Colchicine: Isolation, LC–MS QTof Screening, and Anticancer Activity Study of Gloriosa superba Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Chemicals
2.2. Super Critical Fluid Extractor
2.3. Super Critical Fluid (CO2) Extraction
2.4. Isolation of Colchicine
2.5. Preparation of Solutions for HPLC Analysis
2.6. HPLC Chromatographic Conditions
2.7. Preparation of Sample Solution for LC–MS QTof Screening
2.8. LC–MS QTof Conditions
2.9. Cell Lines and Culture Condition
2.10. Cell Cytotoxicity Assay
2.11. Cell Migration Assay
2.12. Cell Cycle Analysis by Flow Cytometry
2.13. Statistical Analysis
3. Results
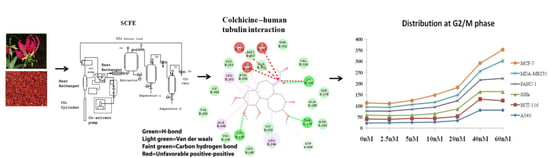
3.1. Super Critical Fluid Extraction
3.2. Isolation and Quantification of Colchicine
3.3. LC–MS QTof Screening of Gloriosa Seeds
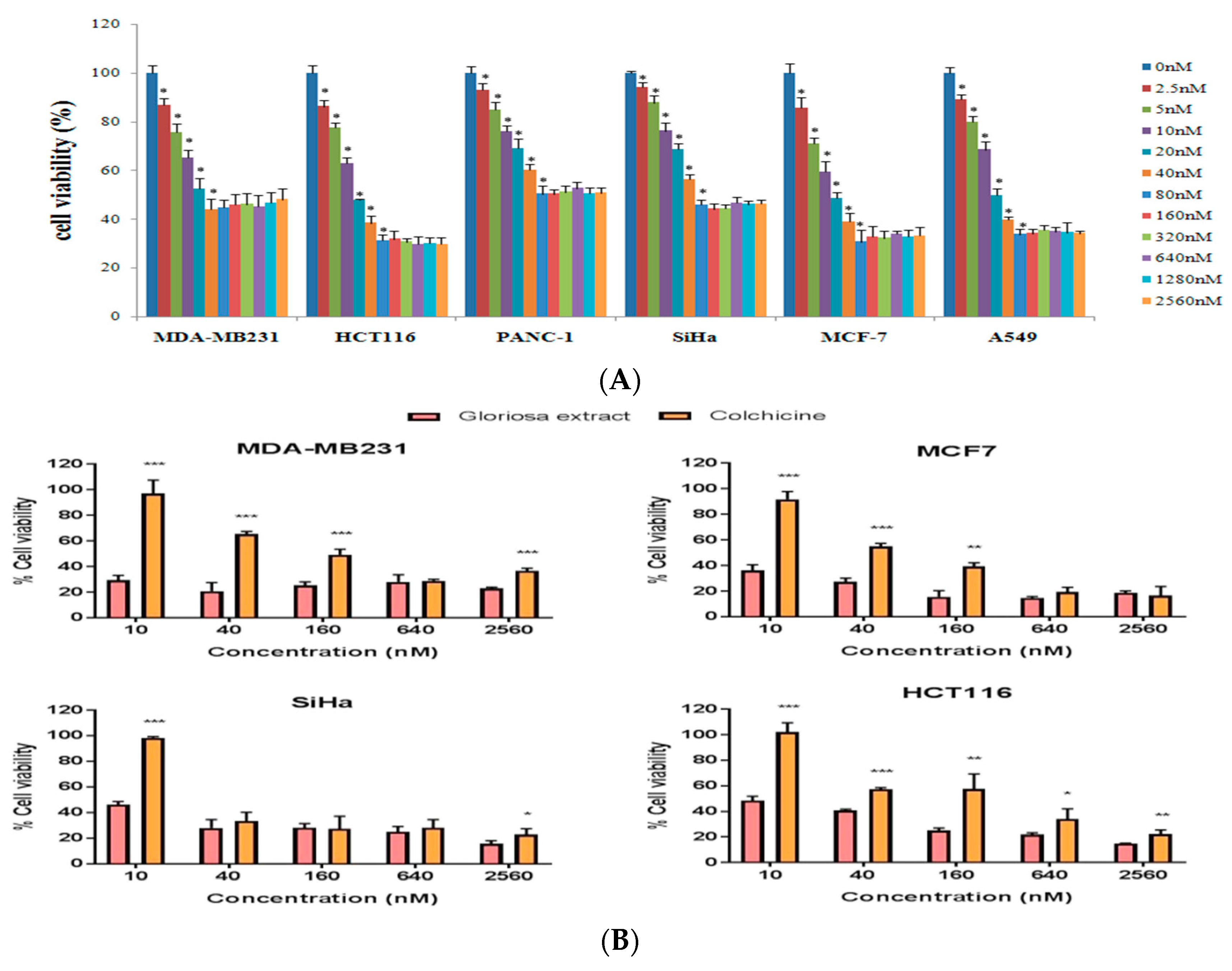
3.4. Effect of Colchicine on Cell Viability
3.5. Effect of Colchicine on Cell Migration
3.6. Analysis of Cell Cycle
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ambasta, S.P. The Useful Plants of India; National Institute of Science Communication: New Delhi, India, 1986; p. 238. [Google Scholar]
- Nadkarni, K.M. Indian Materia Medica, Vol. I.–II; Popular Prakashan Private Limited: Mnmbai, India, 1976; pp. 1–968. [Google Scholar]
- Pulliah, T. Medicinal plants in India, vol. I; Regency Publications: New Delhi, India, 2002; pp. 269–270. [Google Scholar]
- Capraro, H.G.; Brossi, A. Tropolonic colchicum alkaloids. The Alkaloids: Chemistry and Pharmacology; Academic Press: New York, NY, USA, 1984; pp. 1–70. [Google Scholar]
- Kuo, M.C.; Chang, S.J.; Hsieh, M.C. Colchicine Significantly Reduces Incident Cancer in Gout Male Patients A 12-Year Cohort Study. Medicine 2015, 94, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Finnie, J.F.; Van Staden, J. Gloriosa superba L. (Flame Lily): Micropropagation and In Vitro Production of colchicines. Biotechnology in Agriculture and Forestry, Medicinal and Aromatic Plants VI; Springer: Berlin/Heidelberg, Germany, 1994; Volume 26, pp. 146–166. [Google Scholar]
- Chopra, R.N.; Nayar, S.L.; Chopra, I.C. Glossary of Indian Medicinal Plants; PID, CSIR: New Delhi, India, 1956; p. 125. [Google Scholar]
- Sarin, Y.K.; Jamwal, P.S.; Gupta, B.K.; Atal, C.K. Colchicine from seeds of Gloriosa superba. Curr. Sci. 1977, 43, 87. [Google Scholar]
- Srivastava, U.C.; Chandra, V. Gloriosa superba Linn. (Kalihari) an important colchicine producing plant. J. Res. Ind. Med. 1977, 10, 92–95. [Google Scholar]
- Bellet, P.; Gaignault, J.C. Gloriosa superba L. production de substances colchiciniques. Ann. Pharm. Fr. 1985, 43, 345–347. [Google Scholar] [PubMed]
- Husek, A.; Sutlupinar, N.; Potesilova, A.; Dvorackova, S.; Hanus, V.; Sedmera, P.; Malon, P.; Simanek, V. Alkaloids and phenolics of three Merendera Species. Phytochemistry 1989, 28, 3217–3219. [Google Scholar] [CrossRef]
- Husek, A.; Sutlupinar, N.; Sedmera, P.; Volgelein, F.; Valka, I.; Simanek, V. Alkaloids and phenolics of Colchicum turcicum. Phytochemistry 1990, 29, 3058–3060. [Google Scholar] [CrossRef]
- Joshi, C.S.; Priya, E.S.; Mathela, C.S. Isolation and anti-inflammatory activity of colchicinoids from Gloriosa superba seeds. Pharm. Biol. 2010, 48, 206–209. [Google Scholar] [CrossRef]
- Ellington, E.; Bastida, J.; Viladomat, F.; Codina, C. Super Critical carbon dioxide extraction of colchicine and related alkaloids from seeds of Colchicum autumnale. Phytochem. Anal. 2003, 4, 164–169. [Google Scholar] [CrossRef]
- Mansoori, G.A.; Schulz, K.; Martinelli, E. Bioseparation using Super Critical Fluid Extraction/Retrograde Condensation. Bio. Technol. 1988, 6, 393–396. [Google Scholar]
- Martinelli, E.; Schulz, K.; Mansoori, G.A. Super Critical Fluid Extraction/ Retrograde Condensation with Application in Biotechnology; CRC Press: Boca Raton, FL, USA, 1991; pp. 451–478. [Google Scholar]
- Lang, Q.Y.; Wai, C.M. Super Critical Fluid Extraction in Herbal and Natural Product Studies—A Practical review. Talanta 2001, 53, 771–782. [Google Scholar] [CrossRef]
- Mohamed, R.S.; Masoori, G.A. The use of Super Critical Fluid Extraction Technology in Food Processing. Food Technol. Mag. 2002, 20, 134–139. [Google Scholar]
- Ndiomu, D.P.; Simpson, C.F. Some Applications of Super Critical Fluid Extraction. Anal. Chim. Acta 1998, 213, 237–243. [Google Scholar] [CrossRef]
- King, M.B.; Bott, T.R. Extraction of Natural Products Using Near-Critical Solvents; Chapman and Hall: London, UK, 1993; pp. 1–49. [Google Scholar]
- Moore, W.N.; Taylor, L.T. Gaining greater selectivity in the Super Critical Fluid Extraction of digoxin from Digitalis lanata leaves. Phytochem. Anal. 1997, 8, 238–243. [Google Scholar] [CrossRef]
- Chester, T.L.; Pinkston, J.D.; Raynie, D.E. Super Critical Fluid chromatography and extraction. Anal. Chem. 1996, 68, 487–514. [Google Scholar] [CrossRef]
- Chester, T.L.; Pinkston, J.D.; Raynie, D.E. Super Critical Fluid Chromatography and Extraction. Anal. Chem. 1998, 70, 301–320. [Google Scholar] [CrossRef]
- Sihvonen, M.; Jarvenpaa, E.; Heitaniemi, V.; Huopalthi, R. Advances in Super Critical carbon dioxide Technologies. Trends Food Sci. Technol. 1999, 10, 217–222. [Google Scholar] [CrossRef]
- Mukhopadhyay, M. Natural Extracts Using Super Critical Carbon Dioxide; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Ravelli, R.B.G.; Gigant, B.; Curmi, P.A.; Jourdain, I.; Lachkar, S.; Sobel, A.; Knossow, M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004, 428, 198–202. [Google Scholar] [CrossRef]
- Richette, P.; Bardin, T. Colchicine for the treatment of gout. Expert Opin. Pharmacother. 2010, 11, 2933–2938. [Google Scholar] [CrossRef] [PubMed]
- Samy, R.P.; Twin, M.M.; Gopalkrishnakone, P.; Ignacimuthu, S. Ethanobotanical survey of folk plants for treatment of snake bites in southern part of Tamilnadu, India. J. Ethnopharmacol. 2008, 115, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Cerquaglia, C.; Diaco, M.; Nucera, G.; La Regina, M.; Montalto, M.; Manna, R. Pharmacological and clinical basis of treatment of Familial Mediterranean Fever (FMF) with colchicine or analogues: An update. Curr. Drug Targets Inflamm. Allergy 2005, 4, 117–124. [Google Scholar] [CrossRef]
- Kallinich, T.; Haffner, D.; Niehues, T.; Huss, K.; Lainka, E.; Neudorf, U.; Schaefer, C.; Stojanov, S.; Timmann, C.; Keitzer, R.; et al. Colchicine use in children and adolescents with Familial Mediterranean Fever: Literature review and consensus statement. Pediatrics 2007, 119, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Wilson, L. Microtubules and actin filaments: Dynamic targets for cancer chemotherapy. Curr. Opin. Cell Biol. 1998, 10, 123–130. [Google Scholar] [CrossRef]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Downing, K.H. Structural basis for the interaction of tubulin with proteins and drugs that affect microtubule dynamics. Ann. Rev. Cell Dev. Biol. 2000, 16, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Rowinsky, E.K.; Donehower, R.C. The clinical pharmacology and use of antimicrotubule agents in cancer chemotherapeutics. Pharmacol. Ther. 1991, 52, 35–84. [Google Scholar] [CrossRef]
- Taylor, E.W. The mechanism of colchicine inhibition of mitosis. I. Kinetics of inhibition and the binding of H3-Colchicine. J. Cell. Biol. 1965, 25, 145–160. [Google Scholar] [CrossRef]
- Yan, L.; Chen, J.; Xiao, M.; Li, W.; Miller, D.D. An Overview of Tubulin Inhibitors That Interact with the Colchicine Binding Site. Pharm. Res. 2012, 29, 2943–2971. [Google Scholar]
- Eigsti, O.J.P.; Dustin, J.R. Colchicine; Iowa State College Press: Ames, Iowa, 1955. [Google Scholar]
- Brancale, A.; Silvestri, R. Indole, a core nucleus for potent inhibitors of tubulin polymerization. Med. Res. Rev. 2007, 27, 209–238. [Google Scholar] [CrossRef]
- Otto, A.M.; Paddenberg, R.; Schubert, S.; Mannherz, H.G. Cell-cycle arrest, micronucleus formation, and cell death in growth inhibition of MCF-7 breast cancer cells by tamoxifen and cisplatin. J. Cancer Res. Clin. Oncol. 1996, 122, 603–612. [Google Scholar] [CrossRef]
- Wang, S.T.; Ho, H.J.; Lin, J.T.; Shiehand, J.J.; Wu, C.Y. Simvastatin-induced cell cycle arrest through inhibition of STAT3/SKP2 axis and activation of AMPK to promote p27 and p21 accumulation in hepatocellular carcinoma cells. Cell Death Dis. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Wu, C.C.; Chuang, Y.H.; Chuang, W.L. Anti-cancer mechanisms of clinically acceptable colchicine concentrations on hepatocellular carcinoma. Life Sci. 2013, 93, 323–328. [Google Scholar] [CrossRef]
- Cocco, G.; Chu, D.C.; Pandolfi, S. Colchicine in clinical medicine. A guide for internists. Eur. J. Intern. Med. 2010, 21, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Karar, M.G.E.; Kuhnert, N. UPLC-ESI-Q-TOF-MS/MS Characterization of phenolics from Crataegusmonogyna and Crataegus laevigata (Hawthorn) leaves, fruits and their herbal derived drops (CrataeguttTropfen). J Chem. Biol. Ther. 2015, 1, 102. [Google Scholar] [CrossRef]
- Luo, J.L.; Lu, F.L.; Liu, Y.C.; Shih, Y.C.; Lo, C.F. Fingerprint analysis of Ginkgo biloba extract and Ginkgo semen in preparations by LC-Q-TOF/MS. J. Food Drug Anal. 2013, 21, 27–39. [Google Scholar]
- Sliwka, L.; Wiktorska, K.; Suchocki, P.; Milczarek, M.; Mielczarek, S.; Lubelska, K.; Cierpial, T.; Łyżwa, P.; Kiełbasiński, P.; Jaromin, A.; et al. The Comparison of MTT and CVS Assays for the Assessment of Anticancer Agent Interactions. PLoS ONE 2016, 11, e0155772. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Wesley, S.D.; Ruba, A.; Rajlashmi, A.R.; Kumaragurubaran, K. Optimization of solvent for effective isolation of colchicines from Gloriosa superba. Nat. Prod. Res. 2007, 21, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Das, A.; Datta, S.; Ganguli, A.; Chakrabarti, G. Colchicine induces autophagy and senescence in lung cancer cells at clinically admissible concentration: Potential use of colchicine in combination with autophagy inhibitor in cancer therapy. Tumour Biol. 2016, 8, 10653–10664. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.A.; Gernert, K.M.; Nettles, J.H.; Aneja, R. Drugs that target dynamic microtubules: A new molecular perspective. Med. Res. Rev. 2011, 31, 443–481. [Google Scholar] [CrossRef]
- Ferron, G.M.; Rochdi, M.; Jusko, W.J.; Scherrmann, J.M. Oral absorption characteristics and pharmacokinetics of colchicine in healthy volunteers after single and multiple doses. J. Clin. Pharmacol. 1996, 36, 874–883. [Google Scholar] [CrossRef]
- Sivakumar, G. Colchicine semisynthetics: Chemotherapeutics for cancer. Curr. Med. Chem. 2013, 20, 892–898. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |







| Exp. No. | Gloriosa Seeds Loaded (Kg) | Pressure of Extractor | Temp (°C) | Flow Rate of Liquid CO2 (g/min) (3% Water as co Solvent) | Yield (G) | Assay of Colchicine (HPLC) (%) |
|---|---|---|---|---|---|---|
| 1 | 3.0 | 200 | 60 | 100 | 1.2 | Nil, waxy |
| 2 | 3.0 | 250 | 60 | 100 | 5.0 | 3.0 |
| 3 | 3.0 | 300 | 60 | 100 | 10.3 | 6.92 |
| 4 | 3.0 | 350 | 60 | 100 | 56.2 | 20.3 |
| 5 | 3.0 | 400 | 60 | 100 | 72.8 | 27.0 |
| 6 | 3.0 | 450 | 60 | 100 | 80.1 | 16.15 |
| S. No. | Component Name | RT (min) | Positive Mode | Negative Mode | ||
|---|---|---|---|---|---|---|
| Observed m/z | Response | Observed m/z | Response | |||
| 1 | Chelidonic acid | 0.91 | 185.0079 | 3211 | - | - |
| 2 | Adenosine | 0.95 | 268.1031 | 151304 | 266.0877 | 1956 |
| 3 | o-Coumaric acid | 0.95 | 165.0537 | 23392 | - | - |
| 3 | Vanillic acid | 2.32 | - | - | 167.0337 | 2640 |
| 4 | Vanillic acid β-D-glucopyranosyl ester | 3.15 | - | - | 329.0864 | 9708 |
| 5 | Catechin 7-O-β-D-glucopyranoside | 4.58 | 453.1383 | 14809 | 451.1238 | 131,920 |
| 6 | (−)-Epicatechin | 5.28 | 291.0857 | 33690 | 289.0699 | 5584 |
| 7 | Curculigoside B | 5.28 | 453.1383 | 23748 | 451.1241 | 109,583 |
| 8 | Caffeic acid | 5.61 | - | - | 179.0335 | 4978 |
| 9 | d-Isoboldine | 5.97 | 328.1536 | 100400 | - | - |
| 10 | Gentiatibetine | 6.07 | 166.0851 | 6893 | - | - |
| 11 | Procyanidin B7 | 6.28 | - | - | 577.1358 | 227,928 |
| 12 | Daidzein | 7.03 | 255.0659 | 4567 | - | - |
| 13 | Colchicoside | 7.11 | 548.2137 | 2177554 | 546.1986 | 213,123 |
| 14 | 2-demethylcolchicine | 7.11 | 386.16 | 556495 | 384.1445 | 1,186,230 |
| 15 | Catechin | 7.21 | 291.0853 | 38106 | 289.0702 | 93,291 |
| 16 | Isoastilbin | 7.27 | - | - | 449.1079 | 28,723 |
| 17 | 6-Hydroxykaempferol | 9.55 | 303.0498 | 3793 | - | - |
| 18 | 1,2-didemethyl colchicine | 9.65 | 372.1443 | 322523 | 370.1283 | 27,794 |
| 19 | Naringin | 10.24 | - | - | 579.1736 | 27,936 |
| 20 | Kaempferol-3-glucuronide | 11.84 | 465.1023 | 7577 | 463.0871 | 8259 |
| 21 | Quercetin-3-O-β-D-glucopyranoside | 12.26 | - | - | 463.0877 | 231,855 |
| 22 | Quercetin | 12.29 | 303.0495 | 96085 | - | - |
| 23 | N-Deacetyl-N-methylcolchicine | 12.61 | 372.1788 | 7489 | - | - |
| 24 | Isoperlolyrine 2 | 12.9 | 265.0964 | 82453 | 263.0809 | 2075 |
| 25 | Colchiceine | 13.12 | 386.1601 | 2336427 | 384.1448 | 1,907,075 |
| 26 | Anthraquinone | 13.2 | 209.0599 | 7183 | - | - |
| 27 | Flavanonol | 13.2 | 241.0854 | 6229 | - | - |
| 28 | 6-Hydroxykaempferol-3-O-glucoside | 14.61 | 465.1025 | 3279 | 463.0876 | 3857 |
| 29 | 6-Methoxykaempferol-3-O-β-D-glucopyranoside | 14.89 | - | - | 477.1037 | 35,244 |
| 30 | Isoquercetin | 16.12 | 465.1023 | 4261 | - | - |
| 31 | 2,3-didemthylcolchicine | 16.66 | 372.1433 | 167107 | 370.1282 | 18,762 |
| 32 | Gloriosine | 17.03 | 386.1598 | 882243 | 384.1441 | 73,037 |
| 33 | 3-demethylcolchicine | 18.4 | 386.1613 | 2596448 | - | - |
| 34 | Colchicine | 18.59 | 400.1744 | 2315151 | - | - |
| 35 | Procyanidin A2 | 18.6 | - | - | 575.1203 | 51,849 |
| 36 | n-deacetylcolchicine | 18.66 | 358.1664 | 821243 | - | - |
| 37 | Morin | 19.1 | - | - | 301.0336 | 52,761 |
| 38 | Luteolin | 19.33 | 287.054 | 6150 | 285.0392 | 6471 |
| 39 | Cornigerine | 20.45 | 384.1434 | 438658 | - | - |
| 40 | Futoenone | 24.41 | 341.1379 | 739283 | - | - |
| 42 | Balanophonin | 28.24 | 357.1324 | 32373 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balkrishna, A.; Das, S.K.; Pokhrel, S.; Joshi, A.; Laxmi; Verma, S.; Sharma, V.K.; Sharma, V.; Sharma, N.; Joshi, C.S. Colchicine: Isolation, LC–MS QTof Screening, and Anticancer Activity Study of Gloriosa superba Seeds. Molecules 2019, 24, 2772. https://doi.org/10.3390/molecules24152772
Balkrishna A, Das SK, Pokhrel S, Joshi A, Laxmi, Verma S, Sharma VK, Sharma V, Sharma N, Joshi CS. Colchicine: Isolation, LC–MS QTof Screening, and Anticancer Activity Study of Gloriosa superba Seeds. Molecules. 2019; 24(15):2772. https://doi.org/10.3390/molecules24152772
Chicago/Turabian StyleBalkrishna, Acharya, Subrata K. Das, Subarna Pokhrel, Alpana Joshi, Laxmi, Sudeep Verma, Vinai K. Sharma, Vinamra Sharma, Niti Sharma, and C. S. Joshi. 2019. "Colchicine: Isolation, LC–MS QTof Screening, and Anticancer Activity Study of Gloriosa superba Seeds" Molecules 24, no. 15: 2772. https://doi.org/10.3390/molecules24152772
APA StyleBalkrishna, A., Das, S. K., Pokhrel, S., Joshi, A., Laxmi, Verma, S., Sharma, V. K., Sharma, V., Sharma, N., & Joshi, C. S. (2019). Colchicine: Isolation, LC–MS QTof Screening, and Anticancer Activity Study of Gloriosa superba Seeds. Molecules, 24(15), 2772. https://doi.org/10.3390/molecules24152772