Diterpenoid Lactones with Anti-Inflammatory Effects from the Aerial Parts of Andrographis paniculata
Abstract
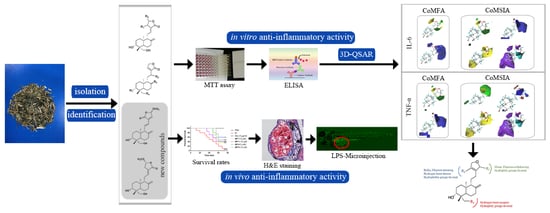
:1. Introduction
2. Results and Discussion
2.1. Identification of Isolated Diterpenoid Lactones
2.2. Anti-Inflammatory Effects of 19 Diterpenoid Lactones in LPS-Induced RAW 264.7 Cells
2.3. D-QSAR Analysis
2.4. New Diterpenoid Lactones Inhibit the Activation of NF-Κb in LPS-Induced RAW 264.7 Cells
2.5. Anti-Inflammatory Effects of New Diterpenoid Lactones in Zebrafish
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material and Reagents
3.3. Extraction and Isolation
3.4. Characterization of AP-1 and AP-4
3.5. Cell Culture
3.6. Cell Viability Assay
3.7. Enzyme-Linked Immunosorbent Assay (ELISA)
3.8. Confocal Microscopy
3.9. D-QSAR Modeling
3.10. Zebrafish Husbandry and Embryo Collection
3.11. LPS-Induced Inflammation Model and Chemical Treatments
3.12. Histopathological Examination of Zebrafish Larvae
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Okhuarobo, A.; Falodun, J.E.; Erharuyi, O.; Imieje, V.; Falodun, A.; Langer, P. Harnessing the medicinal properties of Andrographis paniculata for diseases and beyond: A review of its phytochemistry and pharmacology. Asian Pac. J. Trop. Dis. 2014, 4, 213–222. [Google Scholar] [CrossRef]
- Subramanian, R.; Asmawi, M.Z.; Sadikun, A. A bitter plant with a sweet future? A comprehensive review of an oriental medicinal plant: Andrographis paniculata. Phytochem. Rev. 2012, 11, 39–75. [Google Scholar] [CrossRef]
- Boninia, S.A.; Premolia, M.; Tambarob, S.; Kumarc, A.; Maccarinellia, G.; Memoa, M.; Mastin, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Memo, M.; Mastinu, A. Cannabimimetic plants: Are they new cannabinoidergic modulators? Planta 2019, 249, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.K.; Taneja, S.C.; Dhar, K.L.; Atal, C.K. Flavonoids of andrographis paniculata. Phytochemistry 1983, 22, 314–315. [Google Scholar] [CrossRef]
- Kleipool, R.J. Constituents of Andrographis paniculata Nees. Nature 1952, 169, 33–34. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, A.; Fry, S.; Barrow, R.A.; Marshall, R.L.; Mukkur, T.K. Modulation of immune response in mice immunised with an inactivated Salmonella vaccine and gavaged with Andrographis paniculata extract or andrographolide. Int. Immunopharmacol. 2007, 7, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Saxena, R.; Saxena, R.P.; Saxena, K.C.; Srivastava, V.; Tandon, J.S. Immunostimulant agents from Andrographis paniculata. J. Nat. Prod. 1993, 56, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Michelsen, K.S.; Wong, M.H.; Ko, B.; Thomas, L.S.; Dhall, D.; Targan, S.R. HMPL-004 (Andrographis paniculata extract) prevents development of murine colitis by inhibiting T-cell proliferation and TH1/TH17 responses. Inflamm. Bowel. Dis. 2013, 19, 151–164. [Google Scholar] [CrossRef]
- Chao, W.W.; Kuo, Y.H.; Lin, B.F. Anti-inflammatory activity of new compounds from Andrographis paniculata by N-F-κB transactivation inhibition. J. Agric. Food Chem. 2010, 58, 2505–2512. [Google Scholar] [CrossRef]
- Chassagne, F.; Haddad, M.; Amiel, A.; Phakeovilay, C.; Manithip, C.; Bourdy, G.; Deharo, E.; Marti, G. A metabolomic approach to identify anti-hepatocarcinogenic compounds from plants used traditionally in the treatment of liver diseases. Fitoterapia 2018, 127, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Sheeja, K.; Kuttan, G. Activation of cytotoxic T lymphocyte responses and attenuation of tumor growth in vivo by Andrographis paniculata extract and andrographolide. Immunopharm. Immunot. 2007, 29, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Sheeja, K.; Guruvayoorappan, C.; Kuttan, G. Antiangiogenic activity of Andrographis paniculata extract and andrographolide. Int. Immunopharmacol. 2007, 7, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Thisoda, P.; Rangkadilok, N.; Pholphana, N.; Worasuttayangkurn, L.; Ruchirawat, S.; Satayavivad, J. Inhibitory effect of Andrographis paniculata extract and its active diterpenoids on platelet aggregation. Eur. J. Pharmacol. 2006, 553, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Tan, B.K. Anti-diabetic property of ethanolic extract of Andrographis paniculata in streptozotocin-diabetic rats. Acta Pharmacol. Sin. 2000, 21, 1157–1164. [Google Scholar] [PubMed]
- Reyes, B.A.; Bautista, N.D.; Tanquilut, N.C.; Anunciado, R.V.; Leung, A.B.; Sanchez, G.C.; Magtoto, R.L.; Castronuevo, P.; Tsukamura, H.; Maeda, K.I. Anti-diabetic potentials of Momordica charantia and Andrographis paniculata and their effects on estrous cyclicity of alloxan-induced diabetic rats. J. Ethnopharmacol. 2006, 105, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Karami, M.; Alimon, A.R.; Goh, Y.M. Effect of vitamin E, Andrographis paniculata and turmeric as dietary antioxidant supplementation on lipid and color stability of goat meat. Small Ruminant. Res. 2011, 97, 67–71. [Google Scholar] [CrossRef]
- Mo, M.X. Overview of clinical application of lianbizhi injection. J. Hunan Univ. Chin. Med. 2005, 25, 63–64. [Google Scholar]
- Guo, W.J.; Liu, W.; Chen, G.; Hong, S.C.; Qian, C.; Xie, N.; Yang, X.L.; Sun, Y.; Xu, Q. Water-soluble andrographolide sulfonate exerts anti-sepsis action in mice through down-regulating p38 MAPK, STAT3, and NF-κB pathways. Int. Immunopharmacol. 2012, 14, 613–619. [Google Scholar] [CrossRef]
- Xu, G. Paniculta lactone dropping pill for treatment of sore throat caused by upper respiratory tract infection. China J. Mod. Med. 2016, 26, 104–107. [Google Scholar]
- Wang, Y.J.; Wang, J.T.; Fan, Q.X.; Geng, J.G. Andrographolide inhibits NF-kappa B activation and attenuates neointimal hyperplasia in arterial restenosis. Cell Res. 2007, 17, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.F.; Yu, C.C.; Liang, H.Y.; Wang, X.K.; Liu, Y.; Li, X.; Ji, K.; Xu, H.; Yang, M.Y.; Liu, K.; et al. Andrographolide derivative CX-10 ameliorates dextran sulphate sodium-induced ulcerative colitis in mice: Involvement of NF-kappa B and MAPK signalling pathways. Int. Immunopharmacol. 2018, 57, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.C.; Chen, C.F.; Chiou, W.F. Andrographolide prevents oxygen radical production by human neutrophils: Possible mechanism(s) involved in its anti-inflammatory effect. Brit. J. Pharmacol. 2002, 135, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.; Qiu, F.; Wei, H.; Qu, G.X.; Yao, X.S. Nine new ent-Labdane diterpenoids from the Aerial parts of Andrographis paniculata. Helv. Chim. Acta 2006, 89, 2654–2664. [Google Scholar] [CrossRef]
- Matsuda, T.; Kuroyanagi, M.; Sugiyama, S.; Umehara, K.; Ueno, A.; Nishi, K. Cell differentiation-inducing diterpenes from Andrographis paniculata nees. Chem. Pharm. Bull. 1994, 42, 1216–1225. [Google Scholar] [CrossRef]
- He, X.J.; Zeng, X.B.; Hu, H.; Wu, Y.X. Cytotoxic biotransformed products from andrographolide by Rhizopus stolonifer ATCC 12939. J. Mol. Catal. B Enzym. 2010, 62, 242–247. [Google Scholar] [CrossRef]
- Zhou, K.L.; Chen, L.X.; Zhuang, Y.L.; Wang, N.L.; Yao, X.S.; Qiu, F. Two new ent-labdane diterpenoid glycosides from the aerial parts of Andrographis paniculata. J. Asian Nat. Prod. Res. 2008, 10, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; AnDong, Z.Y. Study on the three-dimensional structure of a new lactone in Andrographis paniculata. Chin. J. Med. Chem. 1997, 7, 270–273. [Google Scholar]
- Shen, Y.H.; Li, R.T.; Xiao, W.L.; Xu, G.; Lin, Z.W.; Zhao, Q.S.; Sun, H.D. ent-Labdane Diterpenoids from Andrographis paniculata. J. Nat. Prod. 2006, 69, 319–322. [Google Scholar] [CrossRef]
- Wang, G.Y.; Wen, T.; Liu, F.F.; Tian, H.Y.; Fan, C.L.; Huang, X.J.; Ye, W.C.; Wang, Y. Two new diterpenoid lactones isolated from Andrographis paniculata. Chin. J. Nat. Med. 2017, 15, 458–462. [Google Scholar] [CrossRef]
- Kallen, K.J. The role of transsignalling via the agonistic soluble IL-6 receptor in human diseases. Biochim. Biophys. Acta 2002, 1592, 323–343. [Google Scholar] [CrossRef] [Green Version]
- Marks, J.D.; Marks, C.B.; Luce, J.M.; Montgomery, A.B.; Turner, J.; Metz, C.A.; Murray, J.F. Plasma tumor necrosis factor in patients with septic shock. Mortality rate, incidence of adult respiratory distress syndrome, and effects of methylprednisolone administration. Am. Rev. Respir. Dis. 1990, 141, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.C.; Ma, X.Q.; Kwan, H.Y.; Tse, K.W.; Cao, H.H.; Su, T.; Shu, X.; Wu, Z.Z.; Yu, Z.L. A herbal formula consisting of Rosae Multiflorae Fructus and Lonicerae Japonicae Flos inhibits inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. J. Ethnopharmacol. 2014, 153, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Kitamura, M. Bidirectional regulation of NF-kappaB by reactive oxygen species: A role of unfolded protein response. Free Radic. Biol. Med. 2013, 65, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.A.; Mastinu, A.; Maccarinelli, G.; Mitola, S.; Premoli, M.; La Rosa., L.R.; Ferrari-Toninelli, G.; Grilli, M.; Memo, M. Cortical Structure Alterations and Social Behavior Impairment in p50-Deficient Mice. Cereb Cortex 2016, 26, 2832–2849. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, Y.; Gray, C.; Vuocolo, T.; Donaldson, L.; Broadway, M.; Tellam, R. Lipopolysaccharide and lipoteichoic acid induce different innate immune responses in bovine mammary epithelial cells. Cytokine 2005, 31, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Eisen, J.S. Zebrafish make a big splash. Cell 1996, 87, 969–977. [Google Scholar] [CrossRef]
- Pichler, F.B.; Laurenson, S.; Williams, L.C.; Dodd, A.; Copp, B.R.; Love, D.R. Chemical discovery and global gene expression analysis in zebrafish. Nat. Biotechnol. 2003, 21, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.L.; Wang, G.Q.; Yang, L.M.; Huang, Z.B.; Zhang, W.Q.; Yu, L.Z. Endotoxin molecule lipopolysaccharide-induced zebrafish inflammation model: A novel screening method for anti-inflammatory drugs. Molecules 2014, 19, 2390–2409. [Google Scholar] [CrossRef]
- Fan, H.Y.; Gao, Z.F.; Ji, K.; Li, X.; Wu, J.B.; Liu, Y.; Wang, X.K.; Liang, H.Y.; Liu, Y.N.; Li, X.T.; et al. The in vitro and in vivo anti-inflammatory effect of osthole, the major natural coumarin from Cnidium monnieri (L.) Cuss, via the blocking of the activation of the NF-κB and MAPK/p38 pathways. Phytomedicine 2019, 18, 1–11. [Google Scholar] [CrossRef]
- Lu, Z.B.; Xie, P.; Zhang, D.M.; Sun, P.H.; Yang, H.Y.; Ye, J.X.; Cao, H.H.; Huo, C.Y.; Zhou, H.L.; Chen, Y.Y.; et al. 3-Dehydroandrographolide protects against lipopolysaccharide-induced inflammation through the cholinergic anti-inflammatory pathway. Biochem. Pharm. 2018, 158, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.S.; Deng, L.J.; Tian, H.Y.; Ruan, Z.X.; Cao, H.H.; Ye, W.C.; Zhang, D.M.; Yu, Z.L. Anti-tumor effects and 3D-quantitative structure-activity relationship analysis of bufadienolides from toad venom. Fitoterapia 2019, 134, 362–371. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |












| No. | 1H | 13C | 1H-1H COSY | HMBC | NOESY |
|---|---|---|---|---|---|
| 1 | 1.45, 1.13 (m) | 39.2 | H-2 | C-10, 20 | − |
| 2 | 1.95 | 29.4 | H-1, 3 | C-1, 3 | − |
| 3 | 3.67 | 80.6 | H-2 | C-19 | H-1, 5, 18 |
| 4 | − | 43.9 | − | − | − |
| 5 | 1.23 (m) | 55.2 | H-6 | C-6, 7, 20 | H-3, 9 |
| 6 | 1.80 (m), 1.45 | 24.1 | H-5, 7 | C-7 | − |
| 7 | 2.41, 2.06 | 37.4 | H-6 | C-5, 9, 17 | − |
| 8 | − | 149.5 | − | − | − |
| 9 | 2.41 | 62.3 | H-11 | C-12, 17, 20 | H-5, 7 |
| 10 | − | 39.6 | − | − | − |
| 11 | 7.28 | 139.1 | H-9, 12 | C-8, 13 | − |
| 12 | 6.30 (d, 15.0) | 121.9 | H-11 | C-9, 14, 16 | H-9, 14 |
| 13 | − | 133.1 | − | − | − |
| 14 | 7.26 | 142.1 | H-15 | C-12, 16 | H-12, 17 |
| 15 | 6.01 (d, 15.0) | 103.3 | H-14 | C-13, 16, 21 | H-21 |
| 16 | − | 170.7 | − | − | − |
| 17 | 4.87 (d, 1.5), 4.72 (d, 1.5) | 109.3 | − | C-7, 9 | H-7 |
| 18 | 1.54 (s) | 24.2 | − | C-3, 5, 19 | H-3 |
| 19 | 4.49 (d, 10.0), 3.69 (d, 10.0) | 64.7 | − | C-3, 4, 5 | H-2, 20 |
| 20 | 0.90 (s) | 16.5 | − | C-1, 5, 9 | H-11, 19 |
| 21 | 3.50 (s) | 57.1 | − | C-15 | − |
| No. | 1H | 13C | 1H-1H COSY | HMBC | NOESY |
|---|---|---|---|---|---|
| 1 | 1.79, 1.28 | 38.1 | H-2 | C-3, 5, 9, 20 | − |
| 2 | 1.79 | 29.0 | H-1, 3 | C-4,10 | − |
| 3 | 3.40 | 80.9 | H-2 | C-5, 18, 19 | H-1, 5 |
| 4 | − | 43.7 | − | − | − |
| 5 | 1.32 | 56.3 | H-6 | C-18, 19, 20 | H-3, 9 |
| 6 | 1.82, 1.37 | 25.2 | H-5, 7 | C-5, 7 | − |
| 7 | 2.43, 2.03 (m) | 39.0 | H-6 | C-5, 6, 8, 17 | − |
| 8 | − | 149.0 | − | − | − |
| 9 | 1.90 (m) | 57.0 | H-11 | C-12, 17, 20 | H-1, 5 |
| 10 | − | 40.0 | − | − | − |
| 11 | 2.39, 2.27 (m) | 26.6 | H-9, 12 | C-8, 12, 13 | H-1, 7, 20 |
| 12 | 6.62 (m) | 143.6 | H-11 | C-14, 16 | H-9, 11 |
| 13 | − | 126.0 | − | − | − |
| 14 | 3.08 (m), 2.69 (m) | 33.6 | H-15 | C-12, 16 | H-11, 15 |
| 15 | 5.55 (dd, 6.5, 2.0) | 104.4 | H-14 | C-14, 16, 21 | H-14 |
| 16 | − | 171.8 | − | − | − |
| 17 | 4.89 (br s), 4.45 (br s) | 108.5 | − | C-7, 9 | H-7 |
| 18 | 1.22 (s) | 23.4 | − | C-3, 5, 19 | H-3 |
| 19 | 4.11 (d, 11.0), 3.36 (d, 11.0) | 65.0 | − | C-3, 5, 18 | H-2, 20 |
| 20 | 0.73 (s) | 15.5 | − | C-1, 5, 9 | H-11, 19 |
| 21 | 3.50 (s) | 57.2 | − | C-15 | − |
| Compound | IL-6 (×10−3 mg) | TNF-α (×10−3 mg) | Compound | IL-6 (×10−3 mg) | TNF-α (×10−3 mg) |
|---|---|---|---|---|---|
| LPS (100 ng/mL) | 1.36 ± 0.01 | 72.13 ± 2.89 | AP-10 (5 μM) | 0.94 ± 0.04 ** | 66.64 ± 0.51 * |
| Dex (5 μM) | 0.31 ± 0.02 ** | 32.21 ± 2.02 ** | AP-10 (10 μM) | 0.94 ± 0.03 ** | 60.34 ± 2.75 ** |
| AP-1 (5 μM) | 0.89 ± 0.02 ** | 63.77 ± 2.11 ** | AP-11 (5 μM) | 1.13 ± 0.01 ** | 81.77 ± 5.00 |
| AP-1 (10 μM) | 0.83 ± 0.03 ** | 53.00 ± 0.59 ** | AP-11 (10 μM) | 1.11 ± 0.01 ** | 66.89 ± 3.23 * |
| AP-2 (5 μM) | 1.11 ± 0.03 ** | 62.72 ± 1.15 ** | AP-12 (5 μM) | 0.93 ± 0.01 ** | 71.07 ± 3.03 |
| AP-2 (10 μM) | 0.69 ± 0.01 ** | 57.05 ± 0.45 ** | AP-12 (10 μM) | 0.81 ± 0.01 ** | 62.33 ± 2.35 ** |
| AP-3 (5 μM) | 0.75 ± 0.01 ** | 51.68 ± 2.18 ** | AP-13 (5 μM) | 1.05 ± 0.02 ** | 87.58 ± 1.60 |
| AP-3 (10 μM) | 0.72 ± 0.01 ** | 50.92 ± 0.93 ** | AP-13 (10 μM) | 1.00 ± 0.02 ** | 80.55 ± 1.27 |
| AP-4 (5 μM) | 0.65 ± 0.01 ** | 59.22 ± 1.74 ** | AP-14 (5 μM) | 1.36 ± 0.04 | 54.00 ± 1.27 ** |
| AP-4 (10 μM) | 0.63 ± 0.01 ** | 56.04 ± 0.71 ** | AP-14 (10 μM) | 1.17 ± 0.01 ** | 49.01 ± 0.25 ** |
| AP-5 (5 μM) | 0.68 ± 0.02 ** | 71.22 ± 0.81 | AP-15 (5 μM) | 1.40 ± 0.03 | 69.27 ± 1.78 * |
| AP-5 (10 μM) | 0.62 ± 0.01 ** | 66.47 ± 0.40 * | AP-15 (10 μM) | 1.32 ± 0.04 | 67.29 ± 0.87 * |
| AP-6 (5 μM) | 0.48 ±.0.01 ** | 41.89 ± 1.21 ** | AP-16 (5 μM) | 1.03 ± 0.01 ** | 64.71 ± 1.98 * |
| AP-6 (10 μM) | 0.15 ± 0.01 ** | 19.75 ± 2.14 ** | AP-16 (10 μM) | 0.95 ± 0.01 ** | 57.23 ± 2.05 ** |
| AP-7 (5 μM) | 1.17 ± 0.01 ** | 75.84 ± 0.20 | AP-17 (5 μM) | 1.19 ± 0.01 ** | 67.02 ± 0.87 * |
| AP-7 (10 μM) | 1.14 ± 0.01 ** | 71.28 ± 0.12 | AP-17 (10 μM) | 1.01 ± 0.01 ** | 61.50 ± 2.99 ** |
| AP-8 (5 μM) | 0.43 ± 0.03 ** | 43.28 ± 1.12 ** | AP-18 (5 μM) | 1.02 ± 0.04 ** | 73.15 ± 0.76 |
| AP-8 (10 μM) | 0.23 ± 0.01 ** | 30.03 ± 1.14 ** | AP-18 (10 μM) | 0.98 ± 0.01 ** | 69.47 ± 0.47 * |
| AP-9 (5 μM) | 1.26 ± 0.01 * | 77.64 ± 3.35 | AP-19 (5 μM) | 1.09 ± 0.04 ** | 76.53 ± 0.91 |
| AP-9 (10 μM) | 1.17 ± 0.01 ** | 75.20 ± 1.10 | AP-19 (10 μM) | 1.07 ± 0.01 ** | 71.70 ± 1.09 |
| IL-6 | TNF-α | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Name | Actual | CoMFA | CoMSIA | Actual | CoMFA | CoMSIA | ||||
| Pred | Res | Pred | Res | Pred | Res | Pred | Res | |||
| AP-1 *# | 6.081 | 6.150 | 0.070 | 6.206 | 0.126 | 4.276 | 4.328 | 0.052 | 4.335 | 0.060 |
| AP-2# | 6.161 | 6.150 | −0.011 | 6.206 | 0.045 | 4.244 | 4.328 | 0.084 | 4.335 | 0.092 |
| AP-3 | 6.143 | 6.042 | −0.101 | 6.116 | −0.027 | 4.145 | 4.149 | 0.005 | 4.289 | −0.004 |
| AP-4 | 6.201 | 6.224 | 0.024 | 6.182 | −0.018 | 4.293 | 4.311 | 0.018 | 4.254 | 0.002 |
| AP-5# | 6.208 | 6.368 | 0.161 | 6.292 | 0.084 | 4.252 | 4.238 | −0.014 | 4.427 | 0.250 |
| AP-6 | 6.824 | 6.661 | −0.163 | 6.674 | −0.150 | 4.178 | 4.524 | 0.346 | 4.706 | 0.001 |
| AP-7 *# | 5.943 | 6.637 | 0.694 | 6.692 | 0.750 | 4.704 | 4.676 | −0.028 | 4.674 | 0.527 |
| AP-8 | 6.639 | 6.696 | 0.058 | 6.735 | 0.097 | 4.147 | 4.664 | 0.517 | 4.522 | −0.000 |
| AP-9 | 5.932 | 5.881 | −0.051 | 5.888 | −0.044 | 4.522 | 4.551 | 0.028 | 4.129 | 0.005 |
| AP-10 | 6.027 | 5.990 | −0.036 | 6.019 | −0.008 | 4.124 | 4.122 | −0.002 | 4.220 | 0.001 |
| AP-11 | 5.955 | 5.934 | −0.021 | 5.956 | 0.001 | 4.220 | 4.232 | 0.013 | 4.173 | −0.002 |
| AP-12 * | 6.092 | 5.898 | −0.194 | 5.890 | −0.202 | 4.175 | 4.161 | −0.014 | 4.205 | −0.001 |
| AP-13 | 6.000 | 6.002 | 0.002 | 5.985 | −0.015 | 4.206 | 4.206 | 0.001 | 4.112 | 0.001 |
| AP-14# | 5.932 | 5.936 | 0.004 | 5.947 | 0.016 | 4.110 | 4.095 | −0.015 | 4.169 | −0.141 |
| AP-15 | 5.8780 | 5.944 | 0.065 | 5.943 | 0.064 | 4.310 | 4.170 | −0.139 | 4.169 | −0.004 |
| AP-16# | 6.022 | 5.989 | −0.033 | 5.974 | −0.049 | 4.172 | 4.183 | 0.011 | 4.152 | −0.091 |
| AP-17 * | 6.000 | 6.057 | 0.062 | 6.036 | 0.040 | 4.242 | 4.125 | −0.117 | 4.211 | 0.000 |
| AP-18 | 6.009 | 6.036 | 0.028 | 5.982 | −0.027 | 4.211 | 4.206 | −0.005 | 4.159 | 0.001 |
| AP-19 | 5.971 | 6.045 | 0.075 | 6.001 | 0.031 | 4.159 | 4.161 | 0.003 | 4.143 | −0.002 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, L.; Zheng, Y.; Deng, L.; Sun, P.; Ye, J.; Wei, X.; Liu, F.; Yu, L.; Ye, W.; Fan, C.; et al. Diterpenoid Lactones with Anti-Inflammatory Effects from the Aerial Parts of Andrographis paniculata. Molecules 2019, 24, 2726. https://doi.org/10.3390/molecules24152726
Gan L, Zheng Y, Deng L, Sun P, Ye J, Wei X, Liu F, Yu L, Ye W, Fan C, et al. Diterpenoid Lactones with Anti-Inflammatory Effects from the Aerial Parts of Andrographis paniculata. Molecules. 2019; 24(15):2726. https://doi.org/10.3390/molecules24152726
Chicago/Turabian StyleGan, Lin, Yuanru Zheng, Lijuan Deng, Pinghua Sun, Jiaxi Ye, Xiduan Wei, Feifei Liu, Linzhong Yu, Wencai Ye, Chunlin Fan, and et al. 2019. "Diterpenoid Lactones with Anti-Inflammatory Effects from the Aerial Parts of Andrographis paniculata" Molecules 24, no. 15: 2726. https://doi.org/10.3390/molecules24152726
APA StyleGan, L., Zheng, Y., Deng, L., Sun, P., Ye, J., Wei, X., Liu, F., Yu, L., Ye, W., Fan, C., Liu, J., & Zhang, W. (2019). Diterpenoid Lactones with Anti-Inflammatory Effects from the Aerial Parts of Andrographis paniculata. Molecules, 24(15), 2726. https://doi.org/10.3390/molecules24152726