing Ionic Liquids with Tunable Functionality
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermophysical Properties
2.2. Solution Properties
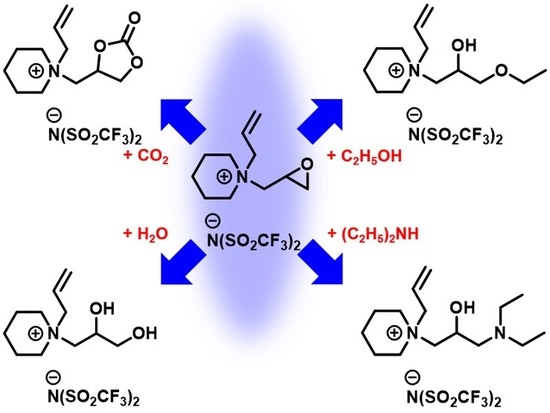
2.3. Carboxylation and Decarboxylation Reactions
2.4. Epoxide-Opening Reactions
3. Materials and Methods
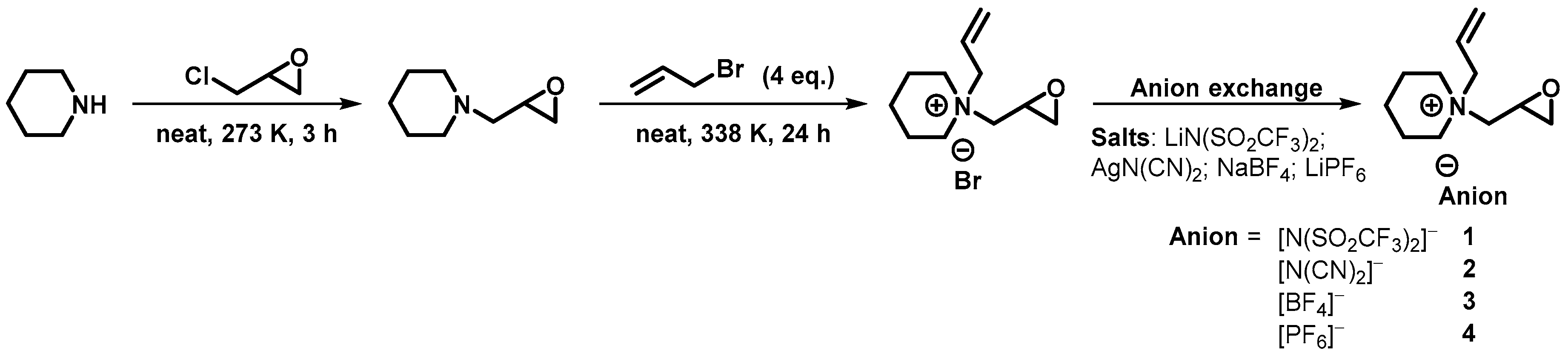
3.1. Synthesis of 1-(Oxiran-2-ylmethyl)piperidine
3.2. Synthesis of 1-Allyl-1-(oxiran-2-ylmethyl)piperidinium Bromide
3.3. General Synthetic Procedure for Organic Salts with Epoxy Moieties
3.3.1. 1-Allyl-1-(oxiran-2-ylmethyl)piperidinium bis(trifluoromethanesulfonyl)amide (1)
3.3.2. 1-Allyl-1-(oxiran-2-ylmethyl)piperidinium dicyanamide (2)
3.3.3. 1-Allyl-1-(oxiran-2-ylmethyl)piperidinium tetrafluoroborate (3)
3.3.4. 1-Allyl-1-(oxiran-2-ylmethyl)piperidinium hexafluorophosphate (4)
3.4. Physicochemical Property Measurements
3.5. Carbonylation of Ionic Liquid 1 Using Gaseous CO2
1-Allyl-1-[(2-oxo-1,3-dioxolan-4-yl)methyl]piperidinium bis(trifluoromethanesulfonyl)amide (5)
3.6. Epoxide-Opening Reaction of Ionic Liquid 1 with Several Reagents
3.6.1. 1-Allyl-1-(3-ethoxy-2-hydroxypropyl)piperidinium bis(trifluoromethanesulfonyl)amide (6)
3.6.2. 1-Allyl-1-(2,3-dihydroxypropyl)piperidinium bis(trifluoromethanesulfonyl)amide (7)
3.6.3. 1-Allyl-1-(3-(diethylamino)-2-hydroxypropyl)piperidinium bis(trifluoromethanesulfonyl)amide (8)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wasserscheid, P.; Welton, T. (Eds.) Ionic Liquids in Synthesis; Wiley: Weinheim, Germany, 2008; ISBN 9783527621200. [Google Scholar]
- Tsuda, T.; Hussey, C.L. Electrochemistry of room-temperature ionic liquids and melts. In Modern Aspects of Electrochemistry 45; White, R.E., Ed.; Springer: New York, NY, USA, 2009; pp. 63–174. ISBN 9781441906557. [Google Scholar]
- Nishimura, N.; Ohno, H. (Eds.) Electrochemical Aspects of Ionic Liquids; Wiley: Hoboken, New Jersey, USA, 2011; ISBN 9781118003350. [Google Scholar]
- Torimoto, T.; Tsuda, T.; Okazaki, K.; Kuwabata, S. New frontiers in materials science opened by ionic liquids. Adv. Mater. 2009, 22, 1196–1221. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Liang, Y.; Liu, W. Ionic liquid lubricants: Designed chemistry for engineering applications. Chem. Soc. Rev. 2009, 38, 2590–2599. [Google Scholar] [CrossRef] [PubMed]
- Somers, A.; Howlett, P.; MacFarlane, D.; Forsyth, M. A review of ionic liquid lubricants. Lubricants 2013, 1, 3–21. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Endres, F.; Abbott, A.; MacFarlane, D.R. (Eds.) Electrodeposition from Ionic Liquids; Wiley: Weinheim, Germany, 2017; ISBN 9783527682720. [Google Scholar]
- Tsuda, T.; Stafford, G.R.; Hussey, C.L. Review–Electrochemical surface finishing and energy storage technology with room-temperature haloaluminate ionic liquids and mixtures. J. Electrochem. Soc. 2017, 164, H5007–H5017. [Google Scholar] [CrossRef]
- Torimoto, T.; Okazaki, K.I.; Kiyama, T.; Hirahara, K.; Tanaka, N.; Kuwabata, S. Sputter deposition onto ionic liquids: Simple and clean synthesis of highly dispersed ultrafine metal nanoparticles. Appl. Phys. Lett. 2006, 89, 243117. [Google Scholar] [CrossRef]
- Kuwabata, S.; Minamimoto, H.; Inoue, K.; Imanishi, A.; Hosoya, K.; Uyama, H.; Torimoto, T.; Tsuda, T.; Seki, S. Three-dimensional micro/nano-scale structure fabricated by combination of non-volatile polymerizable RTIL and FIB irradiation. Sci. Rep. 2014, 4, 3722. [Google Scholar] [CrossRef]
- Tsuda, T.; Nemoto, N.; Kawakami, K.; Mochizuki, E.; Kishida, S.; Tajiri, T.; Kushibiki, T.; Kuwabata, S. SEM observation of wet biological specimens pretreated with room-temperature ionic liquid. Chembiochem 2011, 12, 2547–2550. [Google Scholar] [CrossRef]
- Uematsu, T.; Baba, M.; Oshima, Y.; Tsuda, T.; Torimoto, T.; Kuwabata, S. Atomic resolution imaging of gold nanoparticle generation and growth in ionic liquids. J. Am. Chem. Soc. 2014, 136, 13789–13797. [Google Scholar] [CrossRef]
- Itoi, M.; Jike, T.; Nishio-Hamane, D.; Udagawa, S.; Tsuda, T.; Kuwabata, S.; Boukheddaden, K.; Andrus, M.J.; Talham, D.R. Direct observation of short-range structural coherence during a charge transfer induced spin transition in a CoFe Prussian blue analogue by transmission electron microscopy. J. Am. Chem. Soc. 2015, 137, 14686–14693. [Google Scholar] [CrossRef]
- Chen, C.Y.; Sano, T.; Tsuda, T.; Ui, K.; Oshima, Y.; Yamagata, M.; Ishikawa, M.; Haruta, M.; Doi, T.; Inaba, M.; et al. In situ scanning electron microscopy of silicon anode reactions in lithium-ion batteries during charge/discharge processes. Sci. Rep. 2016, 6, 36153. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, X.; Zhou, Q.; Li, X.; Zhang, X.; Li, S. Ionic Liquids: Physicochemical Properties; Elsevier Science: Amsterdam, The Netherlands, 2009; ISBN 9780080959078. [Google Scholar]
- Marcus, Y. Ionic Liquid Properties: From Molten Salts to RTILs; Springer International Publishing: Cham, Switzerland, 2016; ISBN 9783319303130. [Google Scholar]
- Maton, C.; De Vos, N.; Stevens, C. V Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963–5977. [Google Scholar] [CrossRef]
- Feng, W.-Q.; Lu, Y.-H.; Chen, Y.; Lu, Y.-W.; Yang, T. Thermal stability of imidazolium-based ionic liquids investigated by TG and FTIR techniques. J. Therm. Anal. Calorim. 2016, 125, 143–154. [Google Scholar] [CrossRef]
- Ohno, H.; Ito, K. Room-temperature molten salt polymers as a matrix for fast ion conduction. Chem. Lett. 2003, 27, 751–752. [Google Scholar] [CrossRef]
- Hirao, M.; Ito, K.; Ohno, H. Preparation and polymerization of new organic molten salts; N-alkylimidazolium salt derivatives. Electrochim. Acta 2000, 45, 1291–1294. [Google Scholar] [CrossRef]
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H. CO2 capture by a task-specific ionic liquid. J. Am. Chem. Soc. 2002, 124, 926–927. [Google Scholar] [CrossRef]
- Tsuda, T.; Kondo, K.; Tomioka, T.; Takahashi, Y.; Matsumoto, H.; Kuwabata, S.; Hussey, C.L. Design, synthesis, and electrochemistry of room-temperature ionic liquids functionalized with propylene carbonate. Angew. Chem. Int. Ed. 2011, 50, 1310–1313. [Google Scholar] [CrossRef]
- Zhang, Z.; Veith, G.M.; Brown, G.M.; Fulvio, P.F.; Hillesheim, P.C.; Dai, S.; Overbury, S.H. Ionic liquid derived carbons as highly efficient oxygen reduction catalysts: First elucidation of pore size distribution dependent kinetics. Chem. Commun. 2014, 50, 1469–1471. [Google Scholar] [CrossRef]
- Zhang, S.; Dokko, K.; Watanabe, M. Carbon materialization of ionic liquids: From solvents to materials. Mater. Horizons 2015, 2, 168–197. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, J.; Zhang, Y.; Deng, Y. Nanoconfined ionic liquids. Chem. Rev. 2016, 117, 6755–6833. [Google Scholar] [CrossRef]
- Vitucci, F.M.; Manzo, D.; Navarra, M.A.; Palumbo, O.; Trequattrini, F.; Panero, S.; Bruni, P.; Croce, F.; Paolone, A. Low-temperature phase transitions of 1-butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide swelling a polyvinylidenefluoride electrospun membrane. J. Phys. Chem. C 2014, 118, 5749–5755. [Google Scholar] [CrossRef]
- Shimizu, Y.; Fujii, K.; Imanari, M.; Nishikawa, K. Phase behavior of a piperidinium-based room-temperature ionic liquid exhibiting scanning rate dependence. J. Phys. Chem. B 2015, 119, 12552–12560. [Google Scholar] [CrossRef]
- Seoane, R.G.; Corderí, S.; Gómez, E.; Calvar, N.; González, E.J.; MacEdo, E.A.; Domínguez, Á. Temperature dependence and structural influence on the thermophysical properties of eleven commercial ionic liquids. Ind. Eng. Chem. Res. 2012, 51, 2492–2504. [Google Scholar] [CrossRef]
- Yoshii, K.; Yamaji, K.; Tsuda, T.; Tsunashima, K.; Yoshida, H.; Ozaki, M.; Kuwabata, S. Physicochemical properties of tri-n-butylalkylphosphonium cation-based room-temperature ionic liquids. J. Phys. Chem. B 2013, 117, 15051–15059. [Google Scholar] [CrossRef]
- Almeida, H.F.D.; Lopes, J.N.C.; Rebelo, L.P.N.; Coutinho, J.A.P.; Freire, M.G.; Marrucho, I.M. Densities and viscosities of mixtures of two ionic liquids containing a common cation. J. Chem. Eng. Data 2016, 61, 2828–2843. [Google Scholar] [CrossRef]
- Angell, C.A. Free volume model for transport in fused salts: Electrical conductance in glass-forming nitrate melts. J. Phys. Chem. 1964, 68, 1917–1929. [Google Scholar] [CrossRef]
- Moynihan, C.T.; Angell, C.A. Mass transport in ionic melts at low temperatures. Chronopotentiometric diffusion coefficients of silver(I), cadmium(II), and thallium(I) in calcium nitrate tetrahydrate. J. Phys. Chem. 1970, 74, 736–742. [Google Scholar] [CrossRef]
- Sienel, G.; Rieth, R.; Rowbottom, K.T. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2012; Volume 139, pp. 139–154. [Google Scholar]
- McDanel, W.M.; Cowan, M.G.; Carlisle, T.K.; Swanson, A.K.; Noble, R.D.; Gin, D.L. Corrigendum to “Cross-linked ionic resins and gels from epoxide-functionalized imidazolium ionic liquid monomers” [Polymer 55 (2014) 3305–3313]. Polymer (Guildf). 2014, 55, 6195. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds may be available from the authors. |







| Entry | FW a | Tmb /K | Tgc /K | Tdd/K | d e/g∙cm-3 | η f/mPa∙s | σ g /mS∙cm−1 | Solubility in Water |
|---|---|---|---|---|---|---|---|---|
| 1 | 462.4 | None | 221.6 | 531.4 | 1.487 | 909 | 0.387 | Insoluble |
| 2 | 248.3 | None | 220.9 | 447.3 | 1.149 | 9890 | 0.228 | Soluble |
| 3 | 269.1 | None | 240.9 | 456.9 | - | - | - | Soluble |
| 4 | 327.3 | 363.5 | None | 471.1 | - | - | - | Insoluble |
| Entry | a/g·cm−3 | b × 104/g·cm−3·K−1 | |R| |
|---|---|---|---|
| 1 | 1.751 | −8.834 | >0.9999 |
| 2 | 1.321 | −5.773 | >0.9999 |
| Entry | Activation Energy/kJ∙mol−1 | |
|---|---|---|
| Absolute Viscosity | Ionic Conductivity | |
| 1 | 41.7 | 34.8 |
| 2 | 50.2 | 36.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuda, T.; Iwasaki, K.; Kumagai, K.; Kuwabata, S. Epoxy-Containing Ionic Liquids with Tunable Functionality. Molecules 2019, 24, 2591. https://doi.org/10.3390/molecules24142591
Tsuda T, Iwasaki K, Kumagai K, Kuwabata S. Epoxy-Containing Ionic Liquids with Tunable Functionality. Molecules. 2019; 24(14):2591. https://doi.org/10.3390/molecules24142591
Chicago/Turabian StyleTsuda, Tetsuya, Kazuki Iwasaki, Kohei Kumagai, and Susumu Kuwabata. 2019. "Epoxy-Containing Ionic Liquids with Tunable Functionality" Molecules 24, no. 14: 2591. https://doi.org/10.3390/molecules24142591
APA StyleTsuda, T., Iwasaki, K., Kumagai, K., & Kuwabata, S. (2019). Epoxy-Containing Ionic Liquids with Tunable Functionality. Molecules, 24(14), 2591. https://doi.org/10.3390/molecules24142591