Soy Isoflavones Ameliorate Fatty Acid Metabolism of Visceral Adipose Tissue by Increasing the AMPK Activity in Male Rats with Diet-Induced Obesity (DIO)
Abstract
1. Introduction
2. Results
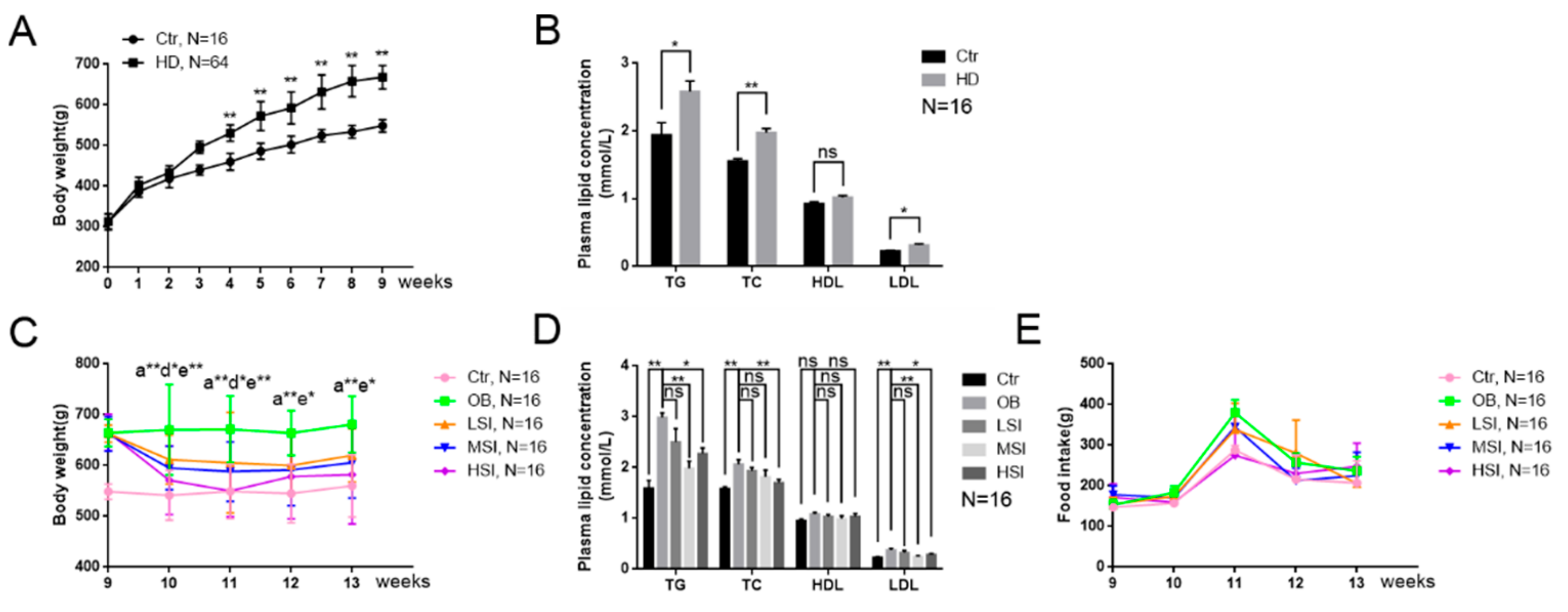
2.1. Soy Isoflavones Reduced Body Weight and Hyperlipidemia in Male Rates with Diet-Induced Obesity (DIO)
2.2. Soy Isoflavones Reduce Adipocyte Hypertrophy and Excessive Accumulation of Lipid in the Visceral Adipose Tissue of Male Rats with DIO
2.3. Soy Isoflavones Regulate Lipid Metabolism in the Visceral Adipose Tissue and Mature Adipocytes
2.4. Soy Isoflavones Enhance the Activity of AMPK
3. Discussion
4. Materials and Methods
4.1. Animal Care and Maintenance
4.2. Body Weight, Fat Weight, Food Intake, and Plasma Measurement Fat Weight
4.3. Histopathologic Evaluation
4.4. Cell Culture, Differentiation, and Treatment
4.5. Cell Viability Assay
4.6. Quantitative Real-Time Reverse-Transcription Polymerase Chain Reaction (Q-RT-PCR)
4.7. Western Blotting
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DIO. | Diet induced obesity |
| Ctr | Control |
| H and E staining | Hematoxylin and eosin staining |
| OB | Obesity control group |
| LSI | Low dose of soy isoflavones |
| MSI | Middle dose of soy isoflavones |
| HSI | High dose of soy isoflavones |
| TG | Triglycerides |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| SREBP-1c | Sterol regulatory element-binding transcription factor 1c |
| ACC | Acetyl-CoA carboxylase |
| ACL | ATP citrate lyase |
| FASN | Fatty acid synthase |
| ATGL | Adipose triglyceride lipase |
| HSL | Hormone sensitive lipase |
| AMPK | AMP-activated protein kinase |
References
- Organization, W.H. Global Strategy on Diet, Physical Activity and Health: Obesity and Overweight Fact sheet. Nutr. Newsl. 2005, 48, 292–302. [Google Scholar]
- Mcpherson, K. Reducing the global prevalence of overweight and obesity. Lancet 2014, 384, 728–730. [Google Scholar] [CrossRef]
- Greenberg, A.S.; Obin, M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006, 83, 461S–465S. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Talavera, O.; Tailleux, A.; Lefebvre, P.; Staels, B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 2017, 152, 1679–1694. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Morak, M.; Schmidinger, H.; Riesenhuber, G.; Rechberger, G.N.; Kollroser, M.; Haemmerle, G.; Zechner, R.; Kronenberg, F.; Hermetter, A. Adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) deficiencies affect expression of lipolytic activities in mouse adipose tissues. Mol. Cell. Proteom. 2012, 11, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, W.; McRae, S.; Marek, G.; Wymer, D.; Pannu, V.; Baylis, C.; Johnson, R.J.; Sautin, Y.Y. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes 2011, 60, 1258–1269. [Google Scholar] [CrossRef]
- Bays, H.E.; González-Campoy, J.M.; Bray, G.A.; Kitabchi, A.E.; Bergman, D.A.; Schorr, A.B.; Rodbard, H.W.; Henry, R.R. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev. Cardiovasc. Ther. 2008, 6, 343–368. [Google Scholar] [CrossRef]
- Schäffler, A.; Schölmerich, J.; Büchler, C. Mechanisms of disease: Adipocytokines and visceral adipose tissue—emerging role in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2005, 2, 273. [Google Scholar] [CrossRef]
- Freedland, E.S. Role of a critical visceral adipose tissue threshold (CVATT) in metabolic syndrome: Implications for controlling dietary carbohydrates: A review. Nutr. Metab. 2004, 1, 12. [Google Scholar] [CrossRef]
- Eun-Jeong, J.; Sung-Won, K.; Byung-Hwa, J.; Seon-Hee, O.; Byung-Hoon, L. Role of the AMPK/SREBP-1 pathway in the development of orotic acid-induced fatty liver. J. Lipid Res. 2011, 52, 1617–1625. [Google Scholar]
- Lee, J.H.; Jung, J.Y.; Jang, E.J.; Jegal, K.H.; Moon, S.Y.; Ku, S.K.; Kang, S.H.; Cho, I.J.; Park, S.J.; Lee, J.R. Combination of honokiol and magnolol inhibits hepatic steatosis through AMPK-SREBP-1c pathway. Exp. Biol. Med. 2015, 240, 508–518. [Google Scholar] [CrossRef]
- Carling, D.; Mayer, F.V.; Sanders, M.J.; Gamblin, S.J. AMP-activated protein kinase: Nature’s energy sensor. Nat. Chem. Biol. 2011, 7, 512. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Shen, Z.; Liang, X.C. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am. J. Physiol. 2010, 298, 364–374. [Google Scholar] [CrossRef]
- Bijland, S.; Mancini, S.J.; Salt, I.P. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin. Sci. 2013, 124, 491–507. [Google Scholar] [CrossRef]
- Zhuo, L.; Mekonnen, M.M.; Hoekstra, A.Y. Consumptive water footprint and virtual water trade scenarios for China—With a focus on crop production, consumption and trade. Environ. Int. 2016, 94, 211–223. [Google Scholar] [CrossRef]
- Kreijkamp-Kaspers, S.; Kok, L.; Grobbee, D.E.; de Haan, E.H.; Aleman, A.; Lampe, J.W.; van der Schouw, Y.T. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: A randomized controlled trial. JAMA 2004, 292, 65–74. [Google Scholar] [CrossRef]
- Lichtenstein, A.H. Soy protein, isoflavones and cardiovascular disease risk. J. Nutr. 1998, 128, 589. [Google Scholar] [CrossRef]
- Imhof, M.; Molzer, S.; Imhof, M. Effects of soy isoflavones on 17β-estradiol-induced proliferation of MCF-7 breast cancer cells. Toxicol. Vitr. 2008, 22, 1452–1460. [Google Scholar] [CrossRef]
- Islam, M.A.; Bekele, R.; Berg, J.H.J.V.; Kuswanti, Y.; Thapa, O.; Soltani, S.; Leeuwen, F.X.R.V.; Rietjens, I.M.C.M.; Murk, A.J. Deconjugation of soy isoflavone glucuronides needed for estrogenic activity. Toxicol. Vitr. 2015, 29, 706–715. [Google Scholar] [CrossRef]
- Lavie, C.J.; Milani, R.V.; Ventura, H.O. Obesity and Cardiovascular Disease: Risk Factor, Paradox, and Impact of Weight Loss. J. Am. Coll. Cardiol. 2009, 53, 1925–1932. [Google Scholar] [CrossRef]
- James, P.T.; Neville, R.; Rachel, L. The obesity epidemic, metabolic syndrome and future prevention strategies. Eur. Soc. Cardiol. Prev. Rehabil. 2004, 11, 3–8. [Google Scholar] [CrossRef]
- Ørgaard, A.; Jensen, L. The effects of soy isoflavones on obesity. Exp. Biol. Med. 2008, 233, 1066–1080. [Google Scholar] [CrossRef]
- Crespillo, A.; Alonso, M.; Vida, M.; Pavón, F.; Serrano, A.; Rivera, P.; Romero-Zerbo, Y.; Fernández-Llebrez, P.; Martínez, A.; Pérez-Valero, V. Reduction of body weight, liver steatosis and expression of stearoyl-CoA desaturase 1 by the isoflavone daidzein in diet-induced obesity. Br. J. Pharmacol. 2011, 164, 1899–1915. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.; Guo, J.; Fu, Z.; Yi, C.; Zhang, M.; Na, X. Soy isoflavone supplementation could reduce body weight and improve glucose metabolism in non-Asian postmenopausal women—A meta-analysis. Nutrition 2013, 29, 8–14. [Google Scholar] [CrossRef]
- Luo, T.; Miranda-Garcia, O.; Sasaki, G.; Wang, J.; Shay, N.F. Genistein and daidzein decrease food intake and body weight gain in mice, and alter LXR signaling in vivo and in vitro. Food Funct. 2018, 9, 6257–6267. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Pan, M.; Ho, C. Anti-obesity molecular mechanism of soy isoflavones: Weaving the way to new therapeutic routes. Food Funct. 2017, 8, 3831–3846. [Google Scholar] [CrossRef]
- Smith, S.R.; Lovejoy, J.C.; Greenway, F.; Ryan, D.; deJonge, L.; de la Bretonne, J.; Volafova, J.; Bray, G.A. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metab. Clin. Exp. 2001, 50, 425–435. [Google Scholar] [CrossRef]
- Brambilla, P.; Manzoni, P.; Sironi, S.; Simone, P.; Del, A.M.; Chiumello, G. Peripheral and abdominal adiposity in childhood obesity. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1994, 18, 795–800. [Google Scholar]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Ascencio, C.; Torres, N.; Isoard-Acosta, F.; Gómez-Pérez, F.J.; Hernández-Pando, R.; Tovar, A.R. Soy protein affects serum insulin and hepatic SREBP-1 mRNA and reduces fatty liver in rats. J. Nutr. 2004, 134, 522–529. [Google Scholar] [CrossRef]
- Beiroa, D.; Imbernon, M.; Gallego, R.; Senra, A.; Herranz, D.; Villarroya, F.; Serrano, M.; Fernø, J.; Salvador, J.; Escalada, J. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 2014, 63, 3346–3358. [Google Scholar] [CrossRef]
- Galic, S.; Fullerton, M.D.; Schertzer, J.D.; Sikkema, S.; Marcinko, K.; Walkley, C.R.; Izon, D.; Honeyman, J.; Chen, Z.-P.; van Denderen, B.J. Hematopoietic AMPK β1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J. Clin. Investig. 2011, 121, 4903–4915. [Google Scholar] [CrossRef]
- Pang, J.; Choi, Y.; Park, T. Ilex paraguariensis extract ameliorates obesity induced by high-fat diet: Potential role of AMPK in the visceral adipose tissue. Arch. Biochem. Biophys. 2008, 476, 178–185. [Google Scholar] [CrossRef]
- Mottillo, E.P.; Desjardins, E.M.; Crane, J.D.; Smith, B.K.; Green, A.E.; Ducommun, S.; Henriksen, T.I.; Rebalka, I.A.; Razi, A.; Sakamoto, K. Lack of adipocyte AMPK exacerbates insulin resistance and hepatic steatosis through brown and beige adipose tissue function. Cell Metab. 2016, 24, 118–129. [Google Scholar] [CrossRef]
- Mulligan, J.D.; Gonzalez, A.A.; Stewart, A.M.; Carey, H.V.; Saupe, K.W. Upregulation of AMPK during cold exposure occurs via distinct mechanisms in brown and white adipose tissue of the mouse. J. Physiol. 2007, 580, 677–684. [Google Scholar] [CrossRef]
- Cederroth, C.R.; Vinciguerra, M.; Gjinovci, A.; Kühne, F.; Klein, M.; Cederroth, M.; Caille, D.; Suter, M.; Neumann, D.; James, R.W. Dietary phytoestrogens activate AMP-activated protein kinase with improvement in lipid and glucose metabolism. Diabetes 2008, 57, 1176–1185. [Google Scholar] [CrossRef]
- Hwang, S.-L.; Kim, H.-N.; Jung, H.-H.; Kim, J.-E.; Choi, D.-K.; Hur, J.-M.; Lee, J.-Y.; Song, H.; Song, K.-S.; Huh, T.-L. Beneficial effects of β-sitosterol on glucose and lipid metabolism in L6 myotube cells are mediated by AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2008, 377, 1253–1258. [Google Scholar] [CrossRef]
- Daval, M.; Diot-Dupuy, F.; Bazin, R.; Hainault, I.; Viollet, B.; Vaulont, S.; Hajduch, E.; Ferré, P.; Foufelle, F. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J. Biol. Chem. 2005, 280, 25250–25257. [Google Scholar] [CrossRef]
- Yuan, H.; Shyy, J.Y.; Martins-Green, M. Second-hand smoke stimulates lipid accumulation in the liver by modulating AMPK and SREBP-1. J. Hepatol. 2009, 51, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Soetikno, V.; Sari, F.R.; Sukumaran, V.; Lakshmanan, A.P.; Harima, M.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin decreases renal triglyceride accumulation through AMPK-SREBP signaling pathway in streptozotocin-induced type 1 diabetic rats. J. Nutr. Biochem. 2013, 24, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Jeyabalan, J.; Aqil, F.; Munagala, R.; Annamalai, L.; Vadhanam, M.V.; Gupta, R.C. Chemopreventive and therapeutic activity of dietary blueberry against estrogen-mediated breast cancer. J. Agric. Food Chem. 2014, 62, 3963–3971. [Google Scholar] [CrossRef] [PubMed]



| Ingredients | Content |
|---|---|
| Corn | 54.0% |
| Fish meal | 6.0% |
| Wheat bran | 14.0% |
| Alfalfa meal | 13.0% |
| Cotton meal | 10.0% |
| Limestone | 1.00% |
| Dicalcium phosphate | 0.2% |
| Sodium chloride | 0.3% |
| Vitamin and mineral | 1.5% |
| Ingredients | Content |
|---|---|
| Basal diet | 69.5% |
| Pork fat | 15% |
| Sucrose | 15% |
| Pig bile | 0.5% |
| Groups | Control (Ctr, n = 16) | Obesity (OB, n = 16) | Low-Dose Soy Isoflavones (LSI, n = 16) | Middle-Dose Soy Isoflavones (MSI, n = 16) | High-Dose Soy Isoflavones (HSI, n = 16) |
|---|---|---|---|---|---|
| Diets | Basal diet | High-fat diet | High-fat diet + 50 mg/kg SIF | High-fat diet + 150 mg/kg SIF | High-fat diet + 450 mg/kg SIF |
| Compounds | Content |
|---|---|
| Daidzin | 50.98% |
| Glycitin | 30.36% |
| Genistein | 8.80% |
| Daidzein | 1.24% |
| Genistin | 0.06% |
| Total isoflavones (HPLC) | 91.64% |
| Gene | Primer Sequence (5′-3′) |
|---|---|
| ACC1 | F: ATTGTGGCTCAAACTGCAGGT |
| R: GCCAATCCACTCGAAGACCA | |
| ACC2 | F: CAACATCCGTCAGACGACCTC |
| R: CGGACTCGTTGGTGATGAAGA | |
| ACL | F: GCAGCACGTGATCCATGAAT |
| R: GTGGGATGCTGGACAACATC | |
| FASN | F: GCATTTCCACAACCCCAACC |
| R: AACGAGTTGATGCCCACGAT | |
| SREBP-1c | F: TGGACTACTAGTGTTGGCCTGCTT |
| R: ATCCAGGTCAGCTTGTTTGCGATG | |
| ATGL | F: TGGCGGCATTTCAGACAACT |
| R: GTCCATCTCGGTAGCCCTGTT | |
| HSL | F: GCGGACCAGCTCTAAAGAAAGA |
| R: TTTCATCCTTCTGCCCCCTAC | |
| AMPK | F: TTCGGGAAAGTGAAGGTGGG |
| R: GGTTCTGGATCTCTCTGCGG | |
| β-actin | F: ACGGTCAGGTCATCACTATCG |
| R: GGCATAGAGGTCTTTACGGATG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.; Huang, C.; Luo, Q.; Liu, W.; Cheng, D.; Li, Y.; Xia, Y.; Li, C.; Tang, L.; Fang, J.; et al. Soy Isoflavones Ameliorate Fatty Acid Metabolism of Visceral Adipose Tissue by Increasing the AMPK Activity in Male Rats with Diet-Induced Obesity (DIO). Molecules 2019, 24, 2809. https://doi.org/10.3390/molecules24152809
Tan J, Huang C, Luo Q, Liu W, Cheng D, Li Y, Xia Y, Li C, Tang L, Fang J, et al. Soy Isoflavones Ameliorate Fatty Acid Metabolism of Visceral Adipose Tissue by Increasing the AMPK Activity in Male Rats with Diet-Induced Obesity (DIO). Molecules. 2019; 24(15):2809. https://doi.org/10.3390/molecules24152809
Chicago/Turabian StyleTan, Jinlong, Chao Huang, Qihui Luo, Wentao Liu, Dongjing Cheng, Yifan Li, Yu Xia, Chao Li, Li Tang, Jing Fang, and et al. 2019. "Soy Isoflavones Ameliorate Fatty Acid Metabolism of Visceral Adipose Tissue by Increasing the AMPK Activity in Male Rats with Diet-Induced Obesity (DIO)" Molecules 24, no. 15: 2809. https://doi.org/10.3390/molecules24152809
APA StyleTan, J., Huang, C., Luo, Q., Liu, W., Cheng, D., Li, Y., Xia, Y., Li, C., Tang, L., Fang, J., Pan, K., Ou, Y., Cheng, A., & Chen, Z. (2019). Soy Isoflavones Ameliorate Fatty Acid Metabolism of Visceral Adipose Tissue by Increasing the AMPK Activity in Male Rats with Diet-Induced Obesity (DIO). Molecules, 24(15), 2809. https://doi.org/10.3390/molecules24152809





