Estimating the Solubility, Solution Thermodynamics, and Molecular Interaction of Aliskiren Hemifumarate in Alkylimidazolium Based Ionic Liquids
Abstract
:1. Introduction
2. Results and Discussion
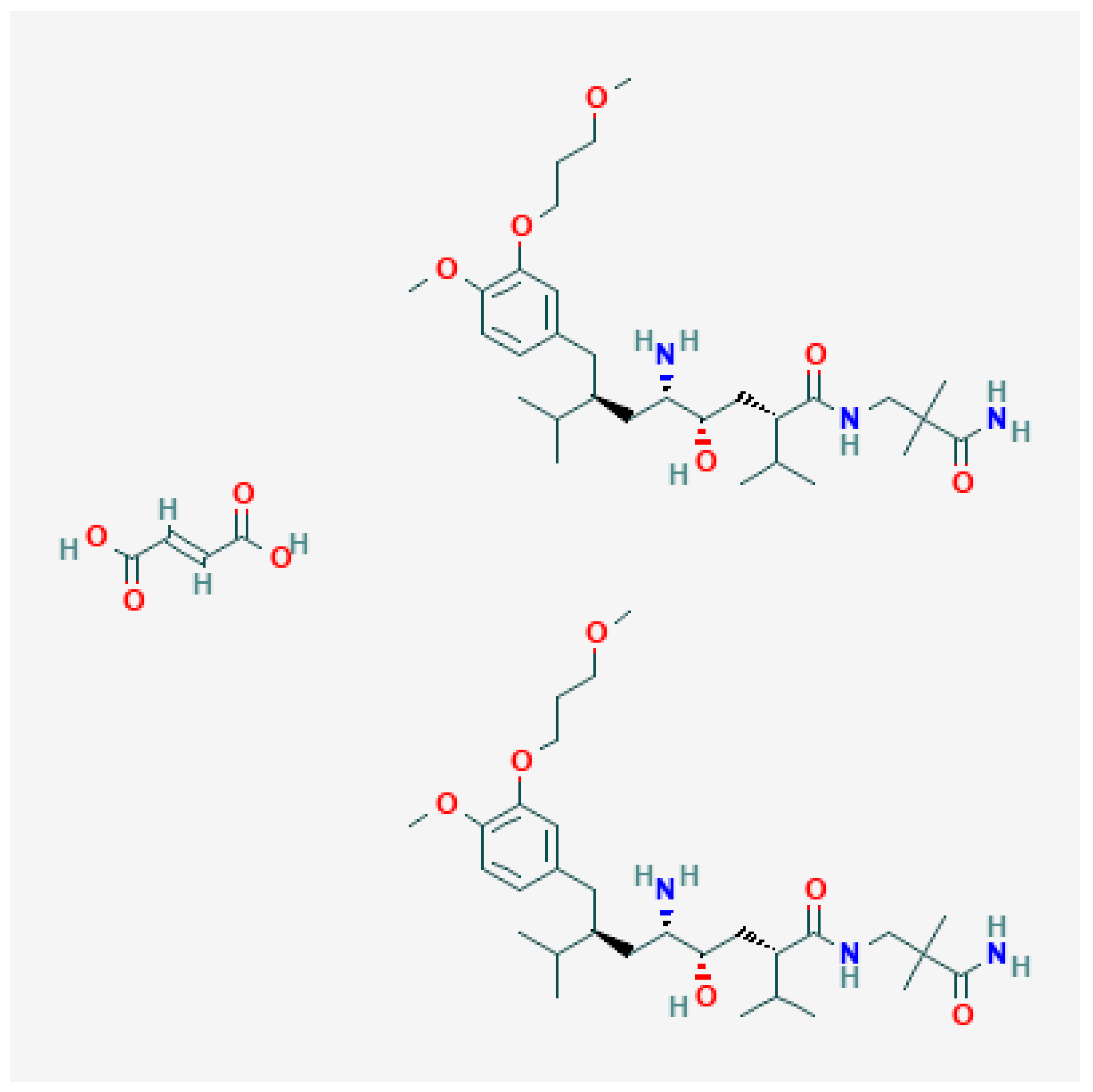
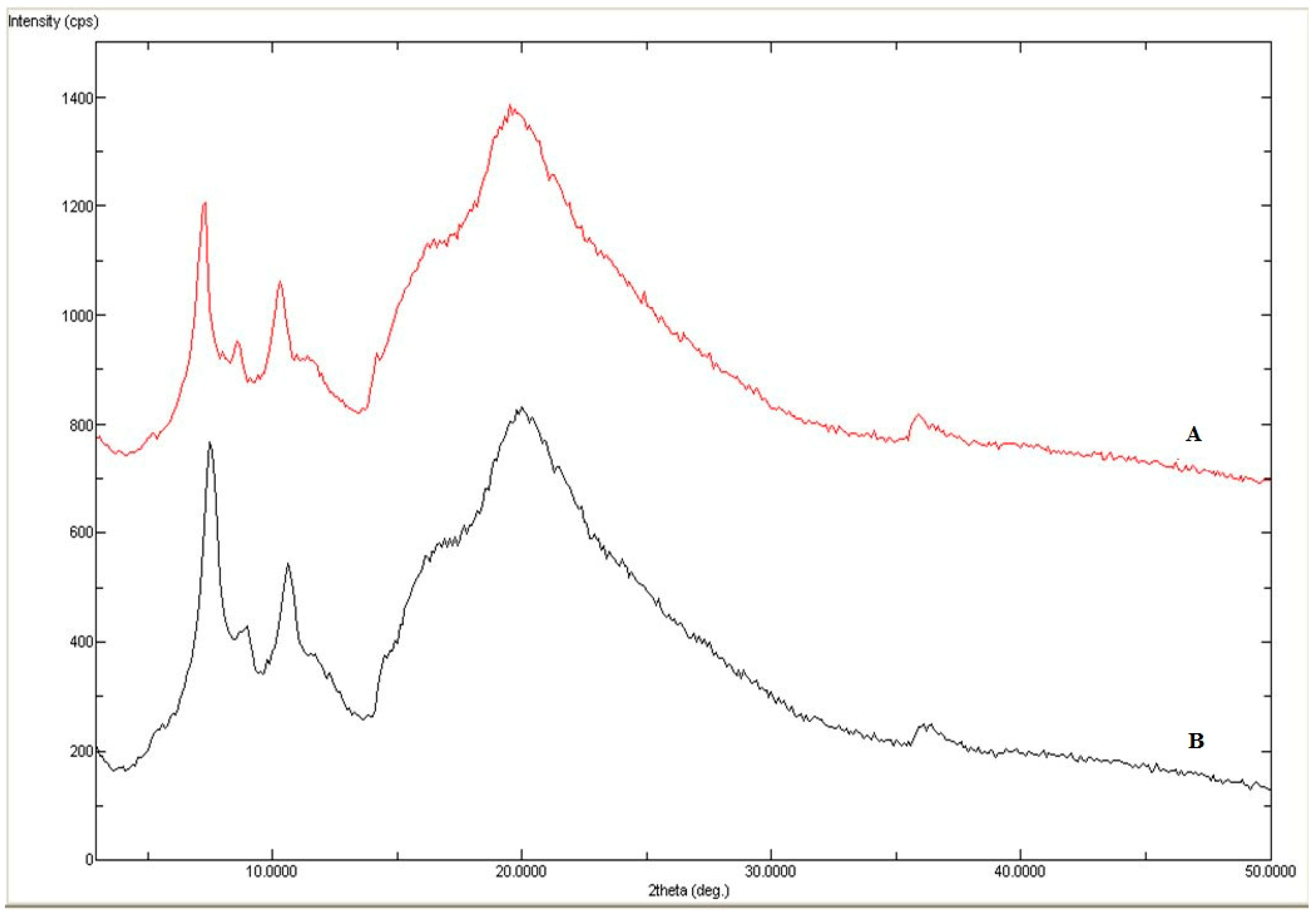
2.1. Solid Phase Characterization of AHF
2.2. Measured Solubilities of AHF
2.3. Hansen Solubility Parameters
2.4. Measurement of Ideal Solubilities and Activity Coefficients
2.5. Correlation of xe Values of AHF
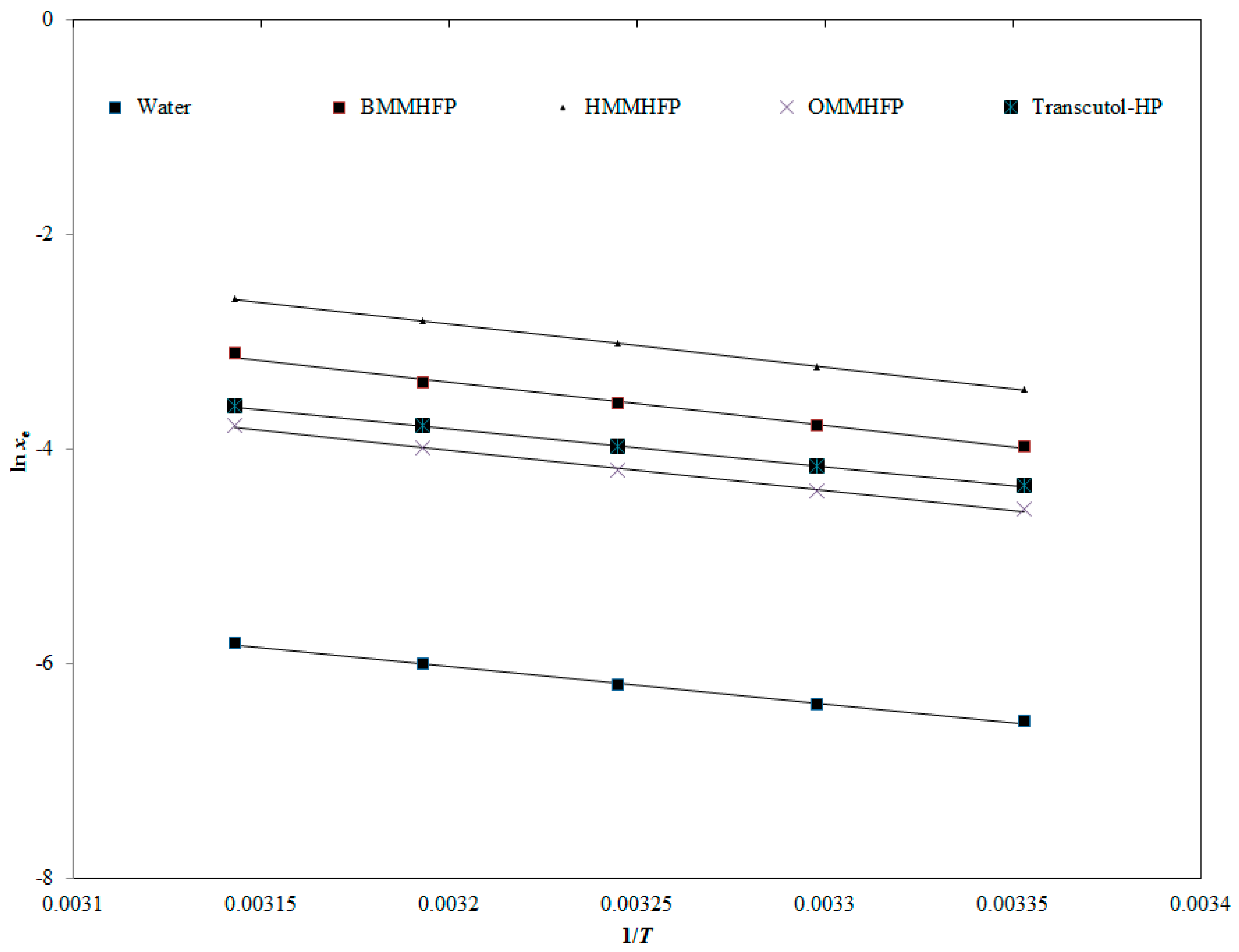
2.6. Measurement of Apparent Thermodynamic Parameters
3. Materials and Methods
3.1. Materials
3.2. Analysis of AHF
3.3. Solid Phase Characterization of AHF
3.4. Solubility Studies of AHF
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pantzaris, N.D.; Karanikolas, E.; Tsiotsios, K.; Velissaris, D. Renin Inhibition with Aliskiren: A Decade of Clinical Experience. J. Clin. Med. 2017, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.M.; Maibaum, J.; Rahuel, J.; Grütter, M.G.; Cohen, N.C.; Rasetti, V.; Rüger, H.; Göschke, R.; Stutz, S.; Fuhrer, W.; et al. Structure-based design of aliskiren, a novel orally effective renin inhibitor. Biochem. Biophys. Res. Commun. 2003, 308, 698–705. [Google Scholar] [CrossRef]
- Waldmeier, F.; Glaenzel, U.; Wirz, B.; Oberer, L.; Schmid, D.; Seiberling, M.; Valencia, J.; Riviere, G.J.; End, P.; Vaidyanathan, S. Absorption, Distribution, Metabolism, and Elimination of the Direct Renin Inhibitor Aliskiren in Healthy Volunteers. Drug. Metab. Dispos. 2007, 35, 1418–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limoges, D.; Dieterich, H.A.; Yeh, C.M.; Vaidyanathan, S.; Howard, D.; Dole, W.P. A study of dose-proportionality in the pharmacokinetics of the oral direct renin inhibitor aliskiren in healthy subjects. Int. J. Clin. Pharmacol. Ther. 2008, 46, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Halett, P.; Welton, T. Room temperature ionic liquids: Solvents for synthesisand catalysis. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, R.; Cheng, T.; Guo, J.; Xian, M.; Liu, H. Imidazolium-based ionic liquids for cellulose pretreatment: Recent progresses and future perspectives. Appl. Microbiol. Biotechnol. 2017, 101, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemicalindustry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P. Ionic liquids in pharmaceutical applications. Annu. Rev. ChemBiomol. Eng. 2014, 5, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Padua, A.A.H. Nanostructural organization in ionic liquids. J. Phys. Chem. B 2006, 110, 3330–3335. [Google Scholar] [CrossRef] [PubMed]
- Berthod, A.; Ruiz-Ángel, M.J.; Carda-Broch, S. Recent advances on ionic liquid uses in separation techniques. J. Chromatogr A 2018, 1559, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Fredlake, C.P.; Crosthwaite, J.M.; Hert, D.G.; Aki, S.N.V.K.; Brennecke, J.F. Thermophysical Properties of Imidazolium-Based Ionic Liquids. J. Chem. Eng. Data 2004, 494, 954–964. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, S.; Li, H.; Liu, J.; Liu, X.; Song, H. In Situ Synthesis ofImidazolium-CrosslinkedIonogels via Debus-RadziszewskiReactionBasedon PAMAM Dendrimers inImidazoliumIonicliquid. Macromol. Rapid. Commun. 2017, 38, 1700415. [Google Scholar] [CrossRef] [PubMed]
- Jaitely, V.; Karatas, A.; Florence, A.T. Water immiscible room temperature ionic liquids: Some properties relevant to their pharmaceutical use. Int. J. Pharm. 2008, 354, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Mizuuchi, H.; Jaitely, V.; Murdan, S.; Florence, A.T. Room temperature ionic liquids and their mixtures: Potential pharmaceutical solvents. Eur. J. Pharm. Sci. 2008, 33, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Badone, D.; Lau, C.K.; Jiandong, Y. Aliskirenhemifumarate, Crystal form and Amorphous Solid. US Patent 14/131,615, 27 November 2014. [Google Scholar]
- Aliskiren (hemifumarate), Cayman Chemicals. Available online: https://www.caymanchem.com/product/19640 (accessed on 28 July 2019).
- Marciniak, A. The Hildebrand Solubility Parameters of Ionic Liquids-Part 2. Int. J. Mol. Sci. 2011, 12, 3553–3575. [Google Scholar] [CrossRef] [PubMed]
- Ruidiaz, M.A.; Delgado, D.R.; Martínez, F.; Marcus, Y. Solubility andpreferential solvation of indomethacin in 1,4-dioxane + water solvent mixtures. Fluid Phase Equailib. 2010, 299, 259–265. [Google Scholar] [CrossRef]
- Manrique, Y.J.; Pacheco, D.P.; Martínez, F. Thermodynamics of mixing and solvation of ibuprofen and naproxen in propylene glycol + water cosolvent mixtures. J. Sol. Chem. 2008, 37, 165–181. [Google Scholar] [CrossRef]
- Shakeel, F.; Imran, M.; Haq, N.A.; Alanazi, F.K.; Alsarra, I.A. Solubility and thermodynamic/solvation behavior of 6-phenyl-4,5-dihydropyridazin-3(2H)-one in different (Transcutol + water) mixtures. J. Mol. Liq. 2017, 230, 511–517. [Google Scholar] [CrossRef]
- Apelblat, A.; Manzurola, E. Solubilities of o-acetylsalicylic, 4-aminosalicylic, 3,5-dinitrosalicylic and p-toluic acid and magnesium-DL-aspartatein water from T = (278–348) K. J. Chem. Thermodyn. 1999, 31, 85–91. [Google Scholar] [CrossRef]
- Manzurola, E.; Apelblat, A. Solubilities of L-glutamic acid, 3-nitrobenzoicacid, acetylsalicylic, p-toluic acid, calcium-L-lactate, calcium gluconate, magnesium-DL-aspartate, and magnesium-L-lactate in water. J. Chem. Thermodyn. 2002, 34, 1127–1136. [Google Scholar] [CrossRef]
- Holguín, A.R.; Rodríguez, G.A.; Cristancho, D.M.; Delgado, D.R.; Martínez, F. Solution thermodynamics of indomethacin in propylene glycol + watermixtures. Fluid Phase Equalib. 2012, 314, 134–139. [Google Scholar] [CrossRef]
- Krug, R.R.; Hunter, W.G.; Grieger, R.A. Enthalpy-entropy compensation. 2. Separation of the chemical from the statistic effect. J. Phys. Chem. 1976, 80, 2341–2351. [Google Scholar] [CrossRef]
- Shakeel, F.; Haq, N.; Raish, M.; Anwer, M.K.; Al-Shdefat, R. Solubility and thermodynamic analysis of sinapic acid in various neat solvents at different temperatures. J. Mol. Liq. 2016, 222, 167–171. [Google Scholar] [CrossRef]
- Wrasse-Sangoi, M.; Secretti, L.T.; Diefenbach, I.F.; Rolim, C.M.B.; Sangoi, M.D. Development and validation of an UV spectrophotometric method for thedetermination of aliskiren in tablets. Quim. Nova 2010, 33, 1330–1334. [Google Scholar] [CrossRef]
- Anwer, M.K.; Al-Mansoor, M.A.; Jamil, S.; Al-Shdefat, R.; Ansari, M.N.; Shakeel, F. Development and evaluation of PLGA polymer based nanoparticles of quercetin. Int. J. BiolMacromol. 2016, 92, 213–219. [Google Scholar] [CrossRef]
- Anwer, M.K.; Mohammad, M.; Fatima, F.; Alshahrani, S.M.; Aldawasari, M.F.; Alalaiwe, A.; Al-Shdefat, R.; Shakeel, F. Solubility, solution thermodynamics and molecular interactions of osimertinib in some pharmaceutically useful solvents. J. Mol. Liq. 2019, 284, 53–58. [Google Scholar] [CrossRef]
- Ahmad, A.; Raish, M.; Alkharfy, K.M.; Alsarra, I.A.; Ahad, A.; Jan, B.L.; Shakeel, F. Solubility, solubility parameters and solution thermodynamics of thymoquinone in different mono solvents. J. Mol. Liq. 2018, 272, 912–918. [Google Scholar] [CrossRef]
- Anwer, M.K.; Al-Shdefat, R.; Jamil, S.; Alam, P.; Abdel-Kader, M.S.; Shakeel, F. Solubility of Bioactive Compound Hesperidin in Six Pure Solvents at (298.15 to333.15) K. J. Chem. Eng. Data 2014, 596, 2065–2069. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Sample | xe | ||||
|---|---|---|---|---|---|
| T = 298.2 K | T = 303.2 K | T = 308.2 K | T = 313.2 K | T = 318.2 K | |
| BMMHFP | 1.89 × 10−2 | 2.29 × 10−2 | 2.80 × 10−2 | 3.42 × 10−2 | 4.47 × 10−2 |
| HMMHFP | 3.22 × 10−2 | 3.95 × 10−2 | 4.91 × 10−2 | 6.08 × 10−2 | 7.46 × 10−2 |
| OMMHFP | 1.04 × 10−2 | 1.24 × 10−2 | 1.51 × 10−2 | 1.86 × 10−2 | 2.29 × 10−2 |
| Transcutol-HP | 1.30 × 10−2 | 1.57 × 10−2 | 1.89 × 10−2 | 2.28 × 10−2 | 2.72 × 10−2 |
| Water | 1.45 × 10−3 | 1.69 × 10−3 | 2.03 × 10−3 | 2.47 × 10−3 | 3.01 × 10−3 |
| xidl | 7.61 × 10−2 | 9.29 × 10−2 | 1.13 × 10−1 | 1.37 × 10−1 | 1.65 × 10−1 |
| Sample | Hansen Solubility Parameters | |||
|---|---|---|---|---|
| δd (MPa1/2) | δp (MPa1/2) | δh (MPa1/2) | δ (MPa1/2) | |
| AHF | 19.20 | 10.80 | 11.90 | 25.00 |
| BMMHFP | 16.40 | 3.90 | 10.40 | 19.80 |
| HMMHFP | 16.30 | 10.60 | 11.60 | 22.70 |
| OMMHFP | 15.50 | 3.30 | 8.10 | 17.80 |
| Transcutol-HP | 16.30 | 7.20 | 11.90 | 21.40 |
| Water | 15.50 | 16.00 | 42.30 | 47.80 |
| Sample | γi | ||||
|---|---|---|---|---|---|
| T = 298.2 K | T = 303.2 K | T = 308.2 K | T = 313.2 K | T = 318.2 K | |
| BMMHFP | 4.02 | 4.04 | 4.02 | 4.01 | 3.70 |
| HMMHFP | 2.37 | 2.35 | 2.30 | 2.26 | 2.22 |
| OMMHFP | 7.26 | 7.49 | 7.48 | 7.36 | 7.24 |
| Transcutol-HP | 5.84 | 5.92 | 5.98 | 5.99 | 6.08 |
| Water | 52.36 | 54.83 | 55.61 | 55.43 | 54.95 |
| Sample | A | B | C | R2 | RMSD (%) | Overall RMSD (%) |
|---|---|---|---|---|---|---|
| BMMHFP | −820.40 | 34062.78 | 123.24 | 0.9990 | 0.91 | |
| HMMHFP | −147.87 | 3229.04 | 23.44 | 0.9999 | 0.74 | |
| OMMHFP | −546.91 | 21723.32 | 82.40 | 0.9996 | 0.57 | 0.62 |
| Transcutol-HP | −77.57 | 370.85 | 12.63 | 0.9998 | 0.76 | |
| Water | −596.20 | 24103.49 | 89.30 | 0.9996 | 0.87 |
| Sample | a | B | R2 | RMSD (%) | Overall RMSD (%) |
|---|---|---|---|---|---|
| BMMHFP | 9.47 | −4013.50 | 0.9929 | 1.54 | |
| HMMHFP | 9.98 | −4003.70 | 0.9997 | 1.70 | |
| OMMHFP | 7.93 | −3730.70 | 0.9964 | 0.74 | 1.42 |
| Transcutol-HP | 7.46 | −3521.40 | 0.9998 | 1.44 | |
| Water | 5.13 | −3485.20 | 0.9953 | 1.69 |
| Sample | ΔsolH0 (kJ mol−1) | ΔsolG0 (kJ mol−1) | ΔsolS0 (J mol−1 K−1) | R2 |
|---|---|---|---|---|
| BMMHFP | 33.41 | 9.11 | 78.90 | 0.9931 |
| HMMHFP | 33.33 | 7.71 | 83.15 | 0.9997 |
| OMMHFP | 31.06 | 10.70 | 66.09 | 0.9965 |
| Transcutol-HP | 29.31 | 10.16 | 62.18 | 0.9998 |
| Water | 29.01 | 15.83 | 42.80 | 0.9954 |
| Material | Molecular Formula | Molar Mass (g mol−1) | CAS Registry No. | Purification Method | Mass Fraction Purity | Analysis Method | Source |
|---|---|---|---|---|---|---|---|
| AHF | C64H110N6O16 | 1219.61 | 173334-58-2 | None | >0.99 | HPLC | Synthesized |
| BMMHFP | C8H15F6N2P | 284.18 | 174501-64-5 | None | >0.99 | HPLC | Sigma Aldrich |
| HMMHFP | C10H19F6N2P | 312.24 | 304680-35-1 | None | >0.99 | HPLC | Sigma Aldrich |
| OMMHFP | C12H23F6N2P | 340.29 | 304680-36-2 | None | >0.99 | HPLC | Fluka Chemica |
| Transcutol-HP | C6H14O3 | 134.17 | 111-90-0 | None | >0.99 | GC | Gattefosse |
| Water | H2O | 18.07 | 7732-18-5 | None | - | - | Milli-Q |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anwer, M.K.; Muqtader, M.; Iqbal, M.; Ali, R.; Almutairy, B.K.; Alshetaili, A.; Alshahrani, S.M.; Aldawsari, M.F.; Alalaiwe, A.; Shakeel, F. Estimating the Solubility, Solution Thermodynamics, and Molecular Interaction of Aliskiren Hemifumarate in Alkylimidazolium Based Ionic Liquids. Molecules 2019, 24, 2807. https://doi.org/10.3390/molecules24152807
Anwer MK, Muqtader M, Iqbal M, Ali R, Almutairy BK, Alshetaili A, Alshahrani SM, Aldawsari MF, Alalaiwe A, Shakeel F. Estimating the Solubility, Solution Thermodynamics, and Molecular Interaction of Aliskiren Hemifumarate in Alkylimidazolium Based Ionic Liquids. Molecules. 2019; 24(15):2807. https://doi.org/10.3390/molecules24152807
Chicago/Turabian StyleAnwer, Md. Khalid, Mohammad Muqtader, Muzaffar Iqbal, Raisuddin Ali, Bjad K. Almutairy, Abdullah Alshetaili, Saad M. Alshahrani, Mohammed F. Aldawsari, Ahmed Alalaiwe, and Faiyaz Shakeel. 2019. "Estimating the Solubility, Solution Thermodynamics, and Molecular Interaction of Aliskiren Hemifumarate in Alkylimidazolium Based Ionic Liquids" Molecules 24, no. 15: 2807. https://doi.org/10.3390/molecules24152807





