Formation of Zearalenone Metabolites in Tempeh Fermentation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Production of Tempeh-Like Products
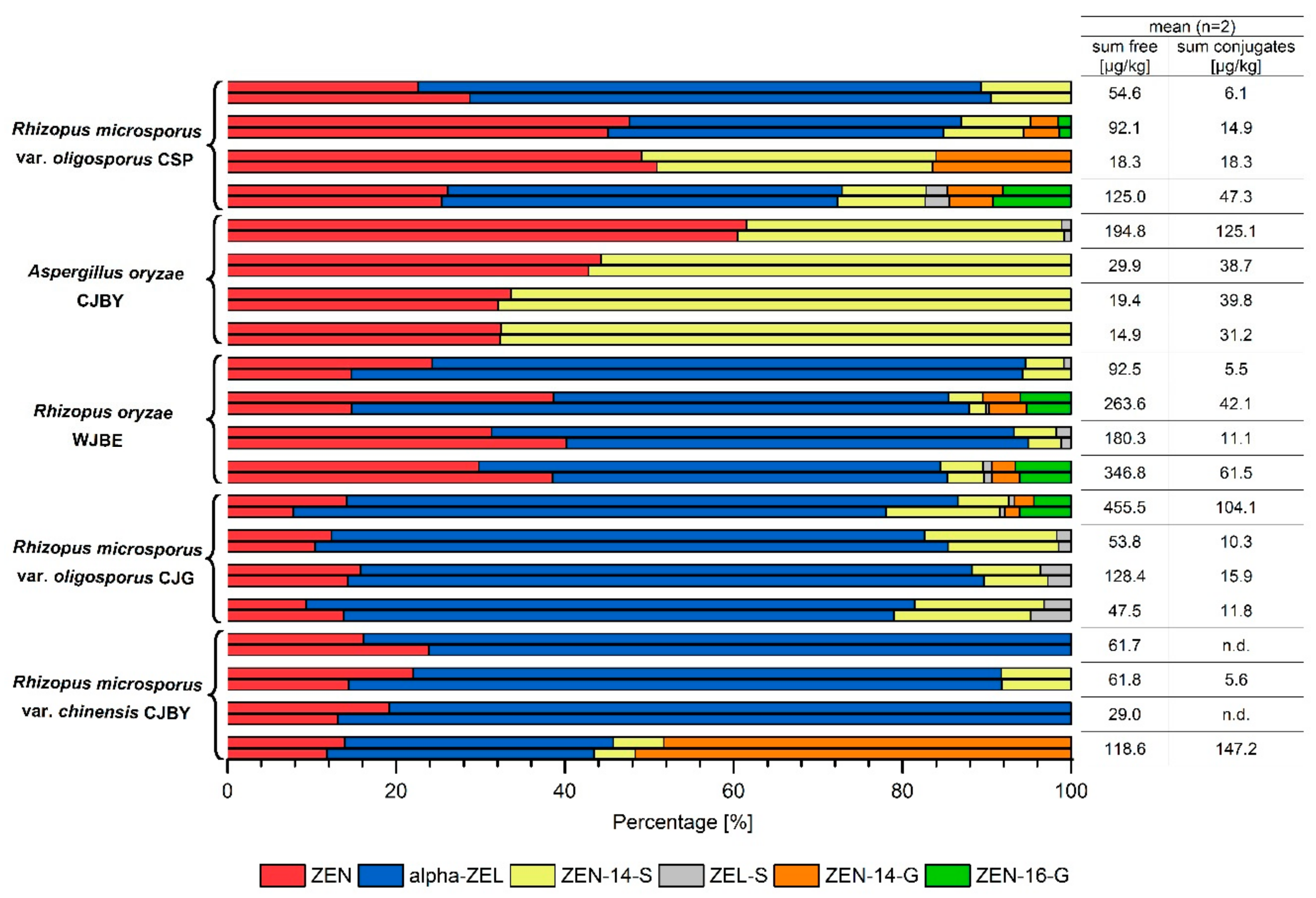
2.2. Zearalenone Metabolization during Fermentation of Tempeh-Like Products
2.3. Analysis of Authentic Tempeh-Like Products
3. Materials and Methods
3.1. Chemicals and Media
3.2. Fungal Strains and Growth Conditions
3.3. Isolation and Identification of Fungal Strains
3.4. Production of Tempeh-Like Products in Laboratory Scale
3.5. Analysis of Tempeh Raw Material, Waste Water, and Tempeh-Like Products
3.6. HPLC-MS/MS Analysis
3.7. Semi-Quantification of α-ZEL-S
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hartanti, A.T.; Rahayu, G.; Hidayat, I. Rhizopus species from fresh tempeh collected from several regions in Indonesia. HAYATI J. Biosci. 2015, 22, 136–142. [Google Scholar] [CrossRef]
- Heskamp, M.L.; Barz, W. Expression of proteases by Rhizopus species during tempeh fermentation of soybeans. Food/Nahrung 1998, 42, 23–28. [Google Scholar] [CrossRef]
- Murata, K.; Ikehata, H.; Miyamoto, T. Studies on the nutritional value of tempeh. J. Food Sci. 1967, 32, 580–596. [Google Scholar] [CrossRef]
- Steinkraus, K.H.; Yap Bwee, H.; Van Buren, J.P.; Provvidenti, M.I.; Hand, D.B. Studies on tempeh–an Indonesian fermented soybean food. J. Food Sci. 1960, 25, 777–788. [Google Scholar] [CrossRef]
- Babu, P.D.; Bhakyaraj, R.; Vidhyalakshmi, R. A low cost nutritious food “tempeh”—A review. World J. Dairy Food Sci. 2009, 4, 22–27. [Google Scholar]
- Ashenafi, M.; Busse, M. Production of tempeh from various indigenous Ethiopian beans. World J. Microbiol. Biotechnol. 1991, 7, 72–79. [Google Scholar]
- Mugula, J.K. Evaluation of the nutritive value of maize-soybean tempe as a potential weaning food in Tanzania. Int. J. Food Sci. Nutr. 1992, 43, 113–119. [Google Scholar] [CrossRef]
- Van der Riet, W.B.; Wight, A.W.; Cilliers, J.J.L.; Datel, J.M. Food chemical analysis of tempeh prepared from South African-grown soybeans. Food Chem. 1987, 25, 197–206. [Google Scholar] [CrossRef]
- Hachmeister, K.A.; Fung, D.Y.C. Tempeh: A mold-modified indigenous fermented food made from soybeans and/or cereal grains. Crit. Rev. Microbiol. 1993, 19, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Mastanjević, K.; Šarkanj, B.; Mastanjević, K.; Šantek, B.; Krstanović, V. Fusarium culmorum mycotoxin transfer from wheat to malting and brewing products and by-products. World Mycotoxin J. 2019, 12, 55–66. [Google Scholar] [CrossRef]
- Mastanjević, K.; Šarkanj, B.; Krska, R.; Sulyok, M.; Warth, B.; Mastanjević, K.; Šantek, B.; Krstanović, V. From malt to wheat beer: A comprehensive multi-toxin screening, transfer assessment and its influence on basic fermentation parameters. Food Chem. 2018, 254, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Dolatabadi, S.; Scherlach, K.; Figge, M.; Hertweck, C.; Dijksterhuis, J.; Menken, S.B.J.; de Hoog, G.S. Fungal Biol. 2016, 120, 393–401. [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Toxins in Fermented Foods: Prevalence and Preventions—A Mini Review. Toxins 2018, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, C.Z. Production of toxic metabolites in Aspergillus niger, Aspergillus oryzae, and Trichoderma reesei: Justification of mycotoxin testing in food grade enzyme preparations derived from the three fungi. Regul. Toxicol. Pharmacol. 2004, 39, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Beardall, J.; Miller, J.D. Natural occurrence of mycotoxins other than aflatoxin in Africa, Asia and South America. Mycotoxin Res. 1994, 10, 21–40. [Google Scholar] [CrossRef]
- Schollenberger, M.; Müller, H.M.; Rüfle, M.; Suchy, S.; Plank, S.; Drochner, W. Natural occurrence of 16 Fusarium toxins in grains and feedstuffs of plant origin from Germany. Mycopathologia 2006, 161, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zeralenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; Van der Saag, P.T.; Van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endokrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Massart, F.; Saggese, G. Oestrogenic mycotoxin exposure and precocious pupertal development. Int. J. Androl. 2010, 33, 369–376. [Google Scholar] [CrossRef]
- De Rodriguez, C.A.S.; Bongiovanni, A.M.; Conde de Borrego, L. An epidemic of precocious development in Puerto Rican children. J. Pediatr. 1985, 107, 393–396. [Google Scholar] [CrossRef]
- Vejdovszky, K.; Schmidt, V.; Warth, B.; Marko, D. Combinatory estrogenic effects between the isoflavone genistein and the mycotoxins zearalenone and alternariol in vitro. Mol. Nutr. Food Res. 2017, 61, 1600526. [Google Scholar] [CrossRef] [PubMed]
- Hueza, I.M.; Raspantini, P.C.F.; Raspantini, L.E.R.; Latorre, A.O.; Górniak, S.L. Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound. Toxins 2014, 6, 1080–1095. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, M.; Humpf, H.U.; Marko, D.; Dänicke, S.; Mally, A.; Berthiller, F.; Klaffke, H.; Lorenz, N. Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxin Res. 2014, 30, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Kovač, M.; Šubarić, D.; Bulaić, M.; Kovač, T.; Šarkanj, B. Yesterday masked, today modified; what do mycotoxins bring next? Arh. Hig. Rada. Toksicol. 2018, 69, 196–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plasencia, J.; Mirocha, C.J. Isolation and characterization of zearalenone sulfate produced by Fusarium spp. Appl. Environ. Microbiol. 1991, 57, 146–150. [Google Scholar] [PubMed]
- Borzekowski, A.; Drewitz, T.; Keller, J.; Pfeifer, D.; Kunte, H.-J.; Koch, M.; Rohn, S.; Maul, R. Biosynthesis and characterization of zearalenone-14-sulfate, zearalenone-14-glucoside and zearalenone-16-glucoside using common fungal strains. Toxins 2018, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, G.; Zill, G.; Wohner, B.; Wallnöfer, P.R. Transformation of the Fusarium mycotoxin zearalenone in maize cell suspension cultures. Naturwissenschaften 1988, 75, 309–310. [Google Scholar] [CrossRef]
- Berthiller, F.; Werner, U.; Sulyok, M.; Krska, R.; Hauser, M.T.; Schuhmacher, R. Liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) determination of phase II metabolites of the mycotoxin zearalenone in the model plant Arabidopsis thaliana. Food Addit. Contam. 2006, 23, 1194–1200. [Google Scholar] [CrossRef]
- Paris, M.P.K.; Schweiger, W.; Hametner, C.; Stückler, R.; Muehlbauer, G.J.; Varga, E.; Krska, R.; Berthiller, F.; Adam, G. Zearalenone-16-O-glucoside: A new masked mycotoxin. J. Agric. Food Chem. 2014, 62, 1181–1189. [Google Scholar] [CrossRef]
- Brodehl, A.; Möller, A.; Kunte, H.-J.; Koch, M.; Maul, R. Biotransformation of the mycotoxin zearalenone by fungi of the genera Rhizopus and Aspergillus. FEMS Microbiol. Lett. 2014, 359, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarc´h, A.; André, F.; Delaforge, M.; Lebrihi, A. Transformation of zearalenone to zearalenone-sulfate by Aspergillus spp. World Mycotoxin J. 2010, 3, 183–191. [Google Scholar] [CrossRef]
- Kamimura, H. Conversion of zearalenone to zearalenone glycoside by Rhizopus sp. Appl Environ Micro 1986, 52, 515–519. [Google Scholar]
- Righetti, L.; Rolli, E.; Galaverna, G.; Suman, M.; Bruni, R.; Dall`Asta, C. Plant organ cultures as masked mycotoxin biofactories: Deciphering the fate of zearalenone in micropropagated durum wheat roots and leaves. PLoS ONE 2017, 12, e0187247. [Google Scholar] [CrossRef] [PubMed]
- Dall´Erta, A.; Cirlini, M.; Dall´Asta, M.; Del Rio, D.; Galaverna, G.; Dall´Asta, C. Masked mycotoxins are efficiently hydrolyzed by human colonic microbiota releasing their aglycones. Chem. Res. Toxicol. 2012, 26, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Binder, S.B.; Schwartz-Zimmermann, H.E.; Varga, E.; Bichl, G.; Michlmayr, H.; Adam, G.; Berthiller, F. Metabolism of zearalenone and its major modified forms in pigs. Toxins 2017, 9, 56. [Google Scholar] [CrossRef]
- Metzler, M.; Pfeiffer, E.; Hildebrand, A.A. Zearalenone and its metabolites as endocrine disrupting chemicals. World Mycotoxin J. 2010, 3, 385–401. [Google Scholar] [CrossRef]
- EFSA. Appropriateness to set a group health-based guidance value for zearalenone and its modified forms. EFSA J. 2016, 14, 4425. [Google Scholar]
- Niermans, K.; Woyzichovski, J.; Kröncke, N.; Benning, R.; Maul, R. Feeding study for the mycotoxins zearalenone in yellow mealworm (Tenebrio molitor) larvae—Investigation of biological impact and metabolis conversion. Mycotoxin Res. 2019, 35, 231–242. [Google Scholar] [CrossRef]
- De Boevre, M.; Di Mavungu, J.D.; Landschoot, S.; Audenaert, K.; Eeckhout, M.; Maene, P.; Haesart, G.; De Saeger, S. Natural occurrence of mycotoxins and their masked forms in food and feed products. World Mycotoxin J. 2012, 5, 207–219. [Google Scholar] [CrossRef]
- Schipper, M.A.A.; Stalpers, J.A. A revision of the genus Rhizopus. 2. The Rhizopus microsporus group. Stud. Mycol. 1984, 25, 20–34. [Google Scholar]
- Liou, G.-Y.; Chen, C.-C.; Chien, C.-Y.; Hsu, W.H. Sporangiospore ornamentation of some species in the genus Rhizopus. Trans. Mycol. Soc. Japan 1991, 32, 535–540. [Google Scholar]
- Zheng, R.Y.; Chen, G.Q.; Huang, H.; Liu, X.Y. A monograph of Rhizopus. Sydowia Horn 2007, 59, 273. [Google Scholar]
- Voigt, K.; Wöstemeyer, J. Phylogeny and origin of 82 zygomycetes from all 54 genera of the Mucorales and Mortierellales based on combined analysis of actin and translation elongation factor EF-1α genes. Genes 2001, 270, 113–120. [Google Scholar] [CrossRef]
- Abia, W.A.; Warth, B.; Ezekiel, C.N.; Sarkanj, B.; Turner, P.C.; Marko, D.; Krska, R.; Sulyok, M. Uncommon toxic microbial metabolite patterns in traditionally home-processed maize dish (fufu) consumed in rural Cameroon. Food Chem. Toxicol. 2017, 107, 10–19. [Google Scholar] [CrossRef]
Sample Availability: Limited sample amounts of the conjugated compounds (ZEN-14-sulfate (ZEN-14-S), α-ZEL-sulfate (ZEL-S), and ZEN-14-glucosides (ZEN-14-G and ZEN-16-G)) are available from the authors. |




| Maize Sample | Content (µg/kg) | Molar Ratio ZEN/ZEN-14-S | |
|---|---|---|---|
| ZEN | ZEN-14-S | ||
| 1 | 38.5 | 5.09 | 9.5 |
| 2 | 102.9 | 10.3 | 12.6 |
| 3 | 216.4 | 7.18 | 37.7 |
| 4 | 220.1 | 9.43 | 29.2 |
| 5 | 65.1 | 4.55 | 17.9 |
| 6 | 1436 | 20.1 | 89.4 |
| 7 | 53.1 | 45.1 | 1.5 |
| 8 | 49.6 | 4.86 | 12.8 |
| 9 | 465.7 | 12.2 | 47.7 |
| Tempeh Fungi | Strain | Origin |
|---|---|---|
| Rhizopus oryzae | WJBE7.84 | West Java |
| Rhizopus microsporus var. chinensis | CJBY16.192 | Central Java |
| Aspergillus oryzae | CJBY22.260 | Central Java |
| Rhizopus microsporus var. oligosporus | CJG27.324 | Central Java |
| Rhizopus microsporus var. oligosporus | CSP71.850 | Central Sulawesi |
| Tempeh-Like Product | Molar Ratio ZEN/Modified ZEN |
|---|---|
| Rhizopus microsporus var. oligosporus CSP (a) | 0.4 |
| Rhizopus microsporus var. oligosporus CSP (b) | 0.9 |
| Rhizopus microsporus var. oligosporus CSP (c) | 1.4 |
| Rhizopus microsporus var. oligosporus CSP (d) | 0.4 |
| Aspergillus oryzae CJBY (a) | 1.9 |
| Aspergillus oryzae CJBY (b) | 0.9 |
| Aspergillus oryzae CJBY (c) | 0.6 |
| Aspergillus oryzae CJBY (d) | 0.6 |
| Rhizopus oryzae WJBE (a) | 0.2 |
| Rhizopus oryzae WJBE (b) | 0.2 |
| Rhizopus oryzae WJBE (c) | 0.7 |
| Rhizopus oryzae WJBE (d) | 0.7 |
| Rhizopus microsporus var. oligosporus CJG (a) | 0.1 |
| Rhizopus microsporus var. oligosporus CJG (b) | 0.1 |
| Rhizopus microsporus var. oligosporus CJG (c) | 0.2 |
| Rhizopus microsporus var. oligosporus CJG (d) | 0.2 |
| Rhizopus microsporus var. chinensis CJBY (a) | 0.3 |
| Rhizopus microsporus var. chinensis CJBY (b) | 0.2 |
| Rhizopus microsporus var. chinensis CJBY (c) | 0.2 |
| Rhizopus microsporus var. chinensis CJBY (d) | 0.2 |
| Sample | Origin | Content (µg/kg) | ||||||
|---|---|---|---|---|---|---|---|---|
| ZEN | α-ZEL | β-ZEL | ZEN-14-S | α-ZEL-S | ZEN-14-G | ZEN-16-G | ||
| TY2 | Yogyakarta, Central Java | - | - | - | - | - | - | - |
| TY4 | Yogyakarta, Central Java | - | - | - | - | - | - | - |
| TB2 | Banyumas, Central Java | - | - | - | - | - | - | - |
| TB3 | Banyumas, Central Java | - | - | - | - | - | - | - |
| TB4 | Banyumas, Central Java | 17.50 | 33.99 | - | - | - | - | - |
| TM3 | Malang, East Java | 9.08 | 43.63 | - | 16.31 | - | - | - |
| TM4 | Malang, East Java | - | - | - | - | - | - | - |
| TM5 | Malang, East Java | - | - | - | - | - | - | - |
| TM6 | Malang, East Java | 24.75 | 28.54 | - | 15.75 | - | - | - |
| TJ1 | Jakarta, West Java | - | - | - | - | - | - | - |
| TJ2 | Jakarta, West Java | - | - | - | - | - | - | - |
| TJ3 | Jakarta, West Java | - | - | - | - | - | - | - |
| TBG1 | Bogor, West Java | - | - | - | - | - | - | - |
| TBG3 | Bogor, West Java | 8.34 | 28.08 | - | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borzekowski, A.; Anggriawan, R.; Auliyati, M.; Kunte, H.-J.; Koch, M.; Rohn, S.; Karlovsky, P.; Maul, R. Formation of Zearalenone Metabolites in Tempeh Fermentation. Molecules 2019, 24, 2697. https://doi.org/10.3390/molecules24152697
Borzekowski A, Anggriawan R, Auliyati M, Kunte H-J, Koch M, Rohn S, Karlovsky P, Maul R. Formation of Zearalenone Metabolites in Tempeh Fermentation. Molecules. 2019; 24(15):2697. https://doi.org/10.3390/molecules24152697
Chicago/Turabian StyleBorzekowski, Antje, Riyan Anggriawan, Maryeni Auliyati, Hans-Jörg Kunte, Matthias Koch, Sascha Rohn, Petr Karlovsky, and Ronald Maul. 2019. "Formation of Zearalenone Metabolites in Tempeh Fermentation" Molecules 24, no. 15: 2697. https://doi.org/10.3390/molecules24152697
APA StyleBorzekowski, A., Anggriawan, R., Auliyati, M., Kunte, H.-J., Koch, M., Rohn, S., Karlovsky, P., & Maul, R. (2019). Formation of Zearalenone Metabolites in Tempeh Fermentation. Molecules, 24(15), 2697. https://doi.org/10.3390/molecules24152697









