Role of Post-Exposure Time in Co(II) Sorption of Higher Concentrations on Electron Irradiated Sheep Wool
Abstract
:1. Introduction
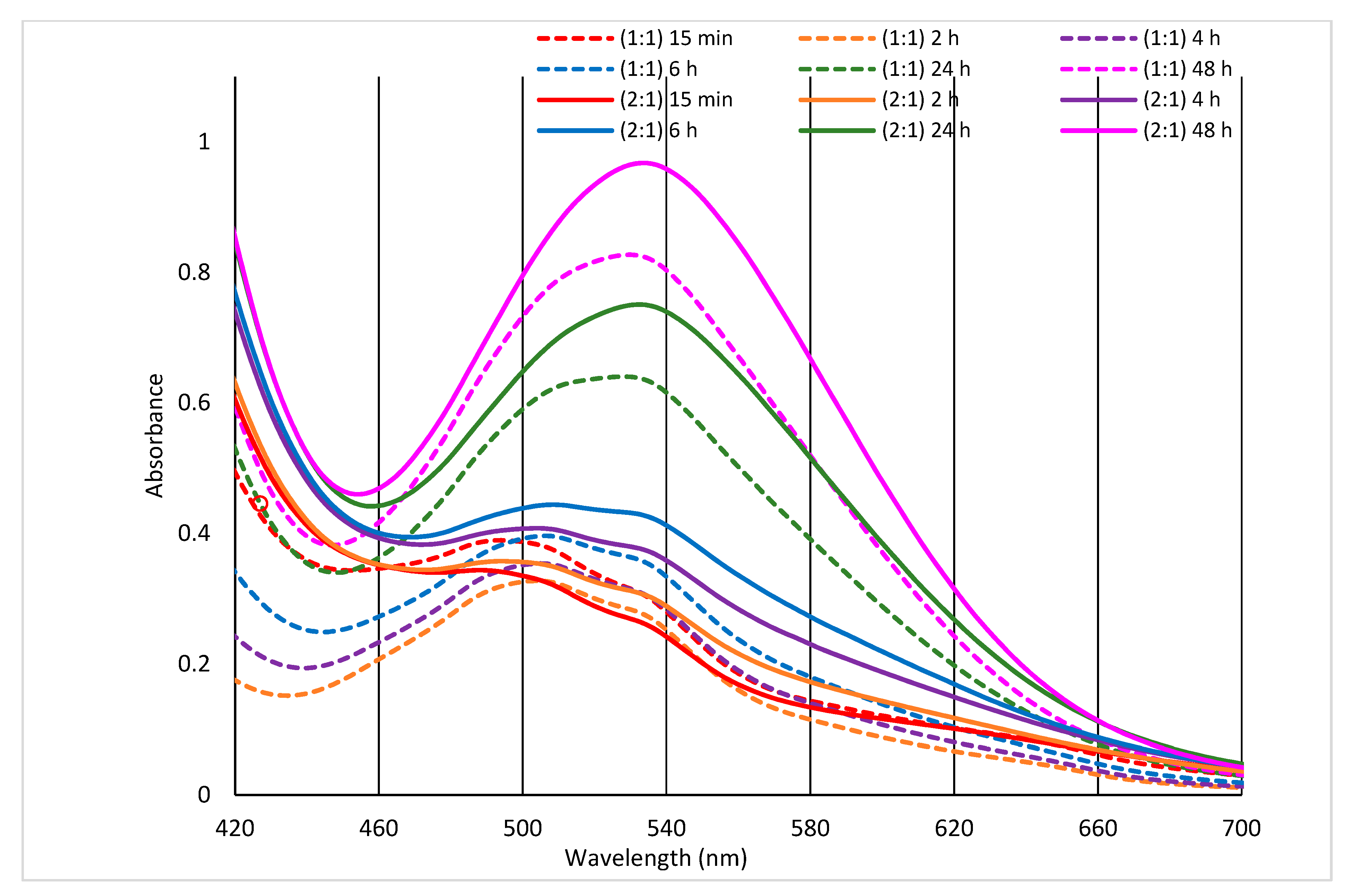
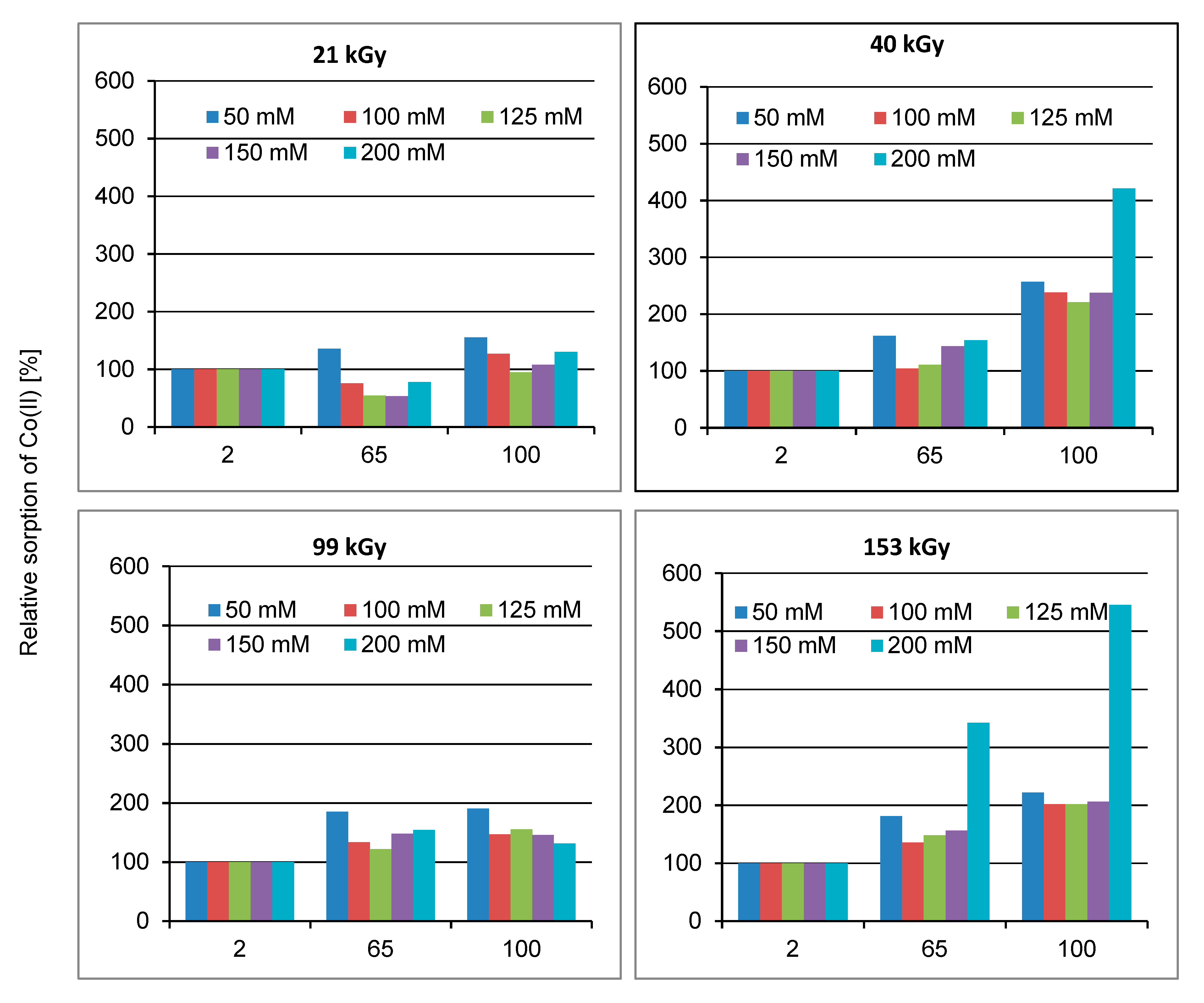
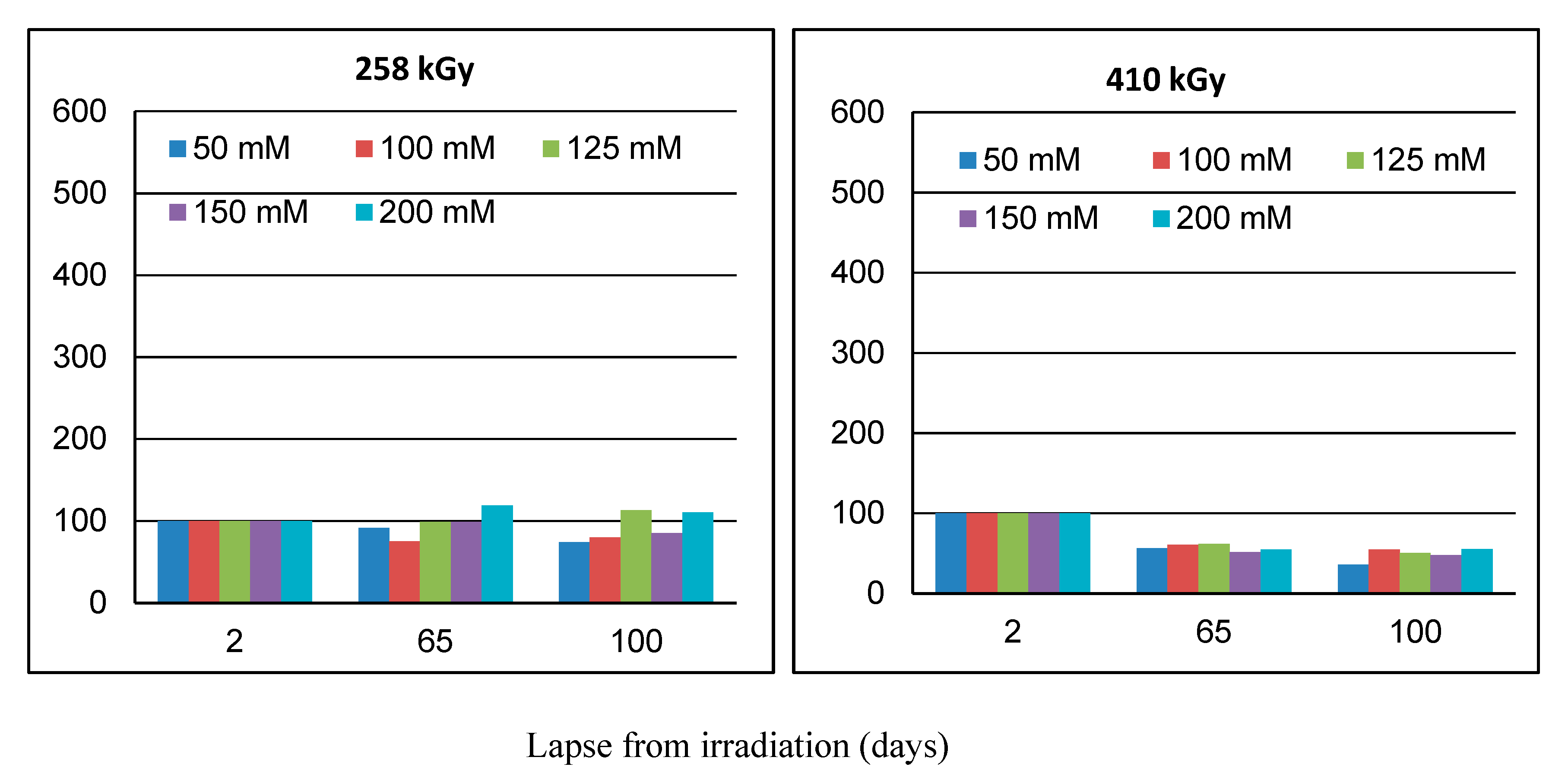
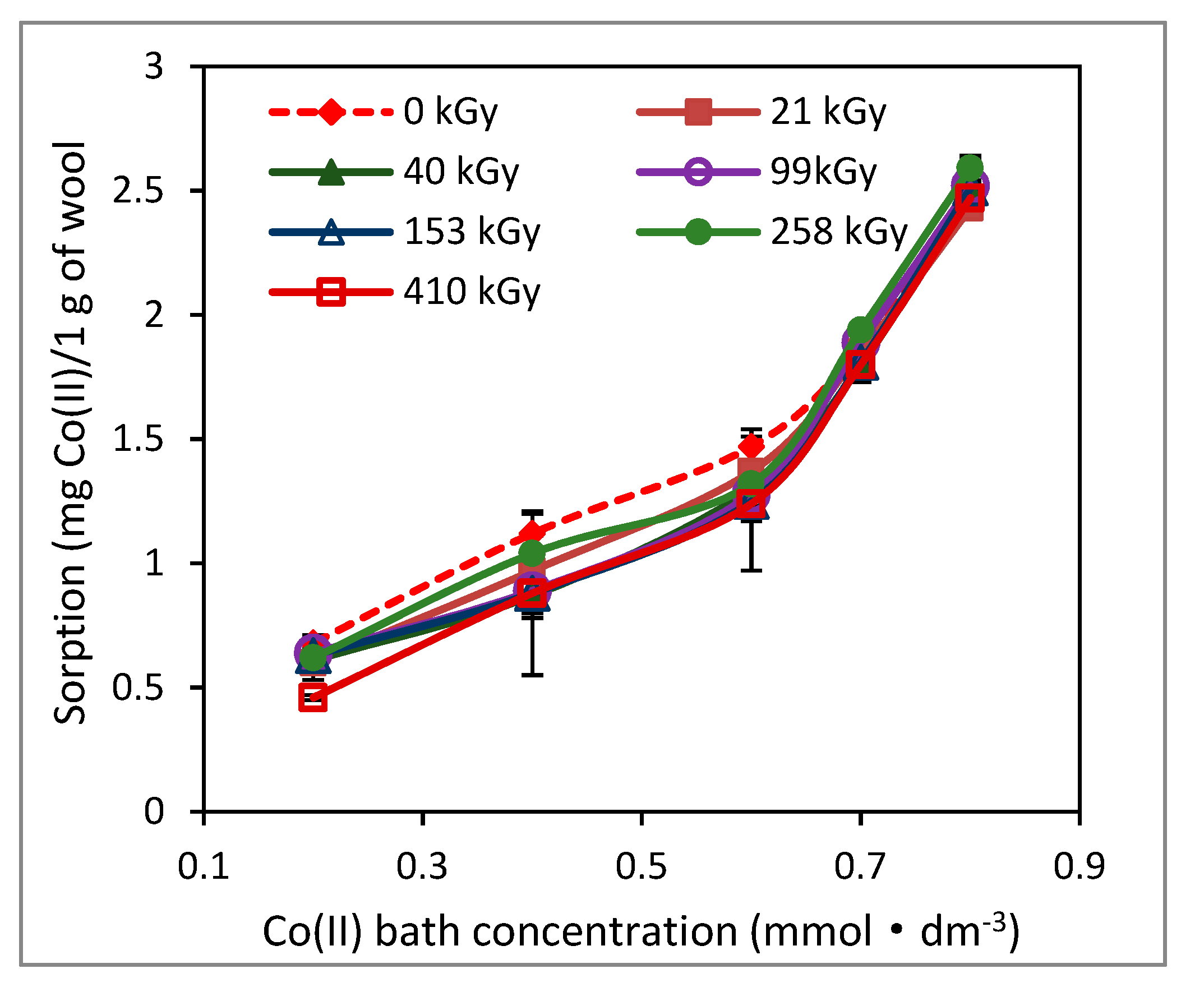
2. Results and Discussion
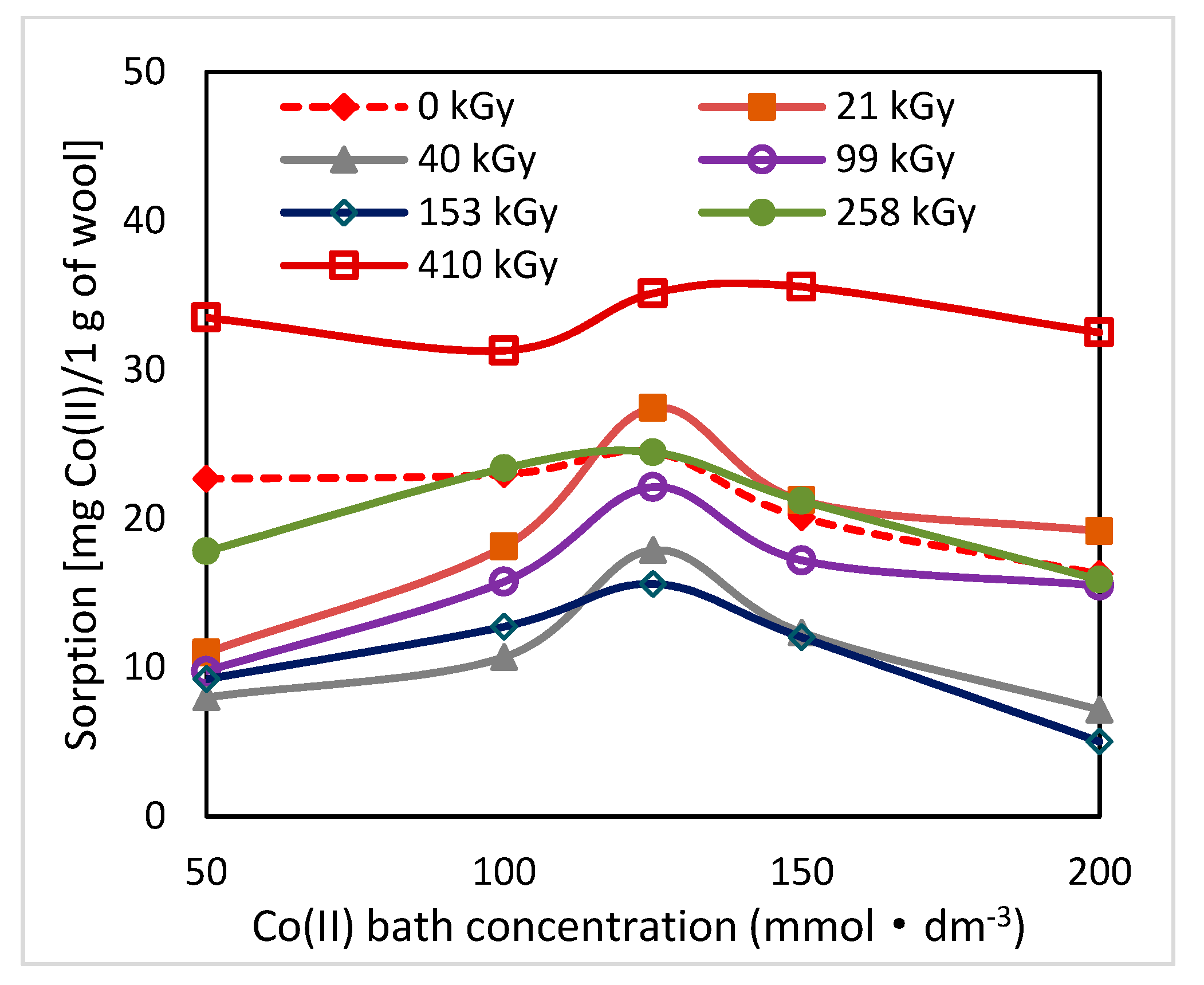
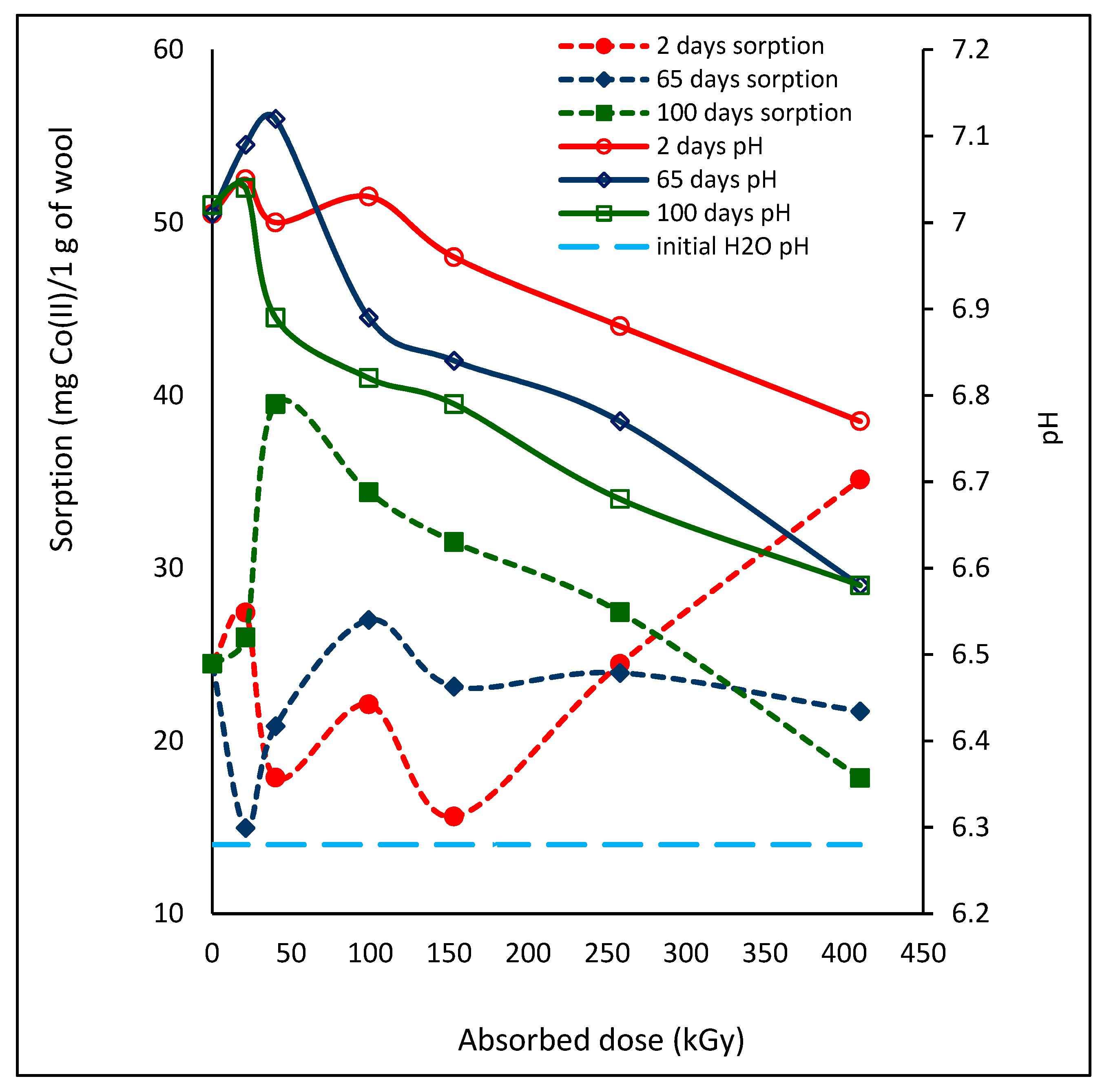
2.1. Effect of Lapse from Wool Exposure on Co(II)-Sorption
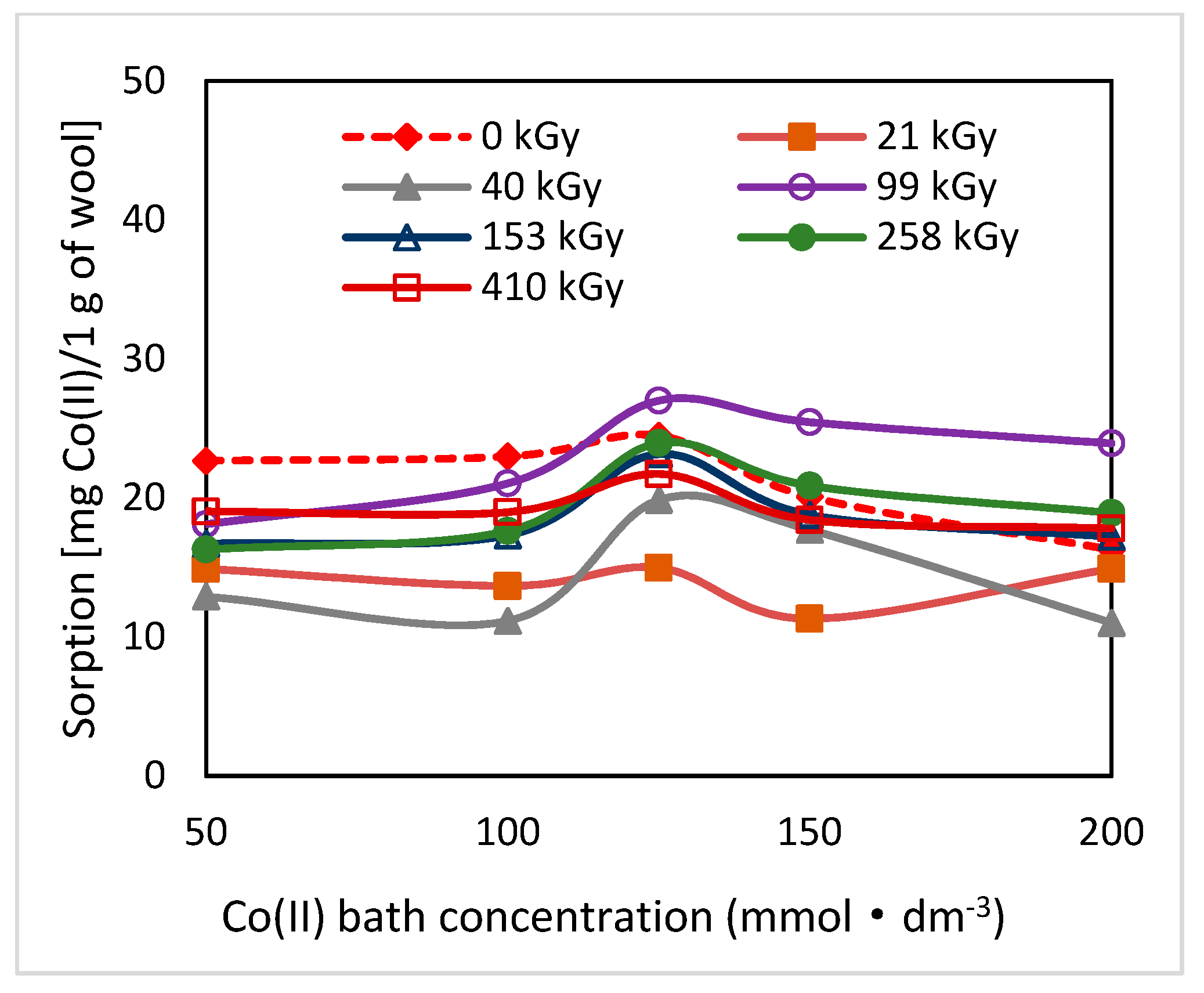
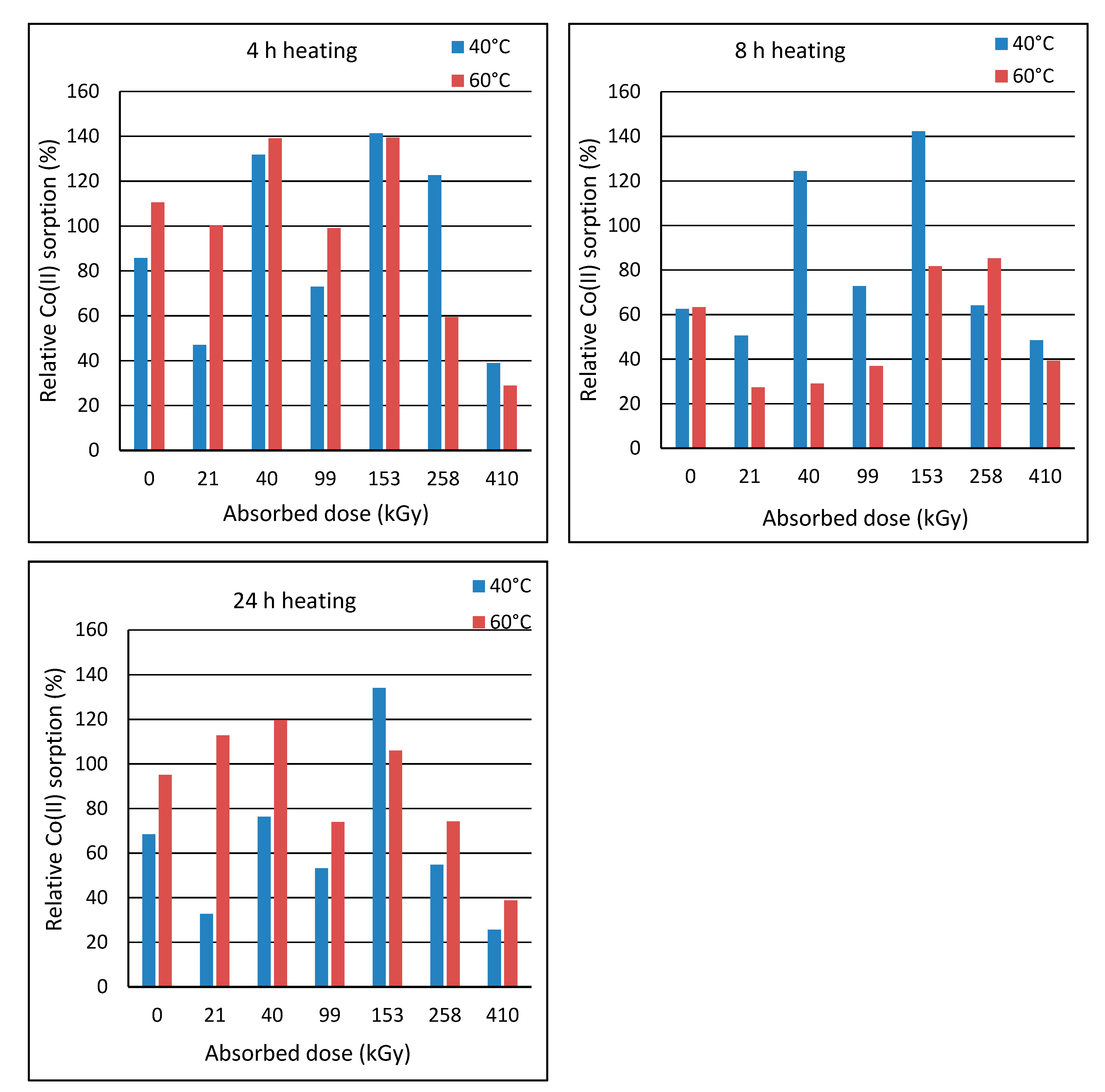
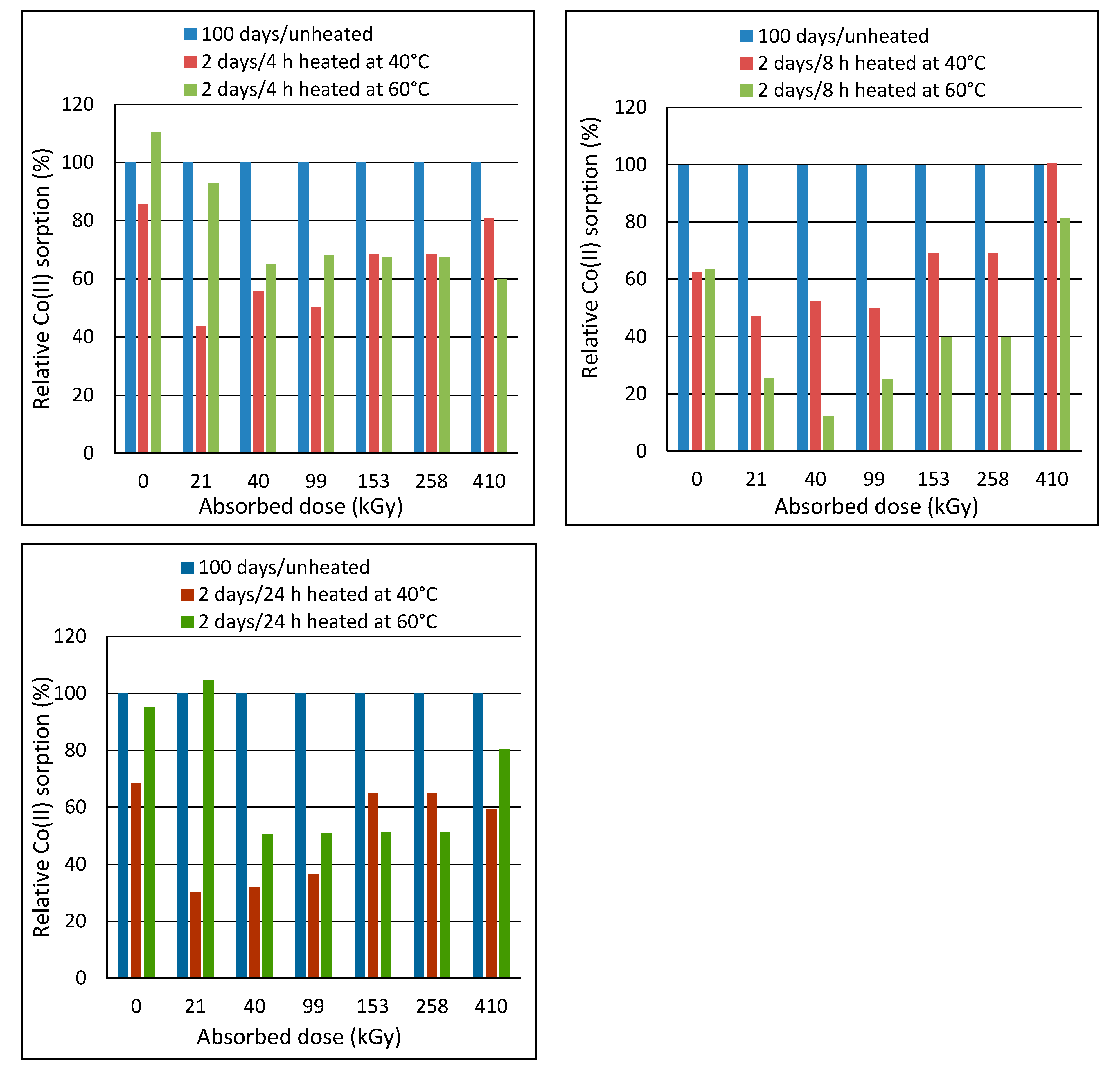
2.2. Effect of Post-Exposure Wool Heating on Co(II)-Sorption
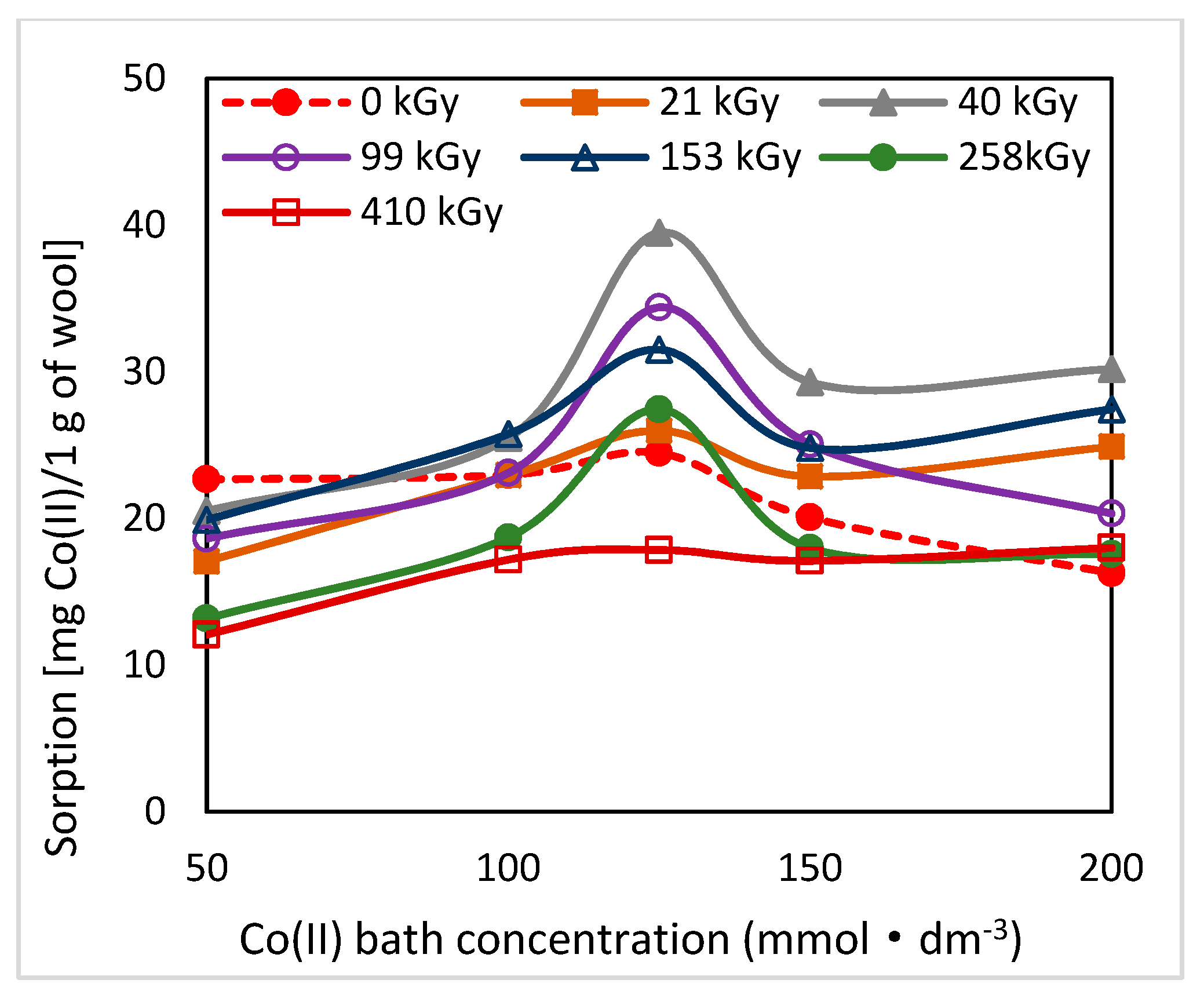
2.3. Effect of Lapse from Wool Exposure on pH of Aqueous Extract
3. Materials and Methods
3.1. Materials
3.2. Electron Beam Irradiation
3.3. Sorption Experiments
- x1 is the mass of Co(II) added in the initial solution (mg),
- x2 is the residual mass of Co(II) in the solution after its contact with the wool sample (mg),
- m is the mass of wool sample taken for analysis (g).
3.4. Spectral Measurements
3.5. Measurement of pH
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gogoi, D.; Shanmugamani, A.G.; Rao, S.V.S.; Kumar, T.; Sinha, P.K. Studies on removal of cobalt from an alkaline waste using synthetic calcium hydroxyapatite. J. Radioanal. Nucl. Ch. 2013, 298, 337–344. [Google Scholar] [CrossRef]
- Narwade, V.N.; Khairnar, R.S.; Kokol, V. In Situ Synthesized Hydroxyapatite—Cellulose Nanofibrils as Biosorbents for Heavy Metal Ions Removal. J. Polym. Environ. 2018, 26, 2130–2141. [Google Scholar] [CrossRef]
- Çiçek, E.; Cojocaru, C.; Zakrzewska-Trznadel, G.; Jaworska, A.; Harasimowicz, M. Response surface methodology for cobalt removal from aqueous solutions using Isparta pumice and zeolite 4A adsorbents. Nukleonika 2008, 53, S121–S128. [Google Scholar]
- Manohar, D.M.; Noeline, B.F.; Anirudhan, T.S. Adsorption performance of Al-pillared bentonite clay for the removal of cobalt(II) from aqueous phase. Appl. Clay Sci. 2006, 31, 194–206. [Google Scholar] [CrossRef]
- Gómez, J.M.; Díez, E.; Bernabé, I.; Sáez, P.; Rodríguez, A. Effective Adsorptive Removal of Cobalt Using Mesoporous Carbons Synthesized by Silica Gel Replica Method. Environ. Processes. 2018, 5, 225–242. [Google Scholar] [CrossRef]
- Saleh, A.S.; Ibrahim, A.G.; Elsharma, E.M.; Metwally, E.; Siyam, T. Radiation grafting of acrylamide and maleic acid on chitosan and effective application for removal of Co(II) from aqueous solutions. Radiat. Phys. Chem. 2018, 144, 116–124. [Google Scholar] [CrossRef]
- Bhaskarapillai, A.; Chandra, S.; Sevilimedu, N.V.; Sellergren, B. Theoretical investigations of the experimentally observed selectivity of a cobalt imprinted polymer. Biosens. Bioelectron. 2009, 25, 558–562. [Google Scholar] [CrossRef]
- Vilvanathan, S.; Shanthakumar, S. Column adsorption studies on nickel and cobalt removal from aqueous solution using native and biochar form of Tectona grandis. Environ. Prog. Sustain. 2017, 36, 1030–1038. [Google Scholar] [CrossRef]
- Nazari, A.M.; Cox, P.W.; Waters, K.E. Biosorption of copper, nickel and cobalt ions from dilute solutions using BSA-coated air bubbles. J. Water Proc. Eng. 2014, 3, 10–17. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Minocha, A.K.; Sillanpää, M. Adsorptive removal of cobalt from aqueous solution by utilizing lemon peel as biosorbent. Biochem. Eng. J. 2010, 48, 181–186. [Google Scholar] [CrossRef]
- Kandah, M.I.; Allawzi, M.A.; Allaboun, H. Improvement of manure adsorption capacity for cobalt removal by chemical treatment with citric acid. Jordan J. Civ. Eng. 2008, 2, 344–354. [Google Scholar]
- Balkaya, N.; Bektas, N. Chromium(VI) sorption from dilute aqueous solutions using wool. Desalin. Water Treat. 2009, 3, 43–49. [Google Scholar] [CrossRef]
- Balköse, D.; Baltacioǧlu, H. Adsorption of heavy metal cations from aqueous solutions by wool fibers. J. Chem. Technol. Biot. 1992, 54, 393–397. [Google Scholar] [CrossRef]
- Ghosh, A.; Collie, S.R. Keratinous materials as novel absorbent systems for toxic pollutants. Defence Sci. J. 2014, 64, 209–221. [Google Scholar] [CrossRef]
- Hanzlíková, Z.; Braniša, J.; Hybler, P.; Šprinclová, I.; Jomová, K.; Porubská, M. Sorption properties of sheep wool irradiated by accelerated electron beam. Chem Pap. 2016, 70, 1299–1308. [Google Scholar] [CrossRef]
- Hanzlíková, Z.; Braniša, J.; Jomová, K.; Fülöp, M.; Hybler, P.; Porubská, M. Electron beam irradiated sheep wool–Prospective sorbent for heavy metals in wastewater. Sep. Purif. Technol. 2018, 19, 3345–3350. [Google Scholar] [CrossRef]
- Porubská, M.; Kleinová, A.; Hybler, P.; Braniša, J. Why Natural or Electron Irradiated Sheep Wool Show Anomalous Sorption of Higher Concentrations of Copper(II). Molecules 2018, 23, 3180. [Google Scholar] [CrossRef] [PubMed]
- Porubská, M.; Hanzlíková, Z.; Braniša, J.; Kleinová, A.; Hybler, P.; Fülöp, M.; Ondruška, J.; Jomová, K. The effect of electron beam on sheep wool. Polym. Degrad. Stabil. 2015, 111, 151–158. [Google Scholar] [CrossRef]
- Hanzlíková, Z.; Lawson, M.K.; Hybler, P.; Fülöp, M.; Porubská, M. Time-Dependent Variations in Structure of Sheep Wool Irradiated by Electron Beam. Adv. Mater. Sci. Eng. 2017. [Google Scholar] [CrossRef]
- Škárka, B.; Ferenčík, M. Biochémia, 3rd ed.; Alfa: Bratislava, Slovakia, 1992; p. 848. [Google Scholar]
- Zhang, X.; Yue, F.; Li, H.; Huang, Y.; Zhang, Y.; Wen, H.; Wang, J. Reversible Oxygenation of α-Amino Acid-Cobalt(II) Complexes. Bioinorg. Chem. Appl. 2016. [Google Scholar] [CrossRef]
- Baryshnikova, A.T.; Minaev, B.F.; Baryshnikov, G.V.; Sun, W.-H. Quantum-chemical studyof the structure and magnetic properties of mono- and binuclear Cu(II) complexes with 1,3-bis(3-(pyrimidin-2-yl)-1H,-1,2,4-triazol-5-yl)propane. Russian J. Inorg. Chem. 2016, 61, 588–593. [Google Scholar] [CrossRef]
- Shulgin, V.F.; Konnik, O.V.; Gusev, A.N.; Boca, R.; Dlhan, L.; Rusanov, E.B.; Alexandrov, G.G.; Eremenko, I.L.; Linert, W. Spacer-armed copper(II) complexes with benzenecarboxylic acids and trifluoroacetylacetone aroylhydrazones. Dalton Trans. 2013, 42, 16878–16886. [Google Scholar] [CrossRef] [PubMed]
- Baryshnikova, G.V.; Minaev, B.F.; Baryshnikova, A.T.; Agren, H. A computational study of structural and magnetic properties of bi- and trinuclear Cu(II) complexes with extremely long Cu—Cu distances. Chem. Phys. 2017, 491, 48–55. [Google Scholar] [CrossRef]
- Wen, G.; Naik, R.; Cookson, P.G.; Smith, S.V.; Liu, X.; Wang, X.G. Wool powders used as sorbents to remove Co2+ ions from aqueous solution. Powder Technol. 2010, 197, 235–240. [Google Scholar] [CrossRef]
- Radetić, M.; Jocić, D.; Jovančić, P.; Rajaković, L.; Thomas, H.; Petrović, Z.L. Recycled-Wool-Based Nonwoven Material as a Sorbent for Lead Cations. J. Appl. Polym. Sci. 2003, 90, 379–386. [Google Scholar] [CrossRef]
- Oae, S.; Doi, J.T. Organic Sulfur Chemistry: Structure and Mechanism; CRC Press: Boca Raton, FL, USA, 1991; p. 433. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braniša, J.; Jomová, K.; Kovalčíková, R.; Hybler, P.; Porubská, M. Role of Post-Exposure Time in Co(II) Sorption of Higher Concentrations on Electron Irradiated Sheep Wool. Molecules 2019, 24, 2639. https://doi.org/10.3390/molecules24142639
Braniša J, Jomová K, Kovalčíková R, Hybler P, Porubská M. Role of Post-Exposure Time in Co(II) Sorption of Higher Concentrations on Electron Irradiated Sheep Wool. Molecules. 2019; 24(14):2639. https://doi.org/10.3390/molecules24142639
Chicago/Turabian StyleBraniša, Jana, Klaudia Jomová, Renáta Kovalčíková, Peter Hybler, and Mária Porubská. 2019. "Role of Post-Exposure Time in Co(II) Sorption of Higher Concentrations on Electron Irradiated Sheep Wool" Molecules 24, no. 14: 2639. https://doi.org/10.3390/molecules24142639
APA StyleBraniša, J., Jomová, K., Kovalčíková, R., Hybler, P., & Porubská, M. (2019). Role of Post-Exposure Time in Co(II) Sorption of Higher Concentrations on Electron Irradiated Sheep Wool. Molecules, 24(14), 2639. https://doi.org/10.3390/molecules24142639